Dual-Responsive “Egg-Box” Shaped Microgel Beads Based on W1/O/W2 Double Emulsions for Colon-Targeted Delivery of Synbiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Zein–Apple Pectin Hybrid Nanoparticles (ZAHPs)
2.3. Characterization of ZAHPs
2.3.1. Particle Size, Polydispersity Index (PDI), and Zeta (ζ)-Potential
2.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.3. Three-Phase Contact Angle (θ)
2.3.4. Scanning Electron Microscopy (SEM)
2.4. Fabrication of W1/O/W2 Emulsions without Synbiotics
2.5. Characterization of W1/O/W2 Emulsions
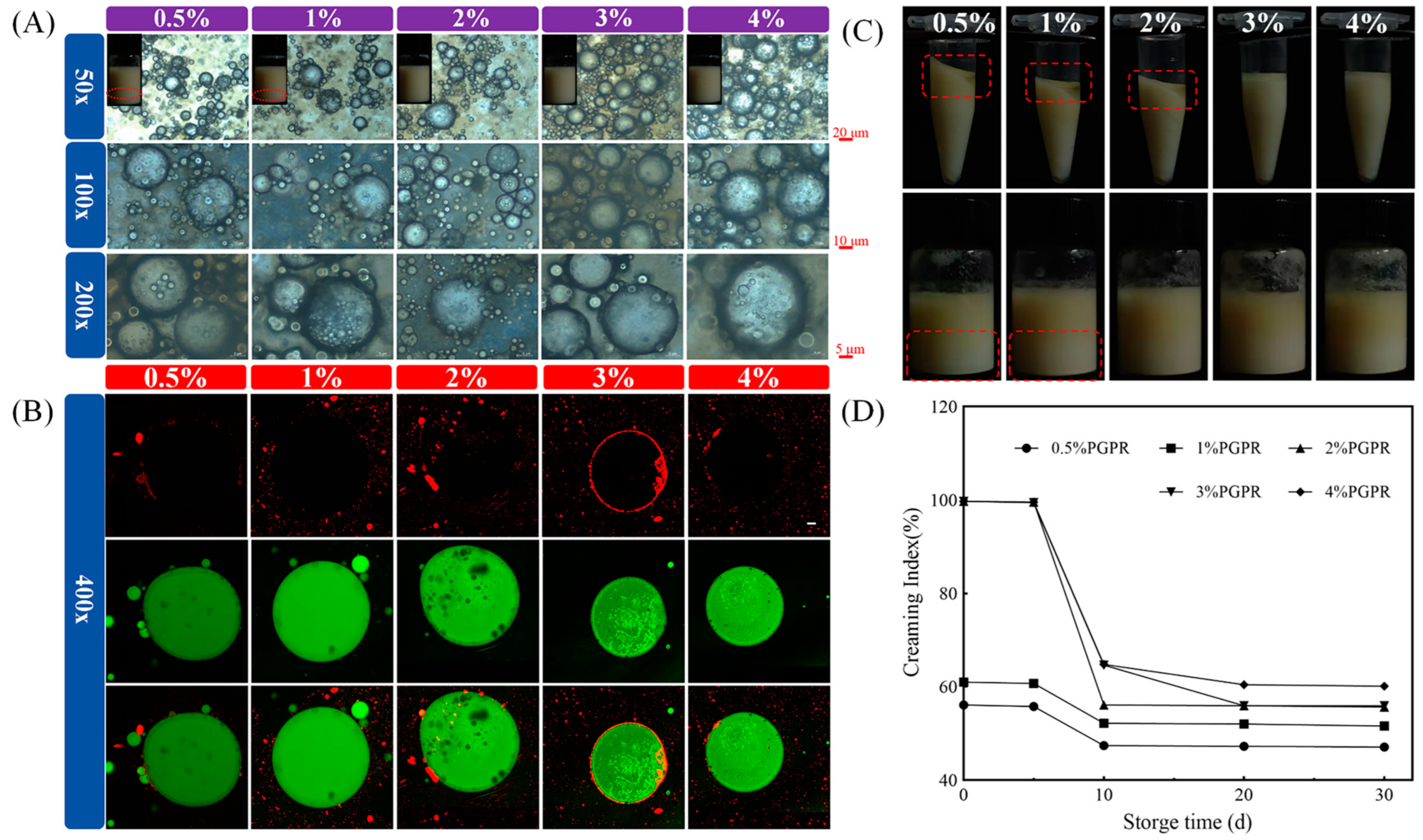
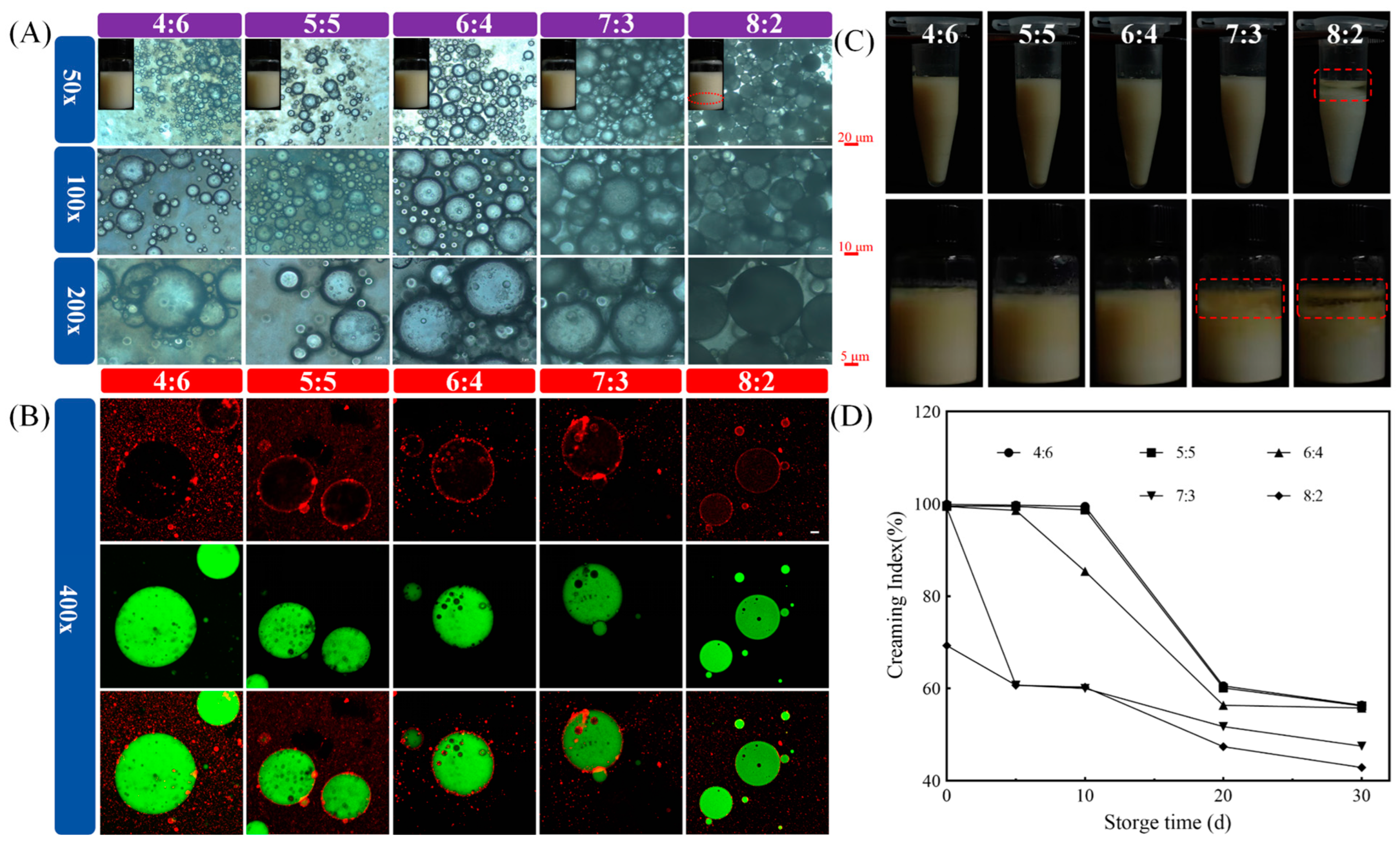
2.5.1. Optical Microstructures and Visible Graphics of W1/O/W2 Emulsions
2.5.2. Confocal Laser Scanning Microscope (CLSM)
2.5.3. Rheology
2.6. Stability Analysis of W1/O/W2 Emulsions
2.6.1. Storage Stability and Creaming Index (CI) of W1/O/W2 Emulsions
2.6.2. Centrifugation Stability
2.7. Configuration of “Egg-Box” Shaped Microgel Beads
2.7.1. Activation and Cultivation of Probiotics
2.7.2. Fabrication of “Egg-Box” Shaped Microgel Beads
2.8. Encapsulation Properties and Characterization of Microgel Beads
2.8.1. Viability of Probiotics in Storage Experiments
2.8.2. Viability of Probiotics in In Vitro Simulated Digestion Experiments
2.9. Statistical Analysis
3. Results and Discussion
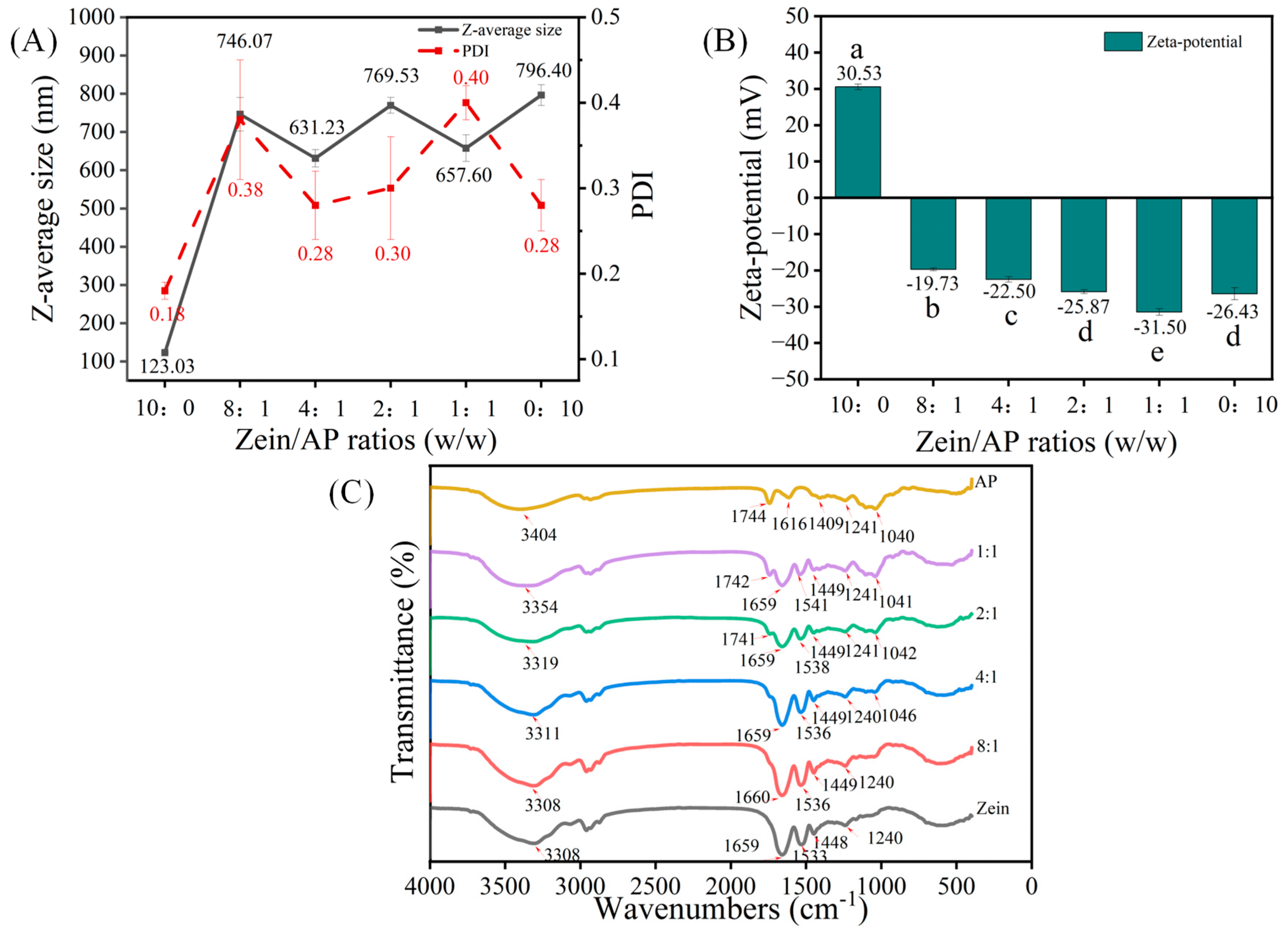
3.1. Characterization of Zein, AP and ZAHPs
3.1.1. Particle Size, ζ-Potential and PDI
3.1.2. FTIR Spectra
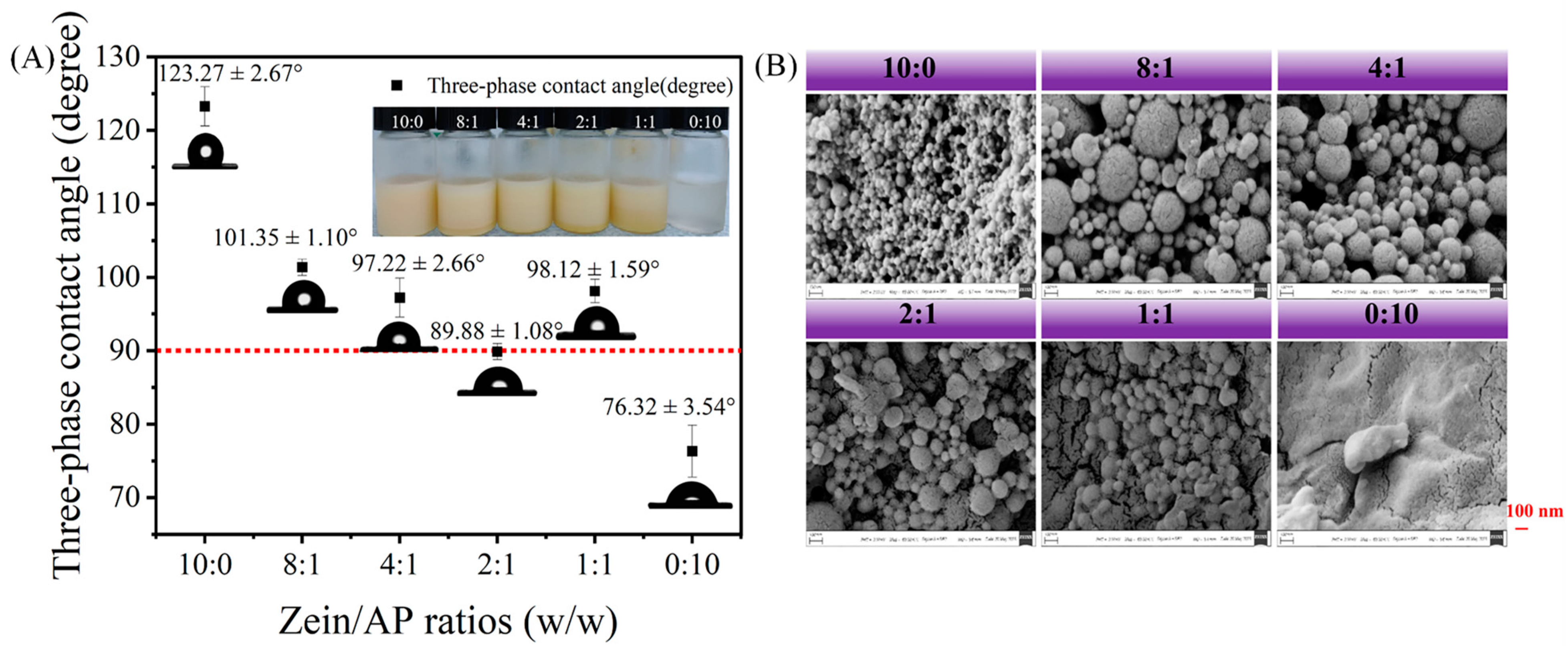
3.1.3. Wettability and Microscopic Morphology
3.2. Effect of Different Preparation Parameters on the Formation of W1/O/W2 Emulsions
3.2.1. PGPR Concentration
3.2.2. The Volume Ratios of W1/O Phase-to-W2 Phase
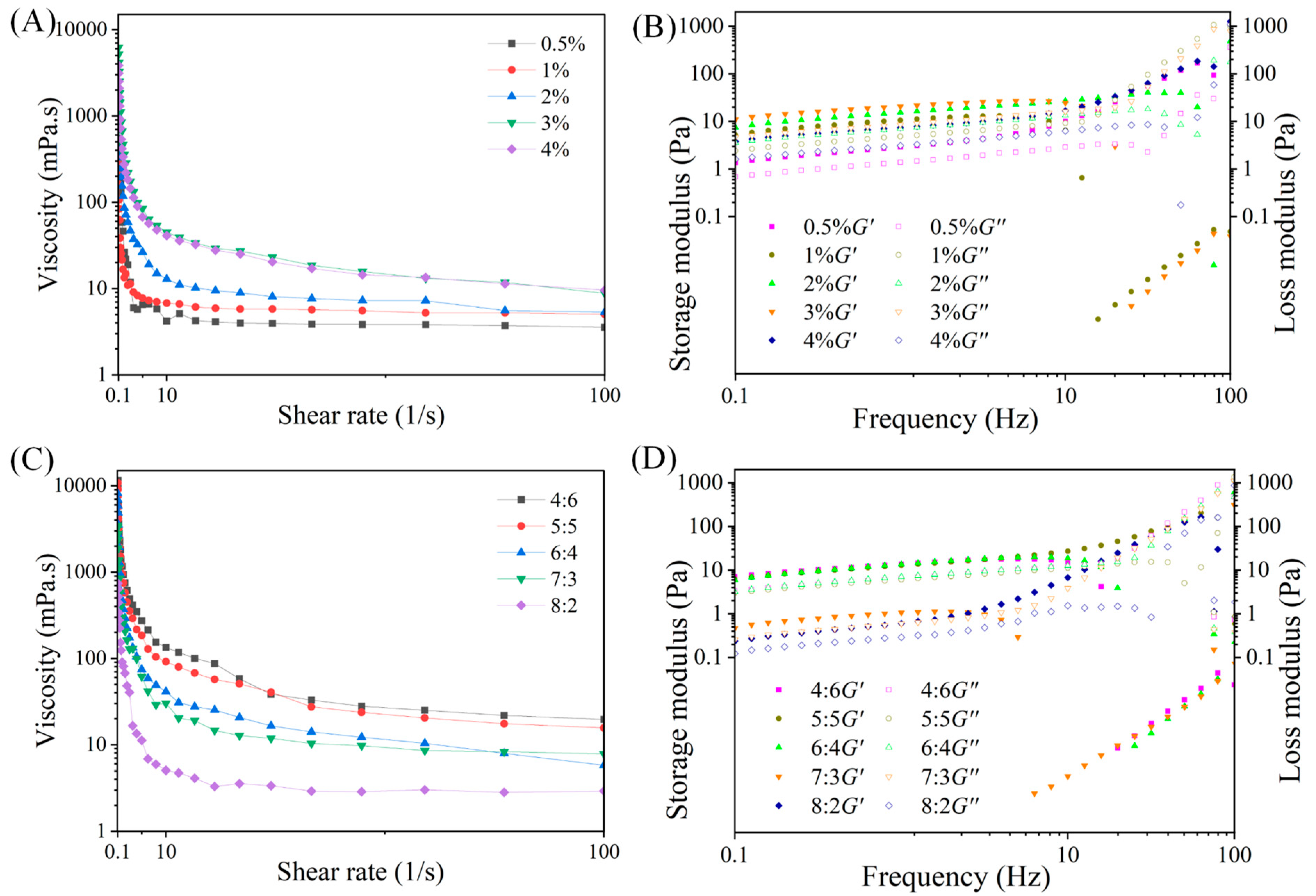
3.3. Rheological Property of W1/O/W2 Emulsions
3.4. Stability and Morphology of “Egg-Box” Shaped Microgel Beads
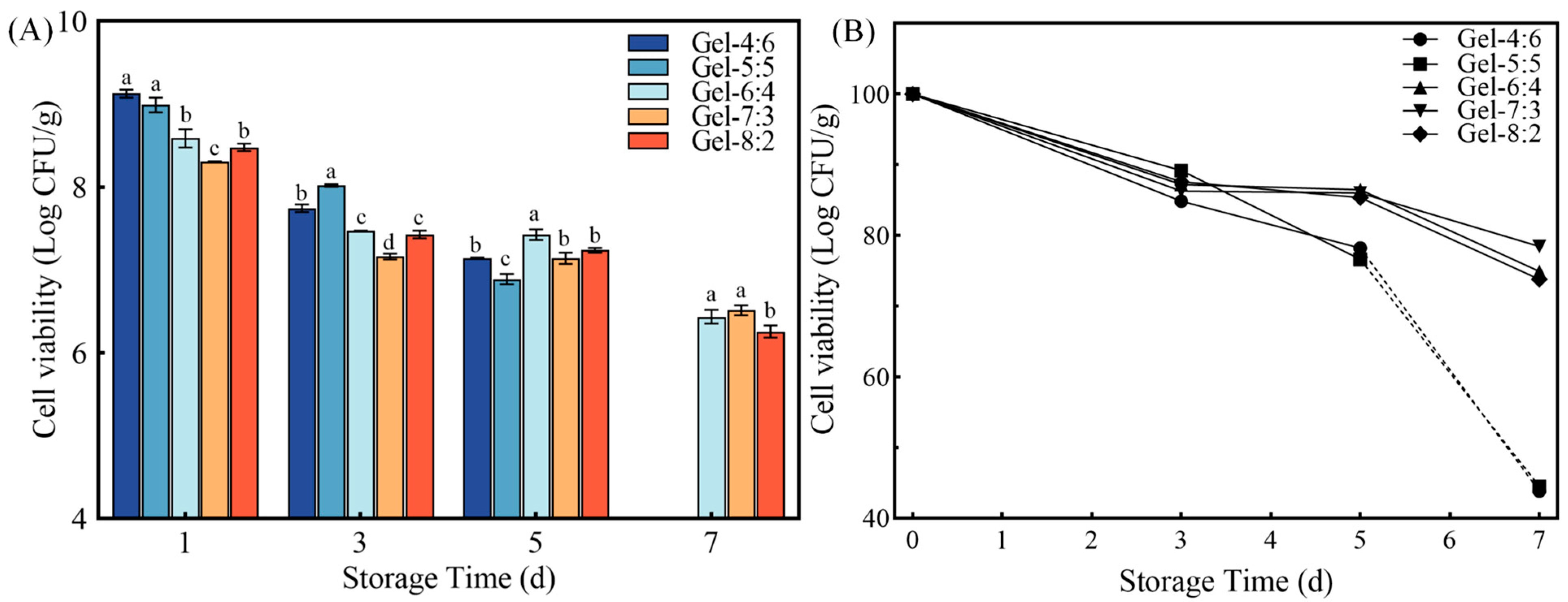
3.4.1. Storage Stability of Microgel Beads
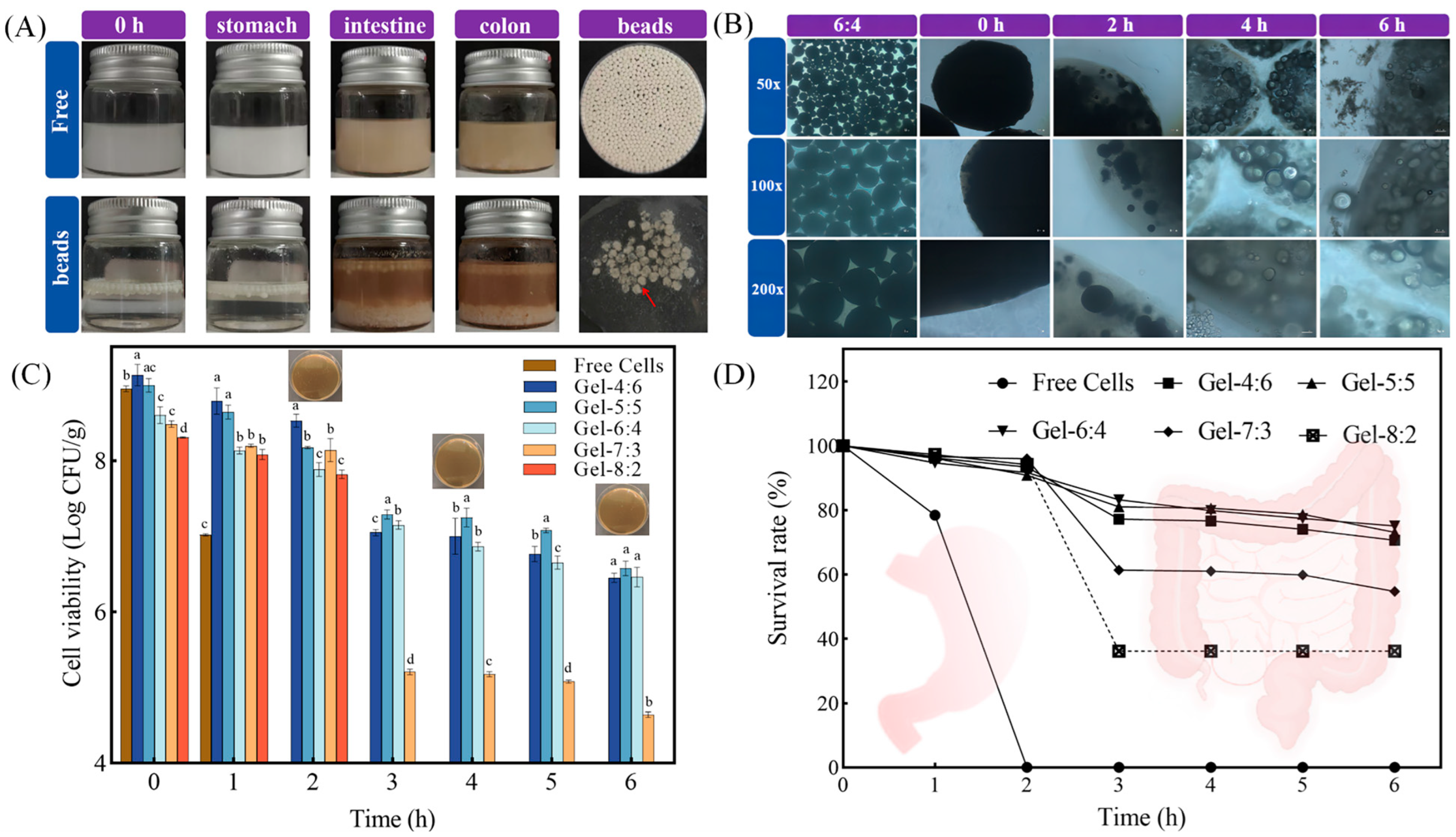
3.4.2. Digestive Stability of Microgel Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flach, J.; van der Waal, M.B.; van den Nieuwboer, M.; Claassen, E.; Larsen, O.F. The underexposed role of food matrices in probiotic products: Reviewing the relationship between carrier matrices and product parameters. Crit. Rev. Food Sci. Nutr. 2018, 58, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating polymers for probiotics delivery systems: Preparation, characterization, and applications. Food Hydrocoll. 2021, 120, 106882. [Google Scholar] [CrossRef]
- Cui, F.; Zhang, H.; Wang, D.; Tan, X.; Li, X.; Li, Y.; Li, J.; Li, T. Advances in the preparation and application of microencapsulation to protect food functional ingredients. Food Funct. 2023, 14, 6766–6783. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, F.; Hashemi, H.; Esteghlal, S.; Hosseini, S.M.H. An Updated Comprehensive Overview of Different Food Applications of W1/O/W2 and O1/W/O2 Double Emulsions. Foods 2024, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, L.; Li, J. Biopolymer-based pickering high internal phase emulsions: Intrinsic composition of matrix components, fundamental characteristics and perspective. Food Res. Int. 2023, 165, 112458. [Google Scholar] [CrossRef]
- Ren, G.; Zhu, Y.; Shi, J.; Liu, J.; He, Y.; Sun, Y.; Zhan, Y.; Lv, J.; Huang, M.; Xie, H. Fabrication of Antioxidant Pickering Emulsion Based on Resveratrol-Grafted Zein Conjugates: Enhancing the Physical and Oxidative Stability. Foods 2022, 11, 3851. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Smruthi, M.R.; Nallamuthu, I.; Anand, T. A comparative study of optimized naringenin nanoformulations using nano-carriers (PLA/PVA and zein/pectin) for improvement of bioavailability. Food Chem. 2022, 369, 130950. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Saurabh, V.; Mahajan, T.; Punia, S.; del Mar Contreras, M.; Kennedy, J.F. Emerging trends in pectin extraction and its anti-microbial functionalization using natural bioactives for application in food packaging. Trends Food Sci. Technol. 2020, 105, 223–237. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Y.; Chen, G.; Shi, Y.; Li, X.; Zhang, H.; Shen, Y. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Sabater, C.; Antón, M.J.; Moreno, F.J.; Riestra, S.; Margolles, A.; Ruiz, L. Prebiotic potential of apple pomace and pectins from different apple varieties: Modulatory effects on key target commensal microbial populations. Food Hydrocoll. 2022, 133, 107958. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Nie, Q.; Hu, J.; Su, W.; Guo, Z.; Zhang, Y.; Nie, S. In vitro assessment of the effect of four polysaccharides on intestinal bacteria of mice with colitis. Food Front. 2023, 4, 1462–1471. [Google Scholar] [CrossRef]
- Qin, X.; Bo, Q.; Qin, P.; Wang, S.; Liu, K. Fabrication of WPI-EGCG covalent conjugates/gellan gum double network emulsion gels by duo-induction of GDL and CaCl2 for colon-controlled Lactobacillus Plantarum delivery. Food Chem. 2023, 404, 134513. [Google Scholar] [CrossRef]
- Qin, X.; Luo, Z.; Li, X. An enhanced pH-sensitive carrier based on alginate-Ca-EDTA in a set-type W1/O/W2 double emulsion model stabilized with WPI-EGCG covalent conjugates for probiotics colon-targeted release. Food Hydrocoll. 2021, 113, 106460. [Google Scholar] [CrossRef]
- Hlaing, S.P.; Kim, J.; Lee, J.; Kwak, D.; Kim, H.; Yoo, J.W. Enhanced viability of probiotics against gastric acid by one-step coating process with poly-L-lysine: In vitro and in vivo evaluation. Pharmaceutics 2020, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, F.; Li, D.; Sun-Waterhouse, D.; Huang, Q. Zein/pectin nanoparticle-stabilized sesame oil Pickering emulsions: Sustainable bioactive carriers and healthy alternatives to sesame paste. Food Bioprocess Technol. 2019, 12, 1982–1992. [Google Scholar] [CrossRef]
- He, X.; Yang, W.; Qin, X. Ultrasound-assisted multilayer Pickering emulsion fabricated by WPI-EGCG covalent conjugates for encapsulating probiotics in colon-targeted release. Ultrason. Sonochem. 2023, 97, 106450. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Elmizadeh, A.; Goli, S.A.H.; Mohammadifar, M.A.; Rahimmalek, M. Fabrication and characterization of pectin-zein nanoparticles containing tanshinone using anti-solvent precipitation method. Int. J. Biol. Macromol. 2024, 260, 129463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kratzer, R.; Murkovic, M.; Eibinger, M.; Charry, E.M.; Li, S.; Zhang, T.; Zhang, X.; Zhang, M.; Chen, H. Fabrication and characterization of a novel zein/pectin/pumpkin seed oil Pickering emulsion and the effects of myricetin on oxidation stability. Int. J. Biol. Macromol. 2023, 253, 127386. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, C.; Yuan, J.; Wu, Y.; Li, F.; Li, D.; Huang, Q. Effects of pectin polydispersity on zein/pectin composite nanoparticles (ZAPs) as high internal-phase Pickering emulsion stabilizers. Carbohydr. Polym. 2019, 219, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Z.; Huang, X.N.; Wu, Z.L.; Yin, S.W.; Zhu, J.H.; Tang, C.H.; Yang, X.Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A. Encapsulation of Orange Pomace Polyphenols in Zein/Pectin Nanoparticles. Master’s Thesis, California State Polytechnic University, Pomona, CA, USA, 2023. [Google Scholar]
- Soltani, S.; Madadlou, A. Two-step sequential cross-linking of sugar beet pectin for transforming zein nanoparticle-based Pickering emulsions to emulgels. Carbohydr. Polym. 2016, 136, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xie, Y.; Li, Z.; Bai, C.; Zou, L.; Liu, W. Novel seamless shell-core microbead for probiotics encapsulation: Influence of gel structure on storage stability and gastrointestinal activity. Food Hydrocoll. 2024, 152, 109908. [Google Scholar] [CrossRef]
- Qin, X.S.; Gao, Q.Y.; Luo, Z.G. Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles. Food Hydrocoll. 2021, 116, 106658. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, X.; Huang, Q. Double emulsion derived from kafirin nanoparticles stabilized Pickering emulsion: Fabrication, microstructure, stability and in vitro digestion profile. Food Hydrocoll. 2017, 62, 230–238. [Google Scholar] [CrossRef]
- Jiang, Z.; Tian, J.; Bai, X.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Improving probiotic survival using water-in-oil-in-water (W1/O/W2) emulsions: Role of fish oil in inner phase and sodium alginate in outer phase. Food Chem. 2023, 417, 135889. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Yin, J.; Chang, F.; Wang, C.; He, X.; Zhang, B. Anthocyanin-loaded double Pickering emulsion stabilized by octenylsuccinate quinoa starch: Preparation, stability and in vitro gastrointestinal digestion. Int. J. Biol. Macromol. 2020, 152, 1233–1241. [Google Scholar] [CrossRef]
- Liang, Z.; Chu, H.; Hou, Z.; Wang, C.; Zhang, G.; Liu, L.; Ma, X.; Li, C.; He, J. W/O/W emulsions stabilized with whey protein concentrate and pectin: Effects on storage, pasteurization, and gastrointestinal viability of Lacticaseibacillus rhamnosus. Int. J. Biol. Macromol. 2023, 232, 123477. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mettu, S.; Rahim, M.A.; Cavalieri, F.; Ashokkumar, M. Insight into the structural, chemical and surface properties of proteins for the efficient ultrasound assisted co-encapsulation and delivery of micronutrients. Food Chem. 2021, 362, 130236. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, U.; Sabikhi, L. Effect of selected stabilizers and processing aids on the stability of a double emulsion encapsulating bitter gourd extract. Food Sci. Eng. 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Yin, M.; Chen, L.; Chen, M.; Yuan, Y.; Liu, F.; Zhong, F. Encapsulation of Lactobacillus rhamnosus GG in double emulsions: Role of prebiotics in improving probiotics survival during spray drying and storage. Food Hydrocoll. 2024, 151, 109792. [Google Scholar] [CrossRef]
- Wang, N.; Fan, H.; Wang, J.; Wang, H.; Liu, T. Fabrication and characterization of curcumin-loaded composite nanoparticles based on high-hydrostatic-pressure-treated zein and pectin: Interaction mechanism, stability, and bioaccessibility. Food Chem. 2024, 446, 138286. [Google Scholar] [CrossRef]
- Liu, R.; Ci, X.; Liu, L.; Wang, X.; Rifky, M.; Liu, R.; Zhang, M. Chitosan entrapping of sodium alginate/Lycium barbarum polysaccharide gels for the encapsulation, protection and delivery of Lactiplantibacillus plantarum with enhanced viability. Int. J. Biol. Macromol. 2024, 260, 129615. [Google Scholar] [CrossRef]
- Thinkohkaew, K.; Jonjaroen, V.; Niamsiri, N.; Panya, A.; Suppavorasatit, I.; Potiyaraj, P. Microencapsulation of probiotics in chitosan-coated alginate/gellan gum: Optimization for viability and stability enhancement. Food Hydrocoll. 2024, 151, 109788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Qin, Y.; Liu, H.; Cheng, K.; Yang, W.; Qin, X. Dual-Responsive “Egg-Box” Shaped Microgel Beads Based on W1/O/W2 Double Emulsions for Colon-Targeted Delivery of Synbiotics. Foods 2024, 13, 2163. https://doi.org/10.3390/foods13142163
He X, Qin Y, Liu H, Cheng K, Yang W, Qin X. Dual-Responsive “Egg-Box” Shaped Microgel Beads Based on W1/O/W2 Double Emulsions for Colon-Targeted Delivery of Synbiotics. Foods. 2024; 13(14):2163. https://doi.org/10.3390/foods13142163
Chicago/Turabian StyleHe, Xian, Yunyun Qin, Haoyue Liu, Kang Cheng, Wanshui Yang, and Xinsheng Qin. 2024. "Dual-Responsive “Egg-Box” Shaped Microgel Beads Based on W1/O/W2 Double Emulsions for Colon-Targeted Delivery of Synbiotics" Foods 13, no. 14: 2163. https://doi.org/10.3390/foods13142163
APA StyleHe, X., Qin, Y., Liu, H., Cheng, K., Yang, W., & Qin, X. (2024). Dual-Responsive “Egg-Box” Shaped Microgel Beads Based on W1/O/W2 Double Emulsions for Colon-Targeted Delivery of Synbiotics. Foods, 13(14), 2163. https://doi.org/10.3390/foods13142163






