Abstract
It is important to eliminate the fishy odor and improve the aroma quality of seafood. In this study, the Saccharina japonica (S. japonica) seedling, which is a new food material, was investigated for the effects of fermentation with Saccharomyces cerevisiae (S. cerevisiae) through sensory evaluation, GC–MS, and odor activity value (OAV) analysis. GC–MS analysis revealed the presence of 43 volatile compounds in the unfermented S. japonica seedling, with 1-octen-3-ol, hexanal, and trans-2,4-decadienal identified as the main contributors to its fishy odor. After fermentation with S. cerevisiae, 26 volatile compounds were identified in the S. japonica seedling. Notably, the major malodorous fish compounds, including 1-octen-3-ol, hexanal and trans-2,4-decadienal, were no longer present. The results indicate that fermentation with S. cerevisiae is an effective method for removing fishy malodor compounds and enhancing the volatile components with fruity, sweet, green, and floral notes in the Saccharina japonica seedling. This process facilitates the elimination of fishy malodor and enhance the fruity, sweet, green, and floral notes of S. japonica seeding and other seaweeds.
1. Introduction
Saccharina japonica (J.E. Areschoug) C.E. Lane, C. Mayes, Druehl & G.W. Saunders, also known as kelp and kombu, is a type of brown algae. S. japonica is a marine product that is rich in nutrients and contains a variety of functional substances, including protein, amino acids, polysaccharides, minerals, and vitamins [1,2]. S. japonica is particularly rich in iodine, and consuming this marine product could help prevent thyroid diseases [3,4]. In addition, S. japonica exhibits a variety of functional properties, including antioxidant, anti-inflammatory, hypoglycemic, and antihypertensive effects [5,6,7,8,9]. Therefore, S. japonica represents an important marine alga with the potential to provide health benefits to humans.
The aroma quality of food has a noticeable effect on the consumption preference of consumers. However, S. japonica products always have a strong fishy odor, which becomes the main barrier to human consumption [10,11]. In order to increase the consumption of S. japonica, it is essential to eliminate its fishy odor [12]. The methods of deodorization mainly include physical, chemical, and biological methods [13]. Compared with physical and chemical methods, biological methods are attracting more and more attention from researchers and manufacturers, due to their safety, environmental friendliness, and ease of implementation. Seo et al. [14] proposed fermenting S. japonica extract with Aspergillus oryzae to reduce its off-flavor. Although researchers have developed effective deodorization processes to remove the off-flavor from extracts and blends of S. japonica, few studies have been conducted to eliminate the fishy malodor from the foods made from S. japonica [14].
Recently, fermentation has been proposed to eliminate the off-flavors of S. japonica and improve the quality of S. japonica food products [15]. Nie et al. [16] demonstrated that the fermentation of S. japonica resulted in the reduction of fishy odorants, including hexanal and nonanal. Zhu et al. [17] demonstrated that fermentation with Saccharomyces cerevisiae could reduce 91% of unsaturated aldehydes from S. japonica. In addition, Lactobacillus plantarum and Acetobacter pasteurianus have also been used to ferment foods with desirable aromas [18,19]. In comparison, fermentation with Saccharomyces cerevisiae not only reduces fishy odors but also enhances fruit and floral aromas [17]. Therefore, researchers prefer to use S. cerevisiae to enhance the flavor of plant, seaweed, and fish products [20,21], which could be a practical method to enhance the flavor quality of food products made from S. japonica.
The S. japonica seedling is the S. japonica harvested at the early growth stage. It has higher nutritional and bioactivity values [22], making it increasingly important in food processing in recent years. However, S. japonica seedlings have a fishy odor, which limits consumer acceptance. Therefore, this study aimed to investigate the effectiveness of fermentation with Saccharomyces cerevisiae in removing the fishy odor from S. japonica seedlings. This study contributes to the understanding of the fishy odor of the novel food material S. japonica seedlings and provides a reference for enhancing the fruity, sweet, green, and floral notes of food products derived from S. japonica seedlings.
2. Materials and Methods
2.1. Chemicals
Standard 1-octen-3-ol (98%), 2-octen-1-ol (97%), 1-octanol (≥99%), anethol (≥99%), phenethyl alcohol (≥99%), 1-nonanol (≥98%), decyl aldehyde (analytical standard), undecanal (analytical standard), 1-octen-3-one (≥98%), 3-acetyl-2-octanone (≥98%), β-ionone (≥96%), 1,3-octadiene (≥95%), undecene (97%), 4-methyl-1-hendecene (97%), 8-heptadecene (98%), hexyl acetate (analytical standard), phenethyl acetate (analytical standard), hexyl butyrate (98%), ethyl caprate (≥99%), ethyl laurate (analytical standard), methyl palmitate (analytical standard), (E)-2-nonenal (≥95%), β-cyclocitral (≥95%), hexanal (95%), nonanal (96%), limonene (97%), and α-terpinene (≥95%) were purchased from Sigma Aldrich (St. Louis, MO, USA). Standard 2,4-dimethylbenzaldehyde (97%) and 2-pentylfuran (≥98%) were obtained from Alfa Aesar Co., Ltd. (Heysham, UK). A standard series of C8–C20 alkanes (analytical standard) was used for retention index (RI) determination, and the internal standard cyclohexanone was purchased from Sigma Co., Ltd. (St. Louis, MO, USA).
2.2. Preparing the Algae and Yeast
A Saccharina japonica seedling was obtained from the FuJian province of China in 2020. It was subsequently divided into 2 cm × 2 cm pieces. Saccharomyces cerevisiae CICC 1464 was purchased from the China Food and Fermentation Industry Research Institute Co., Ltd. (Beijing, China). The S. cerevisiae strain was activated in a 250 mL flask containing 50 mL of malt extract medium (malt extract 130 g/L and chloramphenicol 0.1 g/L) using a ZQZY-CF shaker (Shanghai Chuzhi Biotechnology Co., Ltd. (Shanghai, China) at 180 rpm and 28 °C for 3 days. The S. cerevisiae cells were harvested by centrifugation at 1500 rpm and 4 °C for 5 min.
2.3. Fermentation Procedure
The fermentation was conducted following the methods described in the literature, with minor modifications [23]. In detail, the yeast seed was adjusted to an optical density at 600 nm (OD600) of 0.8 with sterilized water. Ten grams of the S. japonica seedling was mixed with 100 mL of activated S. cerevisiae solution, followed by fermentation at 28 °C and 150 rpm for 120 min. The fermented S. japonica seedling was then removed and washed three times with 20 times the volume of distilled water. The seaweed was then dried at 40 °C to a moisture content of 9.5%.
2.4. Sensory Evaluation of Odor
According to the relevant literature [23] and ISO 13299:2016-05 [24], quantitative descriptive analysis (QDA) was used for the sensory evaluation of the odor. Ten panelists (5 males and 5 females) aged 22–26 years were trained to become familiar with the intensity of “fishy”, “floral”, “green”, “fatty”, “earthy”, “fruity” and “sweet” notes. For the sensory evaluation of the samples, five grams of S. japonica seedling was placed in a 100 mL Erlenmeyer flask, and the sensory evaluation was carried out at 26 ± 2 °C in a clean environment. After each sniff, the evaluators needed a 20 s gap with fresh air to refresh their olfactory fatigue. The panelists rated the sample on a scale of 0–9, with 0 representing no perceived attribute intensity and 9 representing very strong attribute intensity. All analyses were conducted in triplicate, and the average value was considered the final score of the sample, which was then visualized on the radar plot. The rights and privacy of all participants were protected during the research. This included ensuring no coercion to participate, providing full disclosure of study requirements and risks, obtaining verbal consent from the participants, refraining from releasing participant data without their knowledge, and allowing the participants to withdraw from the study at any time.
2.5. GC-MS Analysis
Thirty milliliters of distilled water and 1 g of S. japonica seedling were added to an extraction vial containing 10 µL of the internal standard (cyclohexanone). The sample was equilibrated at 60 °C for 30 min, followed by extraction for 30 min with a 50/30 µm DVB/CAR/PDMS extraction fiber.
A QP2010 gas chromatography–mass spectrometry (GC-MS) system (Shimadzu, Kyoto, Japan) was equipped with an Rtx-5MS column (60 m × 0.32 mm × 0.25 µm, Restek Corporation, Bellefonte, PA, USA). Helium (99.999% purity) was used as the carrier gas. The column flow rate was 3.16 mL/min in splitless injection mode. The injection port temperature was 230 °C. The column temperature was 40 °C at the start, increased to 100 °C at a rate of 6 °C/min, and then further increased to 230 °C at a rate of 5 °C/min and held for 5 min. The temperatures of the ion source and the interface were 220 °C and 250 °C, respectively [23]. The MS was set to electron impact (EI) ionization mode with a mass scan range from 35 to 500 amu, and the electron energy was set at 70 eV. The solvent delay time was 3 min.
Mass spectra were compared to the NIST library (NIST11, NIST11s, FFNSC1.3) to identify relevant molecules. The compounds with a mass spectrometry match greater than 80% were screened out. The identification was then confirmed by combining the base peak, characteristic ion peak, and retention index (RI). The retention index was calculated based on a standard mixture of n-alkanes (C8-C20) using Equation (1):
In this equation, RIx is the retention index of the component to be measured, n is the number of carbon atoms in the n-alkanes, RTx is the retention time of each odorant (x), and RTn and RTn+1 are the retention times of the n-alkanes eluted before and after the odorant (x) under identical chromatographic circumstances conditions.
The volatile compounds with chemical standards were quantitatively analyzed using their respective calibration curves. The volatile compounds without chemical standards were tentatively identified, and their concentrations were approximately estimated using the internal standard according to Equation (2):
In this equation, Cx represents the concentration of the compound to be tested, Px represents the peak area of the compound being tested, Ci represents the concentration of the internal standard, and Pi represents the peak area of the internal standard.
2.6. Analysis of odor Activity Value (OAV)
OAV was calculated by dividing the concentration of the volatile compounds by their odor threshold in water using Equation (3). The threshold value was obtained from the literature [25]. If OAV ≥ 1, the compound is considered to be an aroma compound that may be a major contributor.
In this equation, Ci is the concentration of volatile compounds and OTi is the odor threshold.
2.7. Statistical Analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) was used to calculate mean and errors and to conduct significance analysis (p ˂ 0.05). Analysis of variance (ANOVA) was performed to determine differences in the amounts of the aroma compounds of the quantified volatile compounds before and after fermentation.
3. Results
3.1. Sensory Evaluation of S. japonica seedling before and after Fermentation
As shown in Figure 1, the S. japonica seedling primarily exhibited a fishy odor, accompanied by subtle notes of green, fatty, earthy, floral, and fruity scents. After fermentation with S. cerevisiae, the fishy odor diminished, while the green, floral, sweet, and fruity notes intensified. The results indicated that fermentation with S. cerevisiae could enhance the aroma of the S. japonica seedling. This finding is consistent with the study of Xu et al. [23].
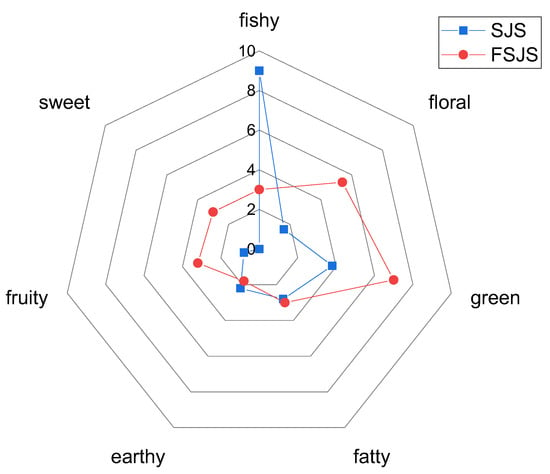
Figure 1.
A spider diagram illustrating the sensory characteristics of the S. japonica seedling before and after fermentation (SJS refers to the S. japonica seedling without fermentation. FSJS is the S. japonica seedling after fermentation. 0 refers to unrecognized, 2 refers to can be recognized, 4 refers to weak, 6 refers to middle, 8 refers to strong, 10 refers to very strong).
3.2. Qualitative and Quantitative Analysis of Volatile Compounds before and after Fermentation
A total of 43 volatile compounds were detected in the unfermented S. japonica seedling, including 6 alcohols, 10 aldehydes, 10 ketones, 4 alkanes, 8 alkenes, 3 esters, and 2 other compounds (Table 1). As shown in Table 1, aldehydes and ketones are the most abundant compounds, followed by alkenes and alcohols. Among the aldehydes, 1-nonanal and hexanal are the most abundant in concentration. Ketones are the second most abundant chemical after aldehydes. Among the ketones, 2,2,6-trimethylcyclohexanone and β-ionone showed relatively high concentrations. Alkenes are the third most abundant chemical after ketones. Among the ketones, 3,5,5-trimethyl-2-hexene and 8-heptadecene exhibited the highest relative abundance in concentration. As for the alcohols, 1-octen-3-ol had the highest concentrations. These findings are consistent with those of Zhu et al. [17].

Table 1.
Quantitative analysis and approximate estimates of volatile compounds (relative to cyclohexanone) in the S. japonica seedling before and after S. cerevisiae fermentation.
The fermented S. japonica seedling was found to contain 26 volatile compounds (Table 1), including 2 alcohols, 6 aldehydes, 2 ketones, 8 alkanes, 3 alkenes, 4 esters, and 1 other compound. Fermentation affects the concentration of volatile compounds. As shown in Figure 2, the quantities of alcohols, aldehydes, alkanes, esters, ketones, and others decrease. Among these, alcohols and alkenes showed a significant decrease. Alkanes are the only volatile compounds that behaved differently, increasing by almost a third. A comparative analysis of the volatile compounds present in the S. japonica seedlings before and after fermentation revealed significant variations in the composition of alcohols, aldehydes, and ketones (Table 1). For example, the content of alcohols such as 1-octen-3-ol, 2-octen-1-ol, 1-octanol, cis-anethol, trans-2-decen-1-ol, and 1-nonanol completely disappeared after fermentation and was replaced by phenethyl alcohol and cedrol. The content of aldehydes, such as hexanal, 2-nonenal, 2,4-dimethylbenzaldehyde, undecanal, and trans-2,4-decadienal, was completely depleted, while the amount of 1-nonanal decreased from 47.4 ± 1.7 to 11.6 ± 1.5 µg/g after fermentation. Most of the ketones disappeared after fermentation, such as 1-octen-3-one, 2,2,6-trimethylcyclohexanone, 3-methyl-2-cyclohexen-1-one, 3,5-octadien-2-one, 3-acetyl-2-octanone, 2,6,6-trimethyl-2-cyclohexanedione, 4-(2,6,6-trimethylcyclohexen-1-yl) butan-2-one, and 5,9-undecadien-2-one, 6,10-dimethyl.
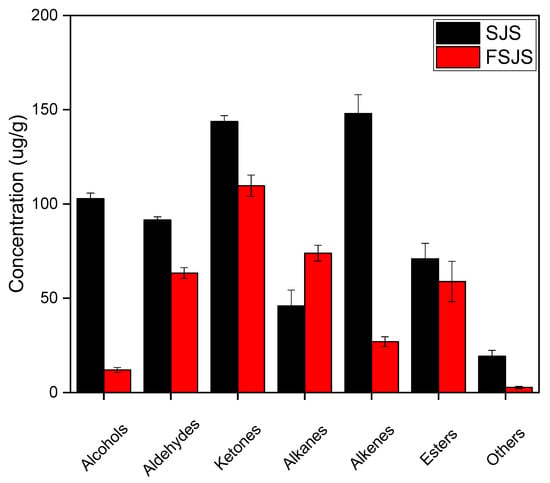
Figure 2.
Volatile compound concentrations in S. japonica seedling before and after fermentation by S. cerevisiae. SJS means unfermented S. japonica seedling; FSJS means fermented S. japonica seedling.
3.3. OAV Analysis of Volatile Compounds of S. japonica Seedling before and after Fermentation
The odor activity value (OAV) is the ratio of the compound concentration to its flavor threshold [26]. An OAV ≥ 1 indicates that the compound is a significant contributor to the overall odor [27]. As shown in Table 2, the S. japonica seedling before fermentation had 14 compounds with OAV ≥ 1, including 1-octen-3-ol, 2-octen-1-ol, 1-octanol, 1-nonanol, hexanal, 1-nonanal, 2-nonenal, 2,4-dimethylbenzaldehyde, decyl aldehyde, β-cyclocitral, trans-2,4-decadienal, dodecyl aldehyde, β-ionone, and 2-pentylfuran. Among these compounds, 1-octen-3-ol, 1-nonanal, and trans-2,4-decadienal, which had fishy odors, had the highest OAV values. Obviously, these three compounds are the main contributors to the fishy odor of the S. japonica seedling. This result is consistent with the findings of Nie et al. [16] and Xu et al. [23].

Table 2.
OAVs of the volatile compounds of the S. japonica seedling before and after fermentation.
After fermentation, the S. japonica seedling had 7 compounds with OAV ≥ 1, including 1-nonanal, decyl aldehyde, β-cyclocitral, dodecyl aldehyde, and β-ionone. Among these volatiles, 1-nonanal exhibited earthy and fatty odors; decyl aldehyde had ocean, cucumber, and herbal scents; β-cyclocitral emitted liquorice, fruity, and fresh aromas; dodecyl aldehyde presented herbal, fatty, and soap odors; and β-ionone showed floral and raspberry odors. Moreover, after fermentation, the OAVs of 1-octen-3-ol, hexanal, and trans-2,4-decadienal decreased to 0, and the OAV of 1-nonanal decreased from 43,093 to 10,541. This indicates that the fishy malodor of the fermented S. japonica seedling was dramatically reduced, while the floral and herbal notes were significantly enhanced.
Combining Figure 1 with Figure 3A, the OAV results are consistent with sensory properties. Unfermented S. japonica seedlings mainly present a fishy odor, which is reflected in OAV of trans-2,4-decadienal, 1-nonanal, and 1-octen-3-ol showing higher values. After fermentation, the sensory properties of the S. japonica seedling exhibited stronger notes of green, floral, sweet, and fruity. The significantly higher levels of β-ionone, β-cyclocitral, and decyl aldehyde in the OAV support their role as contributors to green, floral, sweet, and fruity notes.
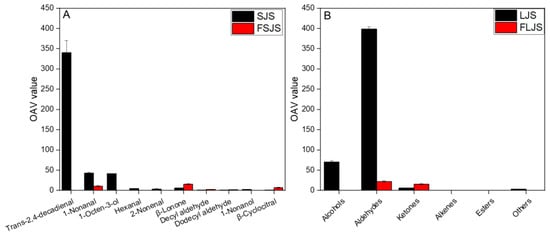
Figure 3.
OAVs of the volatile compounds of the S. japonica seedling before and after S. cerevisiae fermentation. SJS means unfermented S. japonica seedling; FSJS means fermented S. japonica seedling (A refers to the OAV value of volatile compounds relate with sensory properties; B refers to the OAV value of alcohols, aldehydes, ketones, alkenes and esters).
Comparing the OAV before and after fermentation, as shown in Figure 3B, it is evident that alcohols, aldehydes, and ketones make significant contributions to the overall aroma. Aldehydes exhibit the highest OAV, indicating that aldehydes are the main contributors to the odor of the S. japonica seedling. After fermentation, the OAV of alcohols decreased to 0, while aldehydes decreased and ketones increased slightly. The change in the OAV of the S. japonica seedling after fermentation indicates that aldehydes and ketones are the primary contributors to the fermented S. japonica seedling odor. The OAV of alkanes, alkenes, and esters equals to 0, essentially indicating that these three compounds do not significantly contribute to the odor of the S. japonica seedling.
4. Discussion
It is important to eliminate the fishy odor in seafood, as this is a practical approach to enhance the flavor quality of seafood and encourage consumption [21,28,29,30]. The S. japonica seedling, which is the S. japonica harvested at the early growth stage and considered a new food material, was first investigated in terms of its aroma profile, aroma-active volatiles, and aroma enhancement through fermentation.
One of the objectives of this study was to identify the volatile compounds that contribute to the off-flavor of the S. japonica seedling. The result of this study, showing that the S. japonica seedling has 43 volatile compounds, is consistent with the findings of Zhu et al. [17]. Among these volatile compounds, 1-nonanal and hexanal were the most abundant in concentration. Furthermore, 1-nonanal, hexanal, and 1-octen-3-ol were the main contributors to the fishy odor of the S. japonica seedling. Nie et al. [16] reported that 1-octen-3-ol is a typical volatile compound contributing to the fishy odor in kelp. Peinado et al. [31] reported that hexanal and nonanal play an important role in determining the fishy odor of seaweed samples. Thus, this study identifies the presence of 1-octen-3-ol, trans-2,4-decadienal, 1-octen-3-one, and hexanal as responsible for the pronounced fishy odor in the S. japonica seedling, which is identical to the fishy odor reported in previous studies by Nie et al. [16] and Peinado et al. [31]. This is the first study on the volatile compounds of the S. japonica seedling that adds to our understanding of aroma volatile compounds in seafood.
The second aim of the study was to investigate the effectiveness of fermentation with S. cerevisiae in enhancing the aroma of the S. japonica seedling. Several groups of volatile compounds increased after fermentation, including β-ionone (108.4 μg/g), β-cyclocitral (20.5 μg/g), D-limonene (8.3 μg/g), dodecyl aldehyde (4.1 μg/g), and decyl aldehyde (7.1 μg/g). OAV results indicated that these volatile compounds contributed floral and fruity notes. The sensory characteristics of the fermented S. japonica seedling showed enhanced notes of green, floral, sweet, and fruity aromas. Thus, this study identifies β-ionone, β-cyclocitral, DL-limonene, dodecyl aldehyde, and decyl aldehyde as the key fragrance components in the S. japonica seedling. Liang et al. [32] reported that Gracilaria lemaneiformis produced floral and fruity aroma compounds after fermentation, with β-cyclocitral being the main aroma contributor. Xu et al. [23] reported that D-limonene is the main sweet contributor of Bangia fusco-purpurea. β-Ionone, β-cyclocitral, D-limonene, dodecyl aldehyde, and decyl aldehyde belong to terpenoids. The increase in terpenoids after fermentation may be due to the mevalonic acid (MVA) pathway in S. cerevisiae. The MVA pathway involves a series of enzymatic reactions starting with acetyl-coenzyme A (acetyl-CoA) and culminating in the production of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) [33,34]. Enzymes pivotal to this pathway include mevalonate kinase, phosphomevalonate kinase, and mevalonic acid dehydrogenase [35]. IPP and DMAPP are the basis for the synthesis of all terpenoid compounds [36].
The results showed that fishy odor contributors such as 1-octen-3-ol, trans-2,4-decadienal, 1-octen-3-one, and hexanal completely disappeared after fermentation with S. cerevisiae. Additionally, the concentration of 1-nonanal decreased from 47.4 ± 1.7 to 11.6 ± 1.5 µg/g. The results indicated that the fishy odor of the S. japonica seedling was dramatically weakened after fermentation with S. cerevisiae. This finding is consistent with the studies of Xu et al. [23] and Liang et al. [32], which demonstrated that fermentation with Saccharomyces cerevisiae effectively eliminated the fishy odor from Bangia fusco-purpurea and Gracilaria lemaneiformis, respectively, confirming the potential of S. cerevisiae fermentation to enhance the flavor profile of various seaweed species such as S. japonica seedlings. This finding is partially consistent with that of Zhu et al. [17], who found that the fishy odorant 1-octen-3-one disappeared from kelp after fermentation with S. cerevisiae, accompanied by the formation of 3-octanone. 1-Octen-3-ol, hexanal, heptanal, nonanal, and 2,4-heptadienal, which have been reported as the fishy odorants [16,23,31], are mainly derived from the oxidation of unsaturated fatty acids [37]. Unsaturated aldehydes and ketones, such as 1-octen-3-one, 2,6-nonadienal, 2,4-decadienal, and 2-nonenal, have been identified as the contributors to the fishy odor of kelp [17]. Microbial fermentation has been utilized to deodorize seaweed, fish, and other seafood [38,39,40], as enone reductase catalyzes the reduction of unsaturated bonds [17]. In addition, the reduction of fishy odor is related to the reactions of ester synthesis, dehydrogenation, reduction, and deformylation–oxygenation catalyzed by enone reductase, oxidases, aldehyde deformylating oxygenase, aldehyde dehydrogenase, ester synthase, aldoketo reductase, alcohol dehydrogenases, epoxide hydrolase, dehydrogenase, and acyltransferase [23]. Moreover, the disappearance of 1-octen-3-ol may be related to the catalysis of enzymes such as ADH, ALDH, ES, and ATF, which can convert 1-octen-3-ol to esters [41]. Enzymes could catalyze the hexanal to alcohol and esters through the reactions of reduction, dehydrogenation, and ester synthesis [42]. In addition, 1-nonanal could be converted to fatty acids and enol through ester synthesis, dehydrogenation, and reduction reactions in yeast [43]. Thus, the removal of the fishy odor and the enhancement of the overall aroma profile could be attributed to the bioreactions involving reduction, dehydrogenation, and ester synthesis during fermentation with S. cerevisiae. This will facilitate the understanding of how microorganism metabolism enhances the flavor of seaweeds, such as S. japonica seedlings. However, it is still unclear as to what the transformation pathways are for the eliminating or removing the fishy odor. Future research is required to explore how the fishy odors evolve during the fermentation process with S. cerevisiae.
5. Conclusions
The new food material S. japonica seedling was sensorily evaluated and found to have a strong fishy odor. Fermentation with S. cerevisiae is effective in eliminating the fishy note and enhancing the fruity, sweet, green, and floral notes of S. japonica. After fermentation, the number of volatile compounds decreased from 43 to 26; compounds such as 1-octen-3-ol, 2-octen-1-ol, hexanal, 2-nonenal, and 1-octen-3-one disappeared, while 1-nonanal decreased from 47.4 to 11.6 μg/g. On the contrary, some volatile compounds representing aroma increased after fermentation: β-ionone increased from 42.8 to 108.4 μg/g, β-cyclocitral from 3.8 to 20.5 μg/g, D-limonene from 0 to 8.3 μg/g, dodecyl aldehyde from 2.3 to 4.1 μg/g, and decyl aldehyde from 3.0 to 7.1 μg/g. The OAV revealed 14 major odor compounds (OAV ≥ 1), including 1-octen-3-ol, 1-nonanal, and trans-2,4-decadienal, which contribute to the strong fishy malodor; and β-ionone, β-cyclocitral, dodecyl aldehyde, and decyl aldehyde, which contribute to the floral and fruity aroma. After fermentation, the OAV of these fishy odorants decreases to 0, while the floral and fruity aroma increases. Fermentation with S. cerevisiae is a practical method to enhance the flavor quality of the new food material S. japonica seedling.
Author Contributions
J.G.: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing. H.N.: Conceptualization, methodology, project administration, writing—review and editing. X.W.: Data curation, investigation. Y.W.: Funding acquisition, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fujian Provinceal Project for Promoting High-Quality Development of Marine and Fishery Industries, funding number: FJHYF-L-2023-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. World cuisine of seaweeds: Science meets gastronomy. Int. J. Gastron. Food S. 2018, 14, 55–65. [Google Scholar] [CrossRef]
- Park, E.; Yu, H.; Lim, J.-H.; Hee Choi, J.; Park, K.-J.; Lee, J. Seaweed metabolomics: A review on its nutrients, bioactive compounds and changes in climate change. Food Res. Int. 2023, 163, 112221. [Google Scholar] [CrossRef] [PubMed]
- Aoe, S.; Yamanaka, C.; Ohtoshi, H.; Nakamura, F.; Fujiwara, S. Effects of Daily Kelp (Laminaria japonica) Intake on Body Composition, Serum Lipid Levels, and Thyroid Hormone Levels in Healthy Japanese Adults: A Randomized, Double-Blind Study. Mar. Drugs 2021, 19, 352. [Google Scholar] [CrossRef] [PubMed]
- Miyai, K.; Tokushige, T.; Kondo, M.; Iodine Res, G. Suppression of Thyroid Function during Ingestion of Seaweed "Kombu" (Laminaria japonoca) in Normal Japanese Adults. Endocr. J. 2008, 55, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, H.; Liu, Y.; Xu, B.; Du, B.; Yang, Y. Effect of Ultrasonic Irradiation on the Physicochemical and Structural Properties of Laminaria japonica Polysaccharides and Their Performance in Biological Activities. Molecules 2023, 28, 8. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Khan, M.N.A.; Hong, Y.-K. Anti-inflammatory Activities of Undaria pinnatifida and Laminaria japonica (Phaeophyta). Can. J. Fish. Aquat.Sci 2007, 10, 127–132. [Google Scholar] [CrossRef]
- Kim, K.-B.-W.-R.; Kim, M.-J.; Ahn, D.-H. Anti-inflammatory Activity of an Ethanol Extract of Laminaria japonica Root on Lipopolysaccharide-induced Inflammatory Responses in RAW 264.7 Cells. Korean J. Food Sci. Technol. 2014, 46, 729–733. [Google Scholar] [CrossRef]
- Tong, A.; Li, Z.; Liu, X.; Ge, X.; Zhao, R.; Liu, B.; Zhao, L.; Zhao, C. Laminaria japonica polysaccharide alleviates type 2 diabetes by regulating the microbiota-gut-liver axis: A multi-omics mechanistic analysis. Int. J. Biol. Macromol. 2023, 258, 128853. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.; Das, G.; Baek, H. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef]
- Li, S.; Hu, M.; Tong, Y.; Xia, Z.; Tong, Y.; Sun, Y.; Cao, J.; Zhang, J.; Liu, J.; Zhao, S.; et al. A review of volatile compounds in edible macroalgae. Food Res. Int. 2023, 165, 112559. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Yu, M.; Jiang, S.; Qi, H. Effects of Different Processing Methods on the Quality and Physicochemical Characteristics of Laminaria japonica. Foods 2023, 12, 1619. [Google Scholar] [CrossRef] [PubMed]
- Ferraces-Casais, P.; Lage-Yusty, M.A.; Rodríguez-Bernaldo de Quirós, A.; López-Hernández, J. Rapid identification of volatile compounds in fresh seaweed. Talanta 2013, 115, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent advances in fishy odour in aquatic fish products, from formation to control. Int. J. Food Sci. Tech. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Bae, H.-N.; Eom, S.-H.; Lim, K.-S.; Yun, I.-H.; Chung, Y.-H.; Jeon, J.-M.; Kim, H.-W.; Lee, M.-S.; Lee, Y.-B.; et al. Removal of off-flavors from sea tangle (Laminaria japonica) extract by fermentation with Aspergillus oryzae. Bioresour. Technol. 2012, 121, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Wang, Z.; Yu, F.; Tang, X.; Su, L.; Zhang, S.; Sun, X.; Li, K.; Zhao, C.; Zhao, L. Changes in metabolite profiles and antioxidant and hypoglycemic activities of Laminaria japonica after fermentation. LWT Food Sci. Technol. 2022, 158, 113–122. [Google Scholar] [CrossRef]
- Nie, J.; Fu, X.; Wang, L.; Xu, J.; Gao, X. Impact of Monascus purpureus fermentation on antioxidant activity, free amino acid profiles and flavor properties of kelp (Saccharina japonica). Food Chem. 2023, 400, 133990. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jiang, B.; Zhong, F.; Chen, J.; Zhang, T. Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica). Foods 2021, 10, 2532. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, N.; Xu, J.; Guo, Q.; Shi, W. Effect of Lactobacillus plantarum and flavourzyme on physicochemical and safety properties of grass carp during fermentation. Food Chem. X 2022, 15, 100392. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, H.; Zhang, K.; Wang, M. Headspace Solid-Phase Microextraction Analysis of Volatile Compounds in Hawthorn Vinegars Fermented by two Strains of Acetobacter. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics (BMEI 2010), Yantai University, Yantai, China, 16–18 October 2010; pp. 2057–2061. [Google Scholar]
- Du, X.; Xu, Y.; Jiang, Z.; Zhu, Y.; Li, Z.; Ni, H.; Chen, F. Removal of the fishy malodor from Bangia fusco-purpurea via fermentation of Saccharomyces cerevisiae, Acetobacter pasteurianus, and Lactobacillus plantarum. J. Food Biochem. 2021, 45, e13728. [Google Scholar] [CrossRef]
- Hosoglu, M.I. Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem. 2018, 240, 1210–1218. [Google Scholar] [CrossRef]
- Ni, H.; Wang, X.; Jiang, Z. Nutritional, taste and texture characteristics of juvenile and adult kelp. Food Sci. 2022, 43, 34–40. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Jiang, Z.-D.; Du, X.-P.; Zheng, M.-J.; Fan-Yang, Y.; Ni, H.; Chen, F. The identification of biotransformation pathways for removing fishy malodor from Bangia fusco-purpurea using fermentation with Saccharomyces cerevisiae. Food Chem. 2022, 380, 132103. [Google Scholar] [CrossRef] [PubMed]
- ISO PN-EN ISO 13299:2016-05; Sensory Analysis-Methodology-General Guidelines for Determining the Sensory Profile. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/obp/ui/#iso:std:iso:13299:ed-2:v1:en (accessed on 15 June 2024).
- Van Gemert, L.J. OdourThesholds. Compilation of Odour Threshold Values in Air, Water and Other Media, 2011 Ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Guo, X.; Schwab, W.; Ho, C.-T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Duan, R.; Wang, Y.; He, Y.; Li, C.; Shen, X.; Li, Y. Effects of different drying temperatures on the profile and sources of flavor in semi-dried golden pompano (Trachinotus ovatus). Food Chem. 2023, 401, 134112. [Google Scholar] [CrossRef] [PubMed]
- Van Durme, J.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the Volatile Composition and Sensory Properties of Five Species of Microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, X.; Yang, K.; Yu, J.; Liang, F.; Wang, C.; Yang, B.; Chen, T.; Li, Z.; Li, X.; et al. Identification and evaluation of fishy odorants produced by four algae separated from drinking water source during low temperature period: Insight into odor characteristics and odor contribution of fishy odor-producing algae. Chemosphere 2023, 324, 138328. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Gonçalves, A.M.M. Novel Technologies for Seaweed Polysaccharides Extraction and Their Use in Food with Therapeutically Applications—A Review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, C.; He, Z.; Lin, X.; Chen, B.; Li, W. Changes in characteristic volatile aroma substances during fermentation and deodorization of Gracilaria lemaneiformis by lactic acid bacteria and yeast. Food Chem. 2023, 405, 134971. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wu, Y.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Efficient synthesis of limonene in Saccharomyces cerevisiae using combinatorial metabolic engineering strategies. J. Agr. Food Chem. 2023, 71, 7752–7764. [Google Scholar] [CrossRef]
- Tricarico, M.; Crovella, S.; Celsi, F. Mevalonate pathway blockade, mitochondrial dysfunction and autophagy: A possible link. Int. J. Mol. Sci. 2015, 16, 16067–16084. [Google Scholar] [CrossRef] [PubMed]
- Miziorko, M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chhatwal, H.; Pandey, A. Deciphering the Complexity of Terpenoid Biosynthesis and Its Multi-level Regulatory Mechanism in Plants. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Ma, R.; Liu, X.; Tian, H.; Han, B.; Li, Y.; Tang, C.; Zhu, K.; Li, C.; Meng, Y. Odor-active volatile compounds profile of triploid rainbow trout with different marketable sizes. Aquacult. Rep. 2020, 17, 100312. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, P.; Xia, W.; Jiang, Q.; Liu, S.; Xu, Y. Characterization of key aroma compounds in low-salt fermented sour fish by gas chromatography-mass spectrometry, odor activity values, aroma recombination and omission experiments. Food Chem. 2022, 397, 133773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, S.; Peng, Y.; Jin, Y.; Xu, D.; Xu, X. Effect of lactic acid bacteria on mackerel (Pneumatophorus japonicus) seasoning quality and flavor during fermentation. Food Biosci. 2021, 41, 100971. [Google Scholar] [CrossRef]
- López-Pérez, O.; Picon, A.; Nuñez, M. Volatile compounds and odour characteristics of seven species of dehydrated edible seaweeds. Food Res. Int. 2017, 99, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.L.; Susanto, A.V.; Keasling, J.D.; Leong, S.S.J.; Chang, M.W. Whole-Cell Biocatalytic and De Novo Production of Alkanes From Free Fatty Acids in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 232–237. [Google Scholar] [CrossRef]
- Park, Y.C.; Shaffer, C.E.H.; Bennett, G.N. Microbial formation of esters. Appl. Microbiol. Biot. 2009, 85, 13–25. [Google Scholar] [CrossRef]
- Vermeulen, N.; Czerny, M.; Gaenzle, M.G.; Schieberle, P.; Vogel, R.F. Reduction of (E)-2-nonenal and (E,E)-2,4-decadienal during sourdough fermentation. J. Cereal Sci. 2007, 45, 78–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).