Sulfated Polysaccharides from Sea Cucumber Cooking Liquid Prevents Obesity by Modulating Gut Microbiome, Transcriptome, and Metabolite Profiles in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CLSPAJ
2.2. Compositional Analysis of CLSPAJ
2.3. Animals and Experimental Design
2.4. Biochemical Analysis of Serum Physiological Indices
2.5. Histological Examination
2.6. Gut Microbiome Analysis
2.7. Gut Metabolite Analysis
2.8. Transcriptome Analysis
2.9. Statistical Analysis
3. Results
3.1. Chemical Characterization of CLSPAJ
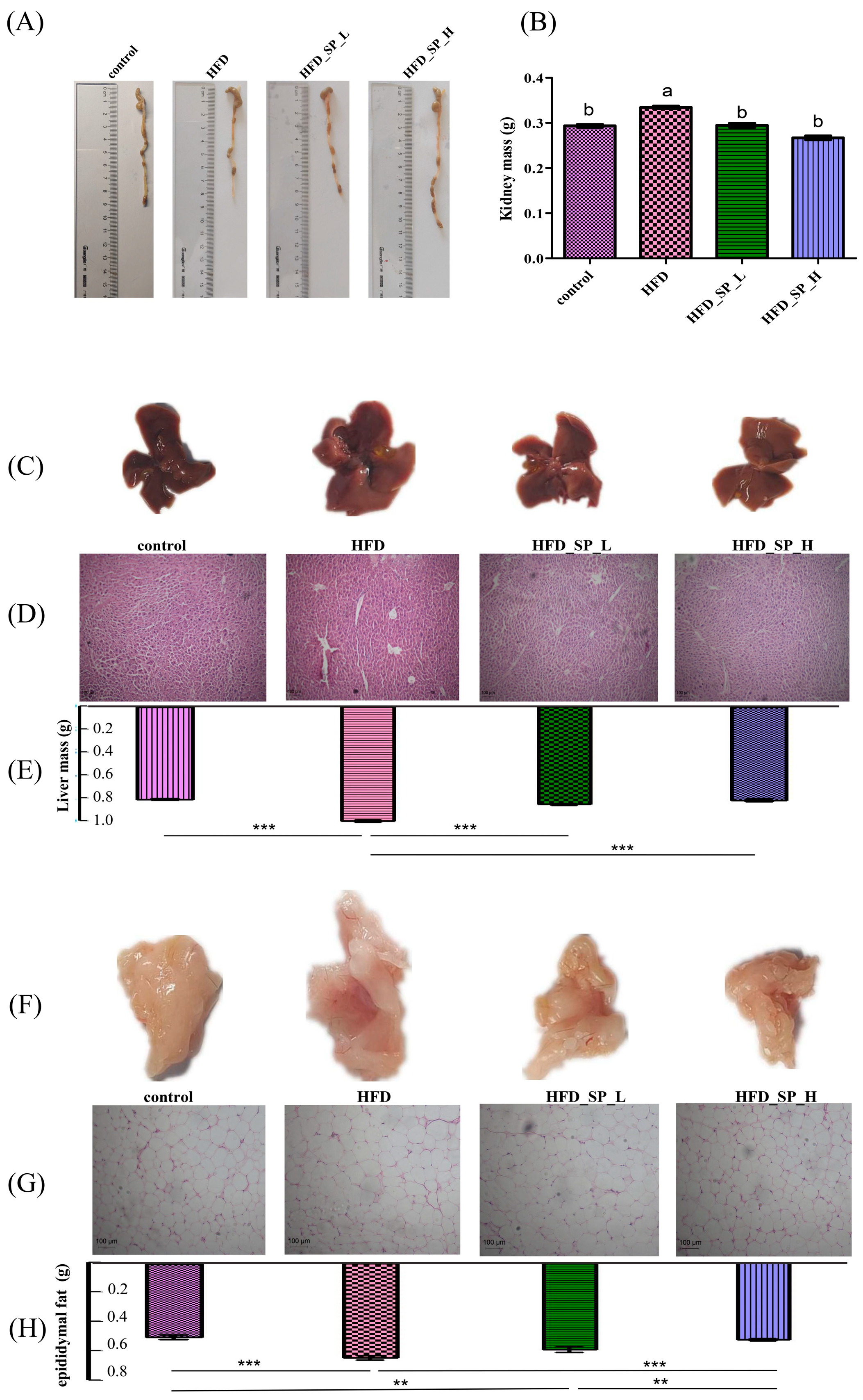
3.2. Effect of CLSPAJ on Body and Organ Weight and Lipid Metabolism
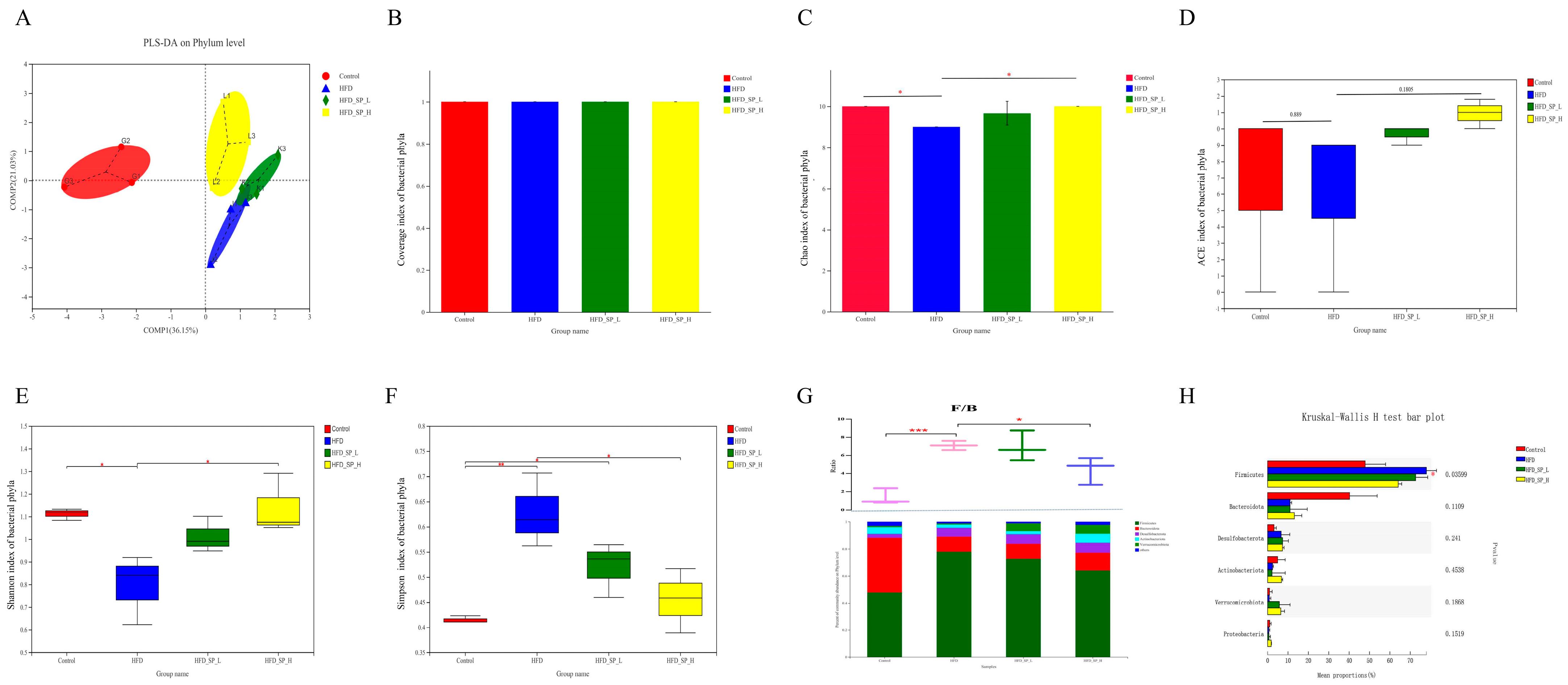
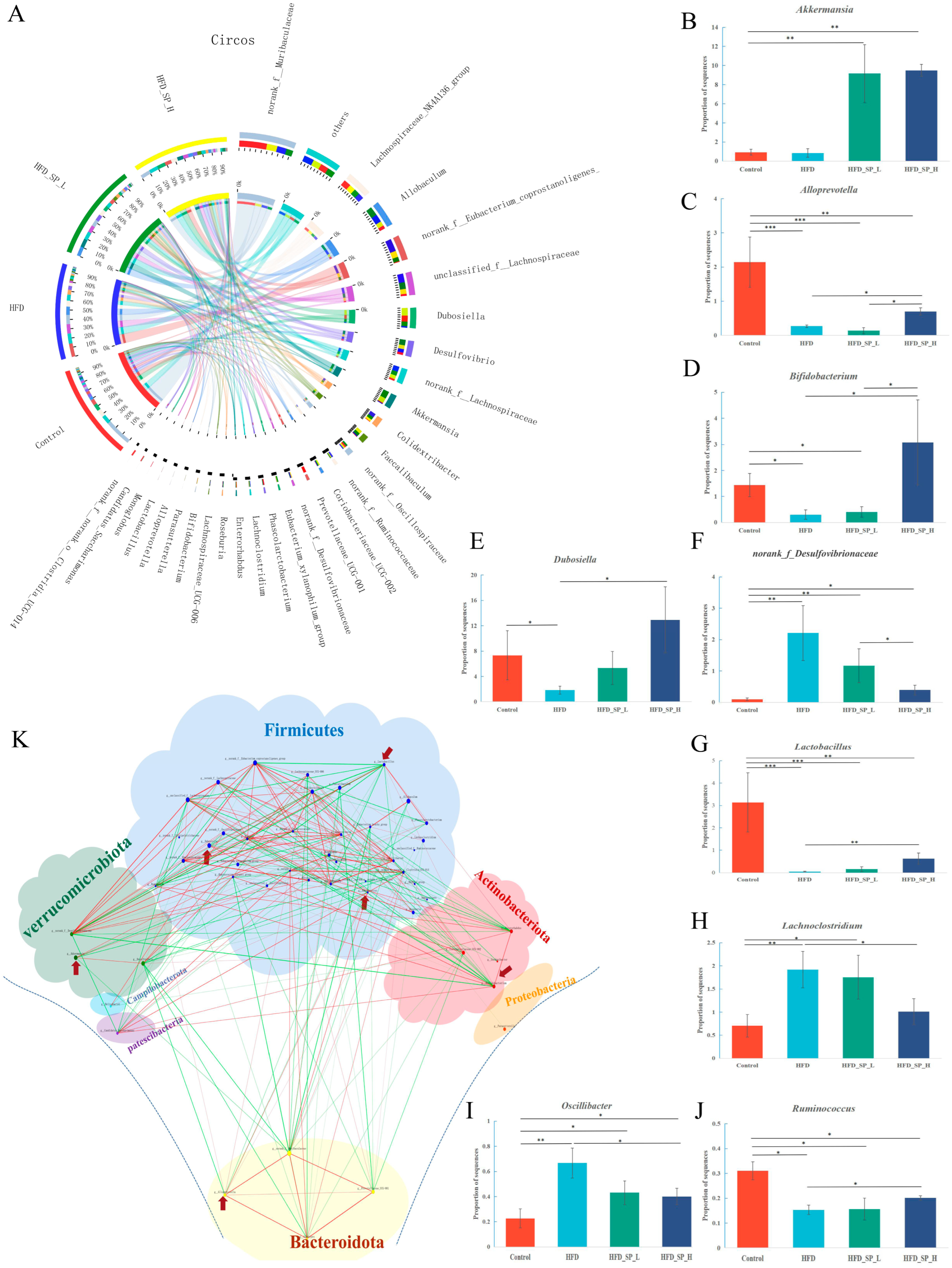
3.3. Effect of CLSPAJ on Gut Microbiota
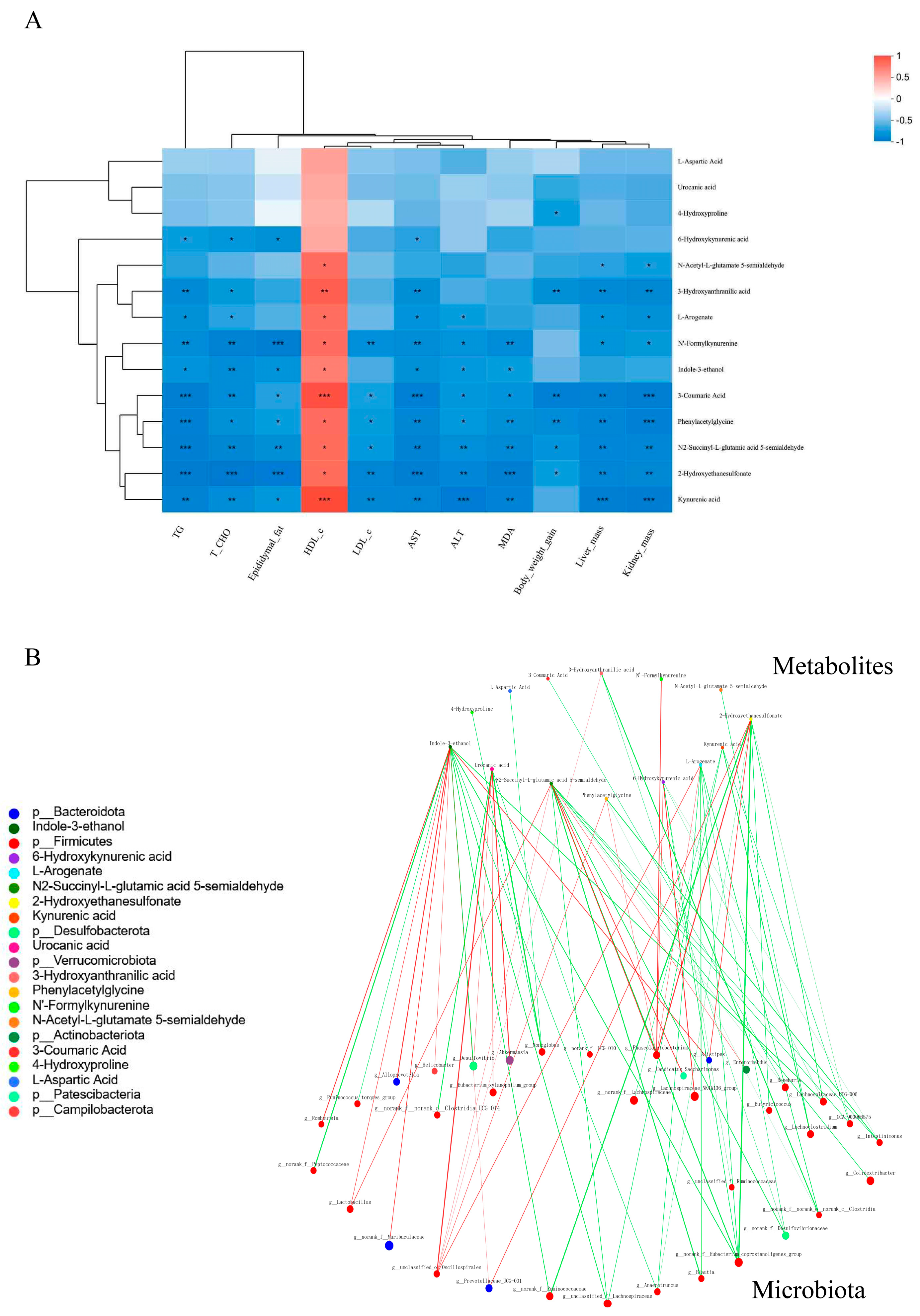
3.4. Effects of CLSPAJ on Alterations of the Metabolic Pathway
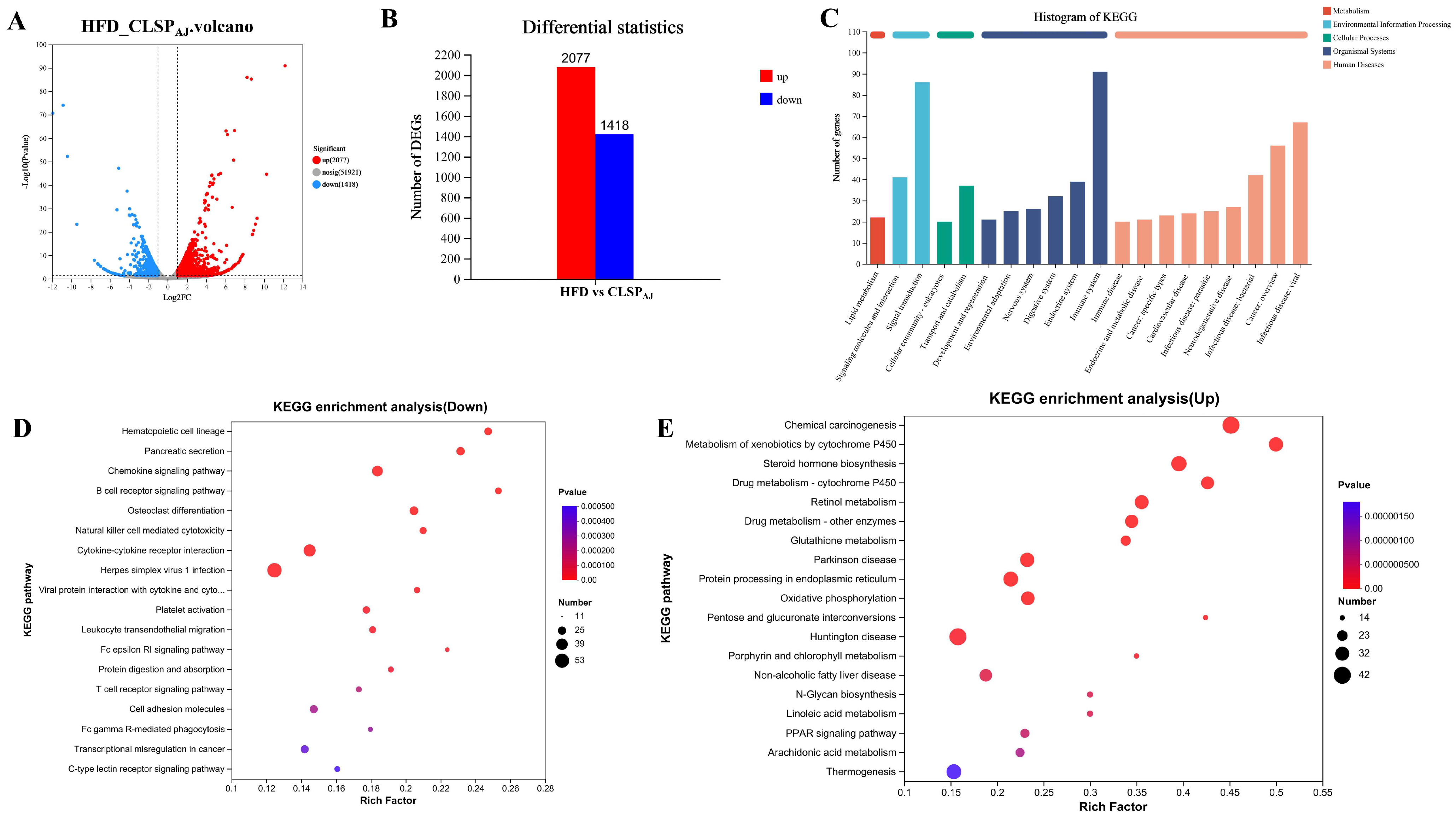
3.5. CLSPAJ Regulated Liver Transcriptome
3.6. Microbiome, Metabolome and Transcriptome Correlation Analysis
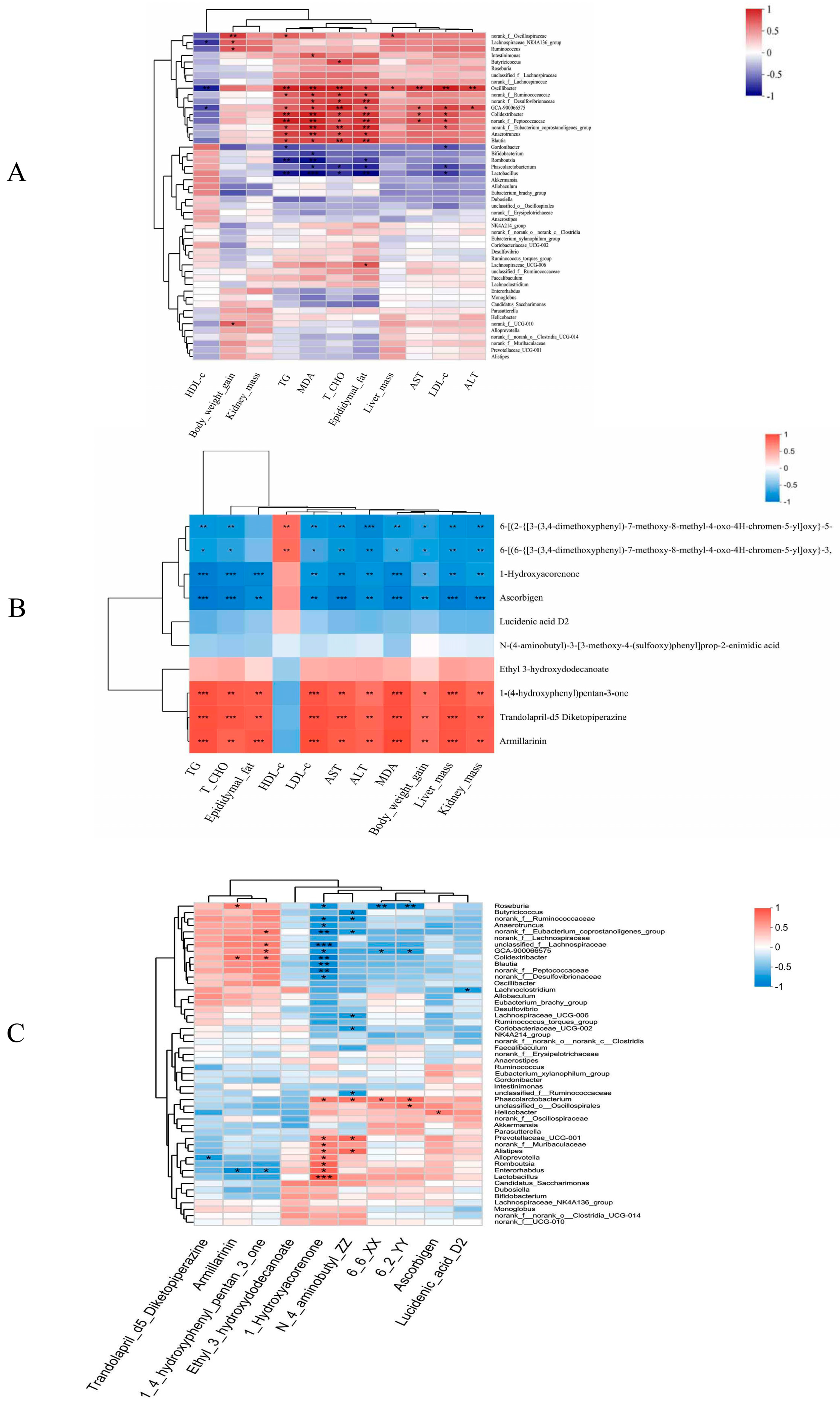
3.6.1. Correlation Analysis of Obesity-Related indices with Microbiome and Metabolite Profiles
3.6.2. CLSPAJ Altered the Gut Microbiome and Related Metabolite Profiles in HFD-Fed Mice
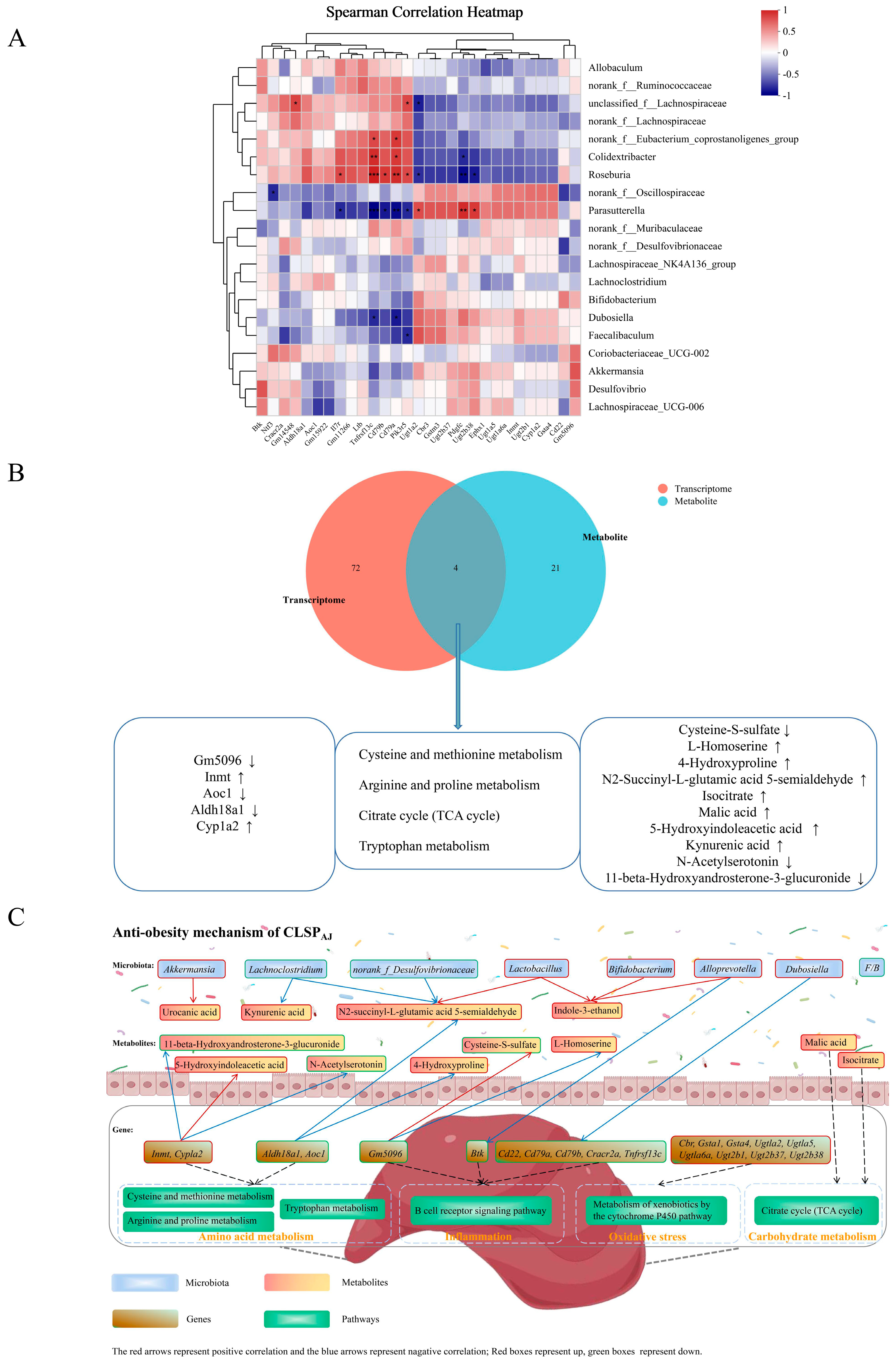
3.6.3. Microbiome–Transcriptome Correlation Analysis
3.6.4. Metabolome–Transcriptome Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Zhang, Y.; Ai, C.; Tian, W.; Wen, C.; Song, S.; Zhu, B. An acidic polysaccharide from Patinopecten yessoensis skirt prevents obesity and improves gut microbiota and metabolism of mice induced by high-fat diet. Food Res. Int. 2022, 154, 110980. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- de Wouters d’Oplinter, A.; Rastelli, M.; Van Hul, M.; Delzenne, N.M.; Cani, P.D.; Everard, A. Gut microbes participate in food preference alterations during obesity. Gut Microbes 2021, 13, 1959242. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H.; et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023, 35, 361–375. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Qi, Y.; Guo, Z.; Lin, Y.; Li, W.; Hu, Y.; Zhao, Q. Proximate composition and nutritional quality of deep sea growth sea cucumbers (Stichopus japonicus) from different origins. J. Sci. Food Agric. 2016, 96, 2378–2383. [Google Scholar] [CrossRef]
- Li, M.; Qi, Y.; Mu, L.; Li, Z.; Zhao, Q.; Sun, J.; Jiang, Q. Effects of processing method on chemical compositions and nutritional quality of ready-to-eat sea cucumber (Apostichopus japonicus). Food Sci. Nutr. 2019, 7, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Wang, F.; Zhou, B.; Sang, X.; Zhao, Q. The nutritional function of active polysaccharides from marine animals: A review. Food Biosci. 2024, 58, 103693. [Google Scholar] [CrossRef]
- Sang, X.; Li, Y.; Tong, Y.; Yu, S.; Song, Z.; Li, S.; Zhao, Q. Research progress on the interaction between sulfated polysaccharides from sea cucumber and gut microbiota with its regulation of glycolipid metabolism. Food Sci. 2023, 44, 321–331. (In Chinese) [Google Scholar]
- Zhang, Y.; Xie, Q.; You, L.; Cheung, P.C.K.; Zhao, Z. Behavior of non-digestible polysaccharides in gastrointestinal tract: A mechanistic review of its anti-obesity effect. eFood 2021, 2, 59–72. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Li, Z.; He, Y.; Li, W.; Wang, Q. Analysis and recovery of polysaccharide and components from sea cucumber processing waste liquid. J. Dalian Ocean 2010, 25, 434–438. (In Chinese) [Google Scholar]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhu, Z.; Wu, S.; Wang, J.; Chen, M.; Liu, W.; Huang, A.; Zhang, J.; Wu, Q.; Ding, Y. Polysaccharides from Cordyceps militaris prevent obesity in association with modulating gut microbiota and metabolites in high-fat diet-fed mice. Food Res. Int. 2022, 157, 111197. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Jiang, P.; Xu, Y.; Zheng, W.; Song, S.; Ai, C. Effect of sulfate group on sulfated polysaccharides-induced improvement of metabolic syndrome and gut microbiota dysbiosis in high fat diet-fed mice. Int. J. Biol. Macromol. 2020, 164, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, Y.; Du, W.; Du, W.; Li, G.; Wu, Y.; Luo, R.; Liu, S.; Fan, J. The potential value of LC-MS non-targeted metabonomics in the diagnosis of follicular thyroid carcinoma. Front. Oncol. 2022, 12, 1076548. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jiang, J.; Li, Y.; Ding, X.; Fang, F.; Chen, L. Quercetin regulates microglia M1/M2 polarization and alleviates retinal inflammation via ERK/STAT3 pathway. Inflammation 2024, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Li, Q.; Shi, W.; Qi, X.; Song, W.; Yang, J. Chain conformation, physicochemical properties of fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus and its in vitro fermentation by human gut microbiota. Carbohydr. Polym. 2020, 228, 115359. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Symonds, M.E. The impact of high intensity interval training on liver fat content in overweight or obese adults: A meta-analysis. Physiol. Behav. 2021, 236, 113416. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef]
- Amor, A.J.; Perea, V. Dyslipidemia in nonalcoholic fatty liver disease. Curr. Opin. Endocrinol. 2019, 26, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Huan, M.; Liu, F.; Zhou, S.; Shen, Q. Alteration of fecal microbiome and metabolome by mung bean coat improves diet-induced non-alcoholic fatty liver disease in mice. Food Sci. Hum. Wellness 2022, 11, 1259–1272. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Sang, Y.; Wang, X.; Wang, S. Metabonomics and the gut microbiome analysis of the effect of 6-shogaol on improving obesity. Food Chem. 2023, 404, 134734. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zu, X.; Wang, Z.; Xu, X.; Liu, G.; Liu, R. Ginsenoside Rc ameliorated atherosclerosis via regulating gut microbiota and fecal metabolites. Front. Pharmacol. 2022, 13, 990476. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Ai, C.; Wen, C.; Dong, X.; Sun, X.; Cao, C.; Zhang, X.; Zhu, B.; Song, S. Gut microbiota response to sulfated sea cucumber polysaccharides in a differential manner using an in vitro fermentation model. Food Res. Int. 2021, 148, 110562. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Choi, Y.; Bose, S.; Seo, J.; Shin, J.H.; Lee, D.; Kim, Y.; Kang, S.G.; Kim, H. Effects of live and pasteurized forms of Akkermansia from the human gut on obesity and metabolic dysregulation. Microorganisms 2019, 9, 2039. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, A.; Neyrinck, D.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simão, A.N.C.; Alfieri, D.F.; Lozovoy, M.A.B.; Mari, N.L.; de Souza, C.H.B.; Dichi, I.; Costa, G.N. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: A randomized trial. Effects of probiotics on metabolic syndrome. Nutrition 2015, 32, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef]
- Liu, J.; Yue, S.; Yang, Z.; Feng, W.; Meng, X.; Wang, A.; Peng, C.; Wang, C.; Yan, D. Oral hydroxysafflor yellow a reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol. Res. 2018, 134, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. 2020, 11, 5024–5039. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, X. Dietary amino acids and the gut-microbiome-immune axis: Physiological metabolism and therapeutic prospects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. Gut microbiota-derived metabolites in obesity: A systematic review. Biosci. Microbiota Food Health 2020, 39, 65–76. [Google Scholar] [CrossRef]
- Sharafedtinov, K.; Plotnikova, O.; Alexeeva, R.; Sentsova, T.; Songisepp, E.; Stsepetova, J.; Smidt, I.; Mikelsaar, M. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients-a randomized double-blind placebo-controlled pilot study. Nutr. J. 2013, 12, 138. [Google Scholar] [CrossRef]
- Leth, M.L.; Ejby, M.; Workman, C.; Ewald, D.A.; Pedersen, S.S.; Sternberg, C.; Bahl, M.I.; Licht, T.R.; Aachmann, F.L.; Westereng, B.; et al. Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat. Microbiol. 2018, 3, 570–580. [Google Scholar] [CrossRef]
- Ogata, Y.; Suda, W.; Ikeyama, N.; Hattori, M.; Ohkuma, M.; Sakamoto, M. Complete genome sequence of Phascolarctobacterium faecium JCM 30894, a succinate-utilizing bacterium isolated from human feces. Microbiol. Resour. Ann. 2019, 8, e01487-18. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Brown, L.; Panchal, S.K. Coffee pulp, a by-product of coffee production, modulates gut microbiota and improves metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Pathogens 2021, 10, 1369. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Kupcova, K.; Havranek, O. B-Cell receptor signaling and beyond: The role of Igα (CD79a)/Igβ (CD79b) in normal and malignant B cells. Int. J. Mol. Sci. 2024, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Hu, H.; Zhao, Y.; Jin, M.; Yang, D.; Yin, J.; Wu, P.; Liu, W.; Li, J. Benzene induces spleen injury through the B cell receptor signaling pathway. Ecotoxicol. Environ. Saf. 2023, 257, 114924. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Rice, L.; Shrimpton, J.; Lawless, D.; Walker, K.; Carter, C.; McKeown, L.; Anwar, R.; Doody, G.M.; Srikanth, S.; et al. Biallelic mutations in calcium release activated channel regulator 2A (CRACR2A) cause a primary immunodeficiency disorder. Elife 2021, 10, e72559. [Google Scholar] [CrossRef]
- Boušová, I.; Košťáková, Š.; Matoušková, P.; Bartikova, H.; Szotakova, B.; Skalova, L. Monosodium glutamate-induced obesity changed the expression and activity of glutathione S-transferases in mouse heart and kidney. Pharmazie 2017, 72, 257–259. [Google Scholar]
- Chartoumpekis, D.V.; Ziros, P.G.; Zaravinos, A.; Iskrenova, R.P.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Sykiotis, G.P.; Habeos, I.G. Hepatic gene expression profiling in Nrf2 knockout mice after long-term high-fat diet-induced obesity. Oxid. Med. Cell. Longev. 2013, 2013, 340731. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Cheng, N.; Zhu, Y.; Li, H.; Zhang, S.; Guo, W.; Ge, G. Pectolinarigenin ameliorates acetaminophen-induced acute liver injury via attenuating oxidative stress and inflammatory response in Nrf2 and PPARa dependent manners. Phytomedicine 2023, 113, 154726. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, P.; Cheng, Y.; Wang, P.; Ma, X.; Liu, M.; Wang, X.; Xu, F. Diet-induced obese alters the expression and function of hepatic drug-metabolizing enzymes and transporters in rats. Biochem. Pharmacol. 2019, 164, 368–376. [Google Scholar] [CrossRef]
- Xing, L.; Fu, L.; Hao, Y.; Miao, Y.; Zhang, W. Xuanwei ham derived peptides exert the anti-inflammatory effect in the dextran sulfate sodium-induced C57BL/6 mice model. Food Biosci. 2022, 48, 101800. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Xing, S.; Li, J.; Liang, D.; Chen, Y.; Abd El-Aty, A.M.; Zhu, B.W.; Liu, D.; Tan, M. Design and preparation of multifunctional astaxanthin nanoparticles with good acid stability and hepatocyte-targeting ability for alcoholic liver injury alleviation. Mater. Today Nano 2024, 25, 100436. [Google Scholar] [CrossRef]
- Oliveras-Cañellas, N.; Castells-Nobau, A.; de la Vega-Correa, L.; Latorre-Luque, J.; Motger-Albertí, A.; Arnoriaga-Rodriguez, M.; Garre-Olmo, J.; Zapata-Tona, C.; Coll-Martínez, C.; Ramió-Torrentà, L.; et al. Adipose tissue coregulates cognitive function. Sci. Adv. 2023, 9, eadg4017. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Hong, G.; Zhu, N.; Liu, Y.; Jiang, Y.; Liu, T. Photodynamic therapy of novel photosensitizer ameliorates TNBS-induced ulcerative colitis via inhibition of AOC1. Front. Pharmacol. 2021, 12, 746725. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ericksen, R.E.; Escande-Beillard, N.; Lee, Q.Y.; Loh, A.; Denil, S.; Steckel, M.; Haegebarth, A.; Wai Ho, T.S.; Chow, P.; et al. Metabolic pathway analyses identify proline biosynthesis pathway as a promoter of liver tumorigenesis. J. Hepatol. 2020, 72, 725–735. [Google Scholar] [CrossRef]
- Larsson, S.C.; Woolf, B.; Gill, D. Appraisal of the causal effect of plasma caffeine on adiposity, type 2 diabetes, and cardiovascular disease: Two sample mendelian randomisation study. BMJ Med. 2023, 2, e000335. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, X.; Guan, X.; Tong, Y.; Wang, F.; Zhou, B.; Li, Y.; Zhao, Q. Sulfated Polysaccharides from Sea Cucumber Cooking Liquid Prevents Obesity by Modulating Gut Microbiome, Transcriptome, and Metabolite Profiles in Mice Fed a High-Fat Diet. Foods 2024, 13, 2017. https://doi.org/10.3390/foods13132017
Sang X, Guan X, Tong Y, Wang F, Zhou B, Li Y, Zhao Q. Sulfated Polysaccharides from Sea Cucumber Cooking Liquid Prevents Obesity by Modulating Gut Microbiome, Transcriptome, and Metabolite Profiles in Mice Fed a High-Fat Diet. Foods. 2024; 13(13):2017. https://doi.org/10.3390/foods13132017
Chicago/Turabian StyleSang, Xue, Xin Guan, Yao Tong, Fuyi Wang, Boqian Zhou, Ying Li, and Qiancheng Zhao. 2024. "Sulfated Polysaccharides from Sea Cucumber Cooking Liquid Prevents Obesity by Modulating Gut Microbiome, Transcriptome, and Metabolite Profiles in Mice Fed a High-Fat Diet" Foods 13, no. 13: 2017. https://doi.org/10.3390/foods13132017
APA StyleSang, X., Guan, X., Tong, Y., Wang, F., Zhou, B., Li, Y., & Zhao, Q. (2024). Sulfated Polysaccharides from Sea Cucumber Cooking Liquid Prevents Obesity by Modulating Gut Microbiome, Transcriptome, and Metabolite Profiles in Mice Fed a High-Fat Diet. Foods, 13(13), 2017. https://doi.org/10.3390/foods13132017





