A Portable Automated Microfluidic Platform for Point-of-Care Testing for Multiple Mycotoxins in Wine
Abstract
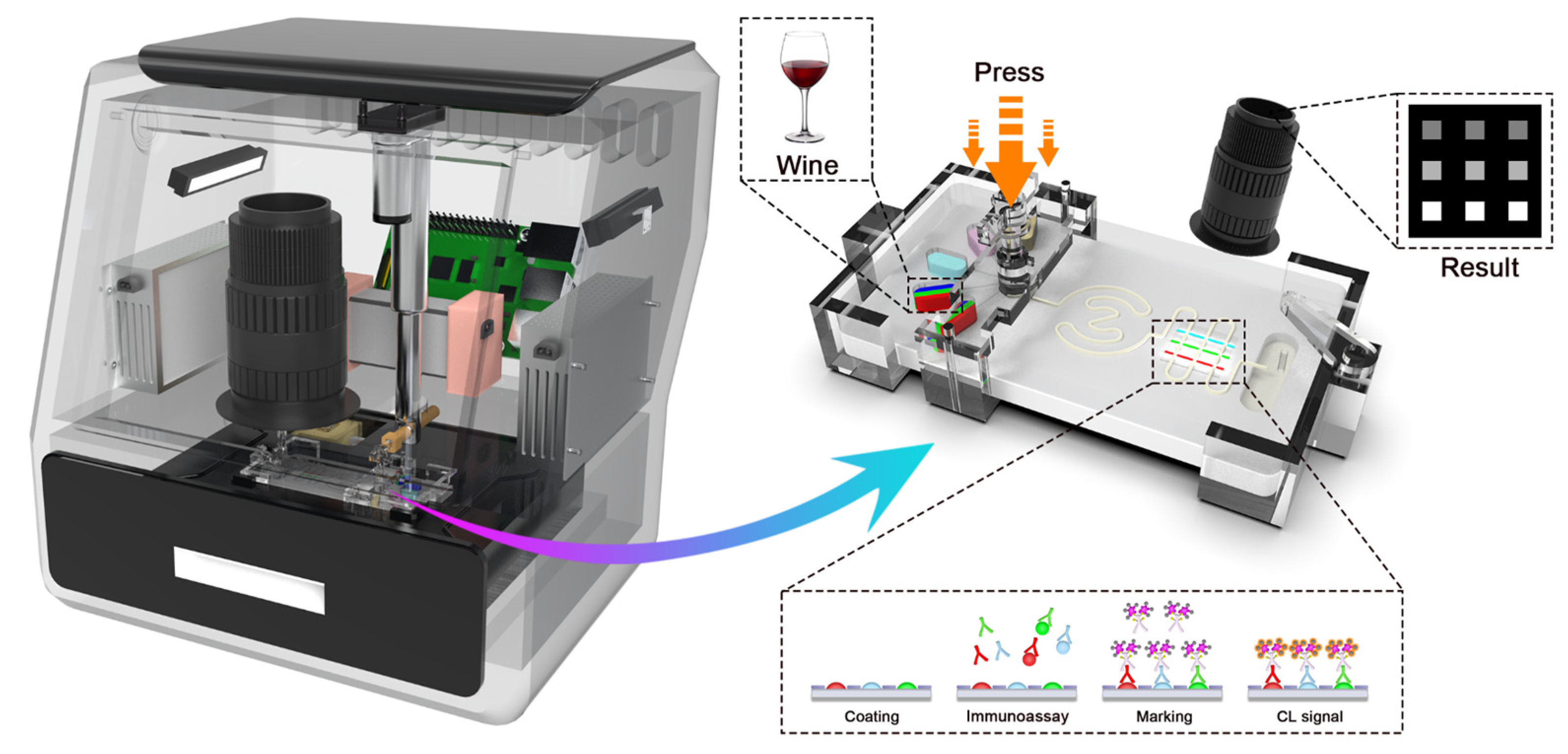
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
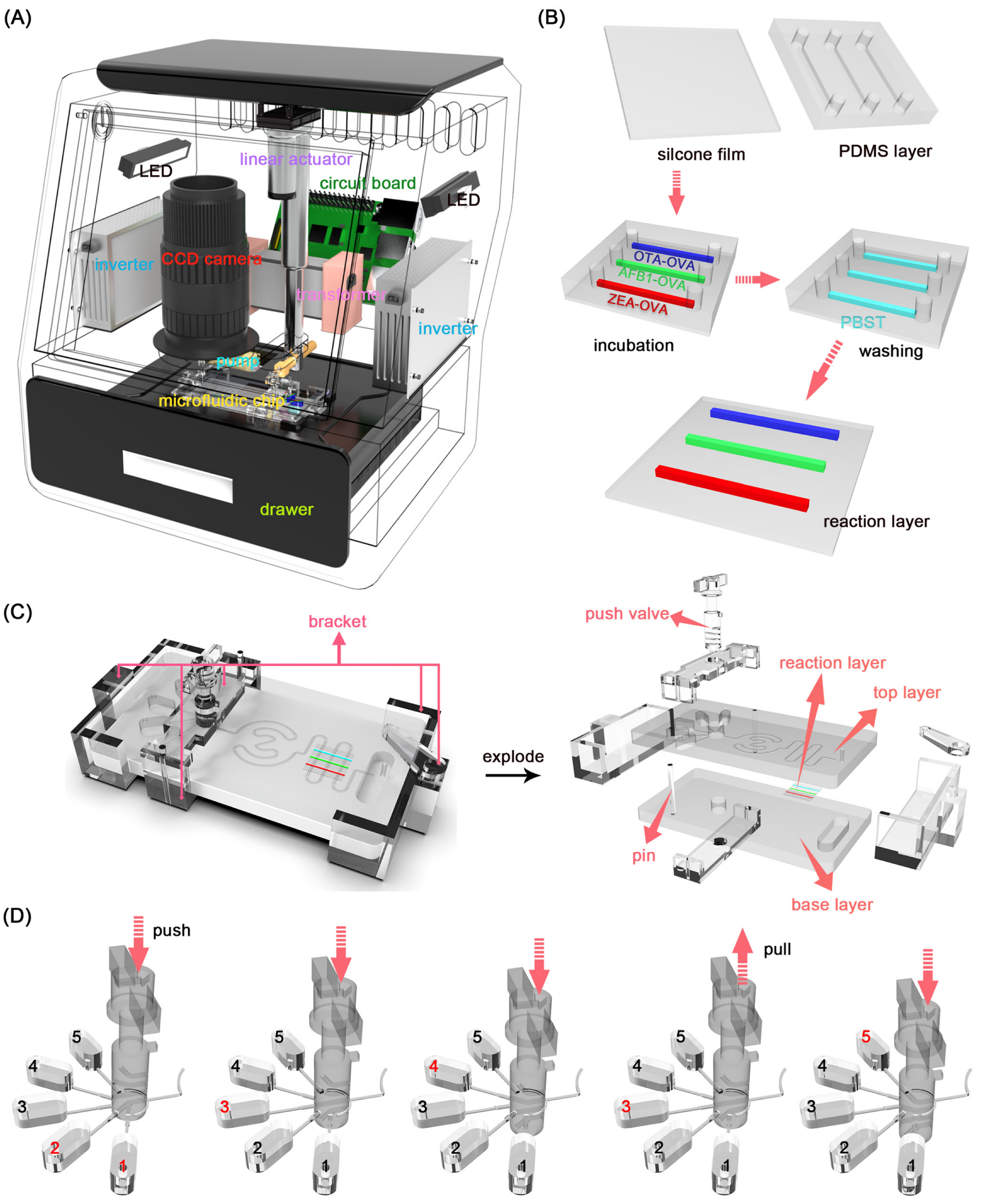
2.2. Design of the Microfluidic Chip
2.3. Fabrication of the Microfluidic Chip
2.4. Lab on a Microfluidic Chip
2.5. Imaging Detection
2.6. Statistical Evaluation
3. Results and Discussion
3.1. The Establishment of PAMP
3.2. The Mixing Performance of Micromixer
3.3. The Microfluidic Chip for Detection
3.4. Optimization of Coating Antigens and Capture Antibodies
3.5. Detecting Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeyeye, S.A.O. Aflatoxigenic fungi and mycotoxins in food: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 709–721. [Google Scholar] [CrossRef]
- Solfrizzo, M. Recent advances on Alternaria mycotoxins. Curr. Opin. Food Sci. 2017, 17, 57–61. [Google Scholar] [CrossRef]
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, X.; Fan, L.; Hu, H. Mechanisms of cell death induction by food-borne mycotoxins. Crit. Rev. Food Sci. Nutr. 2017, 58, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- Le, W.; Pengxue, S.; Mingna, L.; Yan, S.; Yangyong, L.; Hailong, T.; Dapeng, W.; Ying, L.; Qipeng, Y.; Jinshui, W. Application of the in-situ biological detoxification polymer for the improvement of AFB1 detoxification. LWT-Food Sci. Technol. 2022, 159, 113239. [Google Scholar] [CrossRef]
- Qileng, A.; Wei, J.; Lu, N.; Liu, W.; Cai, Y.; Chen, M.; Lei, H.; Liu, Y. Broad-specificity photoelectrochemical immunoassay for the simultaneous detection of ochratoxin A, ochratoxin B and ochratoxin C. Biosens. Bioelectron. 2018, 106, 219–226. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, L.; Chen, J.; Wang, W.; Zhang, R.; Li, Y.; Zhang, Q.; Wang, W. Degradation mechanism for Zearalenone ring-cleavage by Zearalenone hydrolase RmZHD: A QM/MM study. Sci. Total Environ. 2019, 709, 135897. [Google Scholar] [CrossRef]
- Stoycho, D.S. Food security, underestimated hazard of joint mycotoxin exposure and management of the risk of mycotoxin contamination. Food Control 2023, 159, 110235. [Google Scholar] [CrossRef]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef]
- Coton, M.; Dantigny, P. Mycotoxin migration in moldy foods. Curr. Opin. Food Sci. 2019, 29, 88–93. [Google Scholar] [CrossRef]
- Patriarca, A. Fungi and mycotoxin problems in the apple industry. Curr. Opin. Food Sci. 2019, 29, 42–47. [Google Scholar] [CrossRef]
- Rong, X.; Sun-Waterhouse, D.; Wang, D.; Jiang, Y.; Li, F.; Chen, Y.; Zhao, S.; Li, D. The Significance of Regulatory MicroRNAs: Their Roles in Toxicodynamics of Mycotoxins and in the Protection Offered by Dietary Therapeutics Against Mycotoxin-Induced Toxicity. Compr. Rev. Food Sci. Food Saf. 2018, 18, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A.D.W. Mycotoxins in spices and herbs—An update. Crit. Rev. Food Sci. Nutr. 2017, 57, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Bessaire, T.; Perrin, I.; Tarres, A.; Bebius, A.; Reding, F.; Theurillat, V. Mycotoxins in green coffee: Occurrence and risk assessment. Food Control 2018, 96, 59–67. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Sun, J.; Wang, L.; Guo, H.; Ye, Y.; Sun, X. Microbial detoxification of mycotoxins in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 62, 4951–4969. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.I.; Gross, M.; Gottschalk, C.; Usleber, E. Investigations on the occurrence of mycotoxins in beer. Food Control 2016, 63, 135–139. [Google Scholar] [CrossRef]
- Thanushree, M.P.; Sailendri, D.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Mycotoxin contamination in food: An exposition on spices. Trends Food Sci. Technol. 2019, 93, 69–80. [Google Scholar] [CrossRef]
- Sun, X.D.; Su, P.; Shan, H. Mycotoxin Contamination of Maize in China. Compr. Rev. Food Sci. Food Saf. 2017, 16, 835–849. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Ferranti, L.S.; Pinheiro, M.; Bertozzi, B.G.; Rocha, L.O. Mycotoxin contamination in cereal-based baby foods. Curr. Opin. Food Sci. 2019, 30, 73–78. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Schatzmayr, D.; Albalat, A.; Mackenzie, S. Mycotoxins in aquaculture: Feed and food. Rev. Aquac. 2018, 12, 145–175. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, J.; Sachdev, T.; Basu, T.; Malhotra, B.D. Recent advances in mycotoxins detection. Biosens. Bioelectron. 2016, 81, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Vargas Medina, D.A.; Bassolli Borsatto, J.V.; Maciel, E.V.S.; Lanças, F.M. Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Keskin, E.; Eyupoglu, O.E. Determination of mycotoxins by HPLC, LC-MS/MS and health risk assessment of the mycotoxins in bee products of Turkey. Food Chem. 2023, 400, 134086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wieczorek, M.N.; Pawliszyn, J. High throughput and automated solid-phase microextraction and determination by liquid chromatography-mass spectrometry for the analysis of mycotoxins in beer. Food Chem. 2023, 426, 136557. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Ramos, A.J.; Sanchis, V.; Cano-Sancho, G. An overview of mycotoxin biomarker application in exposome-health studies. Curr. Opin. Food Sci. 2021, 39, 31–35. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Aldars-García, L.; Ramos, A.J.; Sanchis, V.; Marín, S. Modeling postharvest mycotoxins in foods: Recent research. Curr. Opin. Food Sci. 2016, 11, 46–50. [Google Scholar] [CrossRef]
- Pagkali, V.; Petrou, P.S.; Makarona, E.; Peters, J.; Haasnoot, W.; Jobst, G.; Moser, I.; Gajos, K.; Budkowski, A.; Economou, A.; et al. Simultaneous determination of Aflatoxin B1, Fumonisin B1 and Deoxynivalenol in beer samples with a label-free monolithically integrated optoelectronic biosensor. J. Hazard. Mater. 2018, 359, 445–453. [Google Scholar] [CrossRef]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef]
- Shuai, Z.; Ting, W.; Jiaxuan, L.; Qing, J.; Rui, X.; Shengqi, W.; Chongwen, W. Difunctional immunochromatographic assay based on magnetic quantum dot for ultrasensitive and simultaneous detection of multiple mycotoxins in foods. Sens. Actuators B Chem. 2022, 359, 131528. [Google Scholar] [CrossRef]
- Patriarca, A.; Fernández Pinto, V. Prevalence of mycotoxins in foods and decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60. [Google Scholar] [CrossRef]
- Murugesan, P.; Brunda, D.K.; Moses, J.A.; Anandharamakrishnan, C. Photolytic and photocatalytic detoxification of mycotoxins in foods. Food Control 2021, 123, 10774. [Google Scholar] [CrossRef]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef]
- Wuzhen, Q.; Siyuan, W.; Lei, W.; Xinge, X.; Shangyi, W.; Yanbin, L.; Ming, L.; Jianhan, L. A portable viable Salmonella detection device based on microfluidic chip and recombinase aided amplification. Chin. Chem. Lett. 2022, 34, 107360. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Santos, D.R.; Chu, V.; Azevedo, A.M.; Aires-Barros, M.R.; Conde, J.P. A point-of-use microfluidic device with integrated photodetector array for immunoassay multiplexing: Detection of a panel of mycotoxins in multiple samples. Biosens. Bioelectron. 2016, 87, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Salafi, T.; Zeming, K.K.; Lim, J.W.; Raman, R.; Seah, A.W.R.; Tan, M.P.; Zhang, Y. Portable Smartphone-Based Platform for Real-Time Particle Detection in Microfluidics. Adv. Mater. Technol. 2018, 4, 1800359. [Google Scholar] [CrossRef]
- Lu, L.; Gunasekaran, S. Dual-channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta 2018, 194, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.G.; Santos, D.R.; Pinto, I.F.; Azevedo, A.M.; Aires-Barros, M.R.; Chu, V.; Conde, J.P. Multiplexed microfluidic fluorescence immunoassay with photodiode array signal acquisition for sub-minute and point-of-need detection of mycotoxins. Lab Chip 2018, 18, 1569–1580. [Google Scholar] [CrossRef]
- Li, F.Y.; Wang, W.; Li, H.L.; Yang, M.; Wu, J.; Zhang, L.; Gao, J.Y.; Pu, Q.S. A low-cost portable microfluidic chemiluminometer for point-of-care testing of adiponectin for early risk assessment of diabetes. Sens. Actuator B-Chem. 2023, 380, 7. [Google Scholar] [CrossRef]
- Lin, X.D.; Wu, H.T.; Zeng, S.Y.; Peng, T.; Zhang, P.; Wan, X.H.; Lang, Y.H.; Zhang, B.; Jia, Y.W.; Shen, R.; et al. A self-designed device integrated with a Fermat spiral microfluidic chip for ratiometric and automated point-of-care testing of anthrax biomarker in real samples. Biosens. Bioelectron. 2023, 230, 9. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.S.; Soares, R.R.G.; Pinto, I.F.; Russom, A. A portable and low-cost centrifugal microfluidic platform for multiplexed colorimetric detection of protein biomarkers. Anal. Chim. Acta 2023, 1245, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, F.; Zhang, W.C.; Shen, W.; Yang, D.; Bian, Z.P.; Cui, H. Chemiluminescence Immunoassays for Simultaneous Detection of Three Heart Disease Biomarkers Using Magnetic Carbon Composites and Three-Dimensional Microfluidic Paper-Based Device. Anal. Chem. 2019, 91, 13006–13013. [Google Scholar] [CrossRef]
- Yin, B.; Yue, W.; Sohan, A.S.M.M.F.; Zhou, T.; Qian, C.; Wan, X. Micromixer with Fine-Tuned Mathematical Spiral Structures. ACS Omega 2021, 6, 30779–30789. [Google Scholar] [CrossRef]
- Yin, B.; Yue, W.; Sohan, A.S.M.M.F.; Wan, X.; Zhou, T.; Shi, L.; Qian, C.; Lin, X. Construction of a desirable hyperbolic microfluidic chip for ultrasensitive determination of PCT based on chemiluminescence. J. Mater. Chem. B 2023, 11, 1978–1986. [Google Scholar] [CrossRef]
- Yin, B.; Wan, X.; Qian, C.; Sohan, A.S.M.M.F.; Wang, S.; Zhou, T. Point-of-Care Testing for Multiple Cardiac Markers Based on a Snail-Shaped Microfluidic Chip. Front. Chem. 2021, 9, 741058. [Google Scholar] [CrossRef]
- He, Y.; Tian, F.Y.; Zhou, J.; Zhao, Q.Y.; Fu, R.J.; Jiao, B.N. Colorimetric aptasensor for ochratoxin A detection based on enzyme-induced gold nanoparticle aggregation. J. Hazard. Mater. 2020, 388, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, B.B.; Ye, Y.F.; Qi, X.Y.; Zhang, Y.T.; Xia, X.L.; Wang, X.L.; Zhou, N.D. A fluorescence and surface-enhanced Raman scattering dual-mode aptasensor for rapid and sensitive detection of ochratoxin A. Biosens. Bioelectron. 2022, 207, 9. [Google Scholar] [CrossRef]
- Yin, L.M.; You, T.Y.; Arslan, M.; El-Seedi, H.R.; Guo, Z.M.; Zou, X.B.; Cai, J.R. Dual-layers Raman reporter-tagged Au@Ag combined with core-satellite assemblies for SERS detection of Zearalenone. Food Chem. 2023, 429, 11. [Google Scholar] [CrossRef]
- Zhou, F.L.; Deng, H.L.; Agarry, I.E.; Hu, J.; Xu, D.H.; Feng, H.; Kan, J.Q.; Cai, T.; Chen, K.W. Determination of multiple mycotoxins in chili powder using cold-induced liquid-liquid extraction and Fe3O4@MWCNTs-NH2 coupled with UPLC-Q-TOF/MS. Food Chem. 2023, 423, 8. [Google Scholar] [CrossRef]
- Jiang, H.; Su, H.; Wu, K.S.; Dong, Z.M.; Li, X.M.; Nie, L.J.; Leng, Y.K.; Xiong, Y.H. Multiplexed lateral flow immunoassay based on inner filter effect for mycotoxin detection in maize. Sens. Actuator B-Chem. 2023, 374, 7. [Google Scholar] [CrossRef]






| ZEA | AFB1 | OTA | ||||||
|---|---|---|---|---|---|---|---|---|
| Spiked Value (ng/mL) | Recovery (%) | RSD (%) | Spiked Value (ng/mL) | Recovery (%) | RSD (%) | Spiked Value (ng/mL) | Recovery (%) | RSD (%) |
| 1.25 | 106.76 | 7.18 | 0.3 | 103.32 | 6.21 | 3 | 103.45 | 10.15 |
| 2.5 | 105.35 | 10.22 | 0.6 | 95.56 | 7.72 | 6 | 104.79 | 7.28 |
| 5 | 109.14 | 12.10 | 1.2 | 96.86 | 8.07 | 9 | 96.46 | 9.08 |
| 10 | 102.16 | 9.91 | 2.4 | 91.39 | 6.99 | 18 | 92.20 | 9.66 |
| 20 | 105.09 | 9.52 | 4.8 | 97.75 | 8.31 | 36 | 95.82 | 8.59 |
| Methods | Colorimetric | Fluorescence | SERS | UPLC-Q-TOF/MS | Fluorescence | Chemiluminescence |
|---|---|---|---|---|---|---|
| Mycotoxins | OTA | OTA | ZEA | AFB1 | ZEA AFB1 OTA | ZEA AFB1 OTA |
| LOD | 2 μg/kg | 0.17 ng/mL | 3 μg/kg | 0.2 μg/kg | 400 ng/mL 5 ng/mL 40 ng/mL | 0.7619 ng/mL 0.1374 ng/mL 0.9183 ng/mL |
| Real samples | grape juice | coffee, wine | corn | chili powder | corn | dry red wine |
| Recovery (%) | 99.4–104.2 | 99.2–100.9 | 90.6–105.3 | 78.8–100.8 | 72.9–105.8% | 91.4–109.1 |
| RSD (%) | <2% | 1.45–4.67 | 2.56–7.62 | 1.6–7.7 | - | 6.21–12.10 |
| References | [47] | [48] | [49] | [50] | [51] | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zeng, S.; Zhu, H.; Wan, X.; Sohan, A.S.M.M.F.; Yin, B. A Portable Automated Microfluidic Platform for Point-of-Care Testing for Multiple Mycotoxins in Wine. Foods 2024, 13, 2066. https://doi.org/10.3390/foods13132066
Liu J, Zeng S, Zhu H, Wan X, Sohan ASMMF, Yin B. A Portable Automated Microfluidic Platform for Point-of-Care Testing for Multiple Mycotoxins in Wine. Foods. 2024; 13(13):2066. https://doi.org/10.3390/foods13132066
Chicago/Turabian StyleLiu, Jun, Shiyu Zeng, Haoyu Zhu, Xinhua Wan, A. S. M. Muhtasim Fuad Sohan, and Binfeng Yin. 2024. "A Portable Automated Microfluidic Platform for Point-of-Care Testing for Multiple Mycotoxins in Wine" Foods 13, no. 13: 2066. https://doi.org/10.3390/foods13132066
APA StyleLiu, J., Zeng, S., Zhu, H., Wan, X., Sohan, A. S. M. M. F., & Yin, B. (2024). A Portable Automated Microfluidic Platform for Point-of-Care Testing for Multiple Mycotoxins in Wine. Foods, 13(13), 2066. https://doi.org/10.3390/foods13132066







