Simultaneous Determination of Aflatoxin B1 and Ochratoxin A in Cereals by a Novel Electrochemical Aptasensor Using Metal–Organic Framework as Signal Carrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Preparation of Signal Probes
2.4. Preparation of the Electrochemical Aptasensor
2.5. Electrochemical Measurement
2.6. Real Sample Analysis
2.7. Statistical Analysis
3. Results
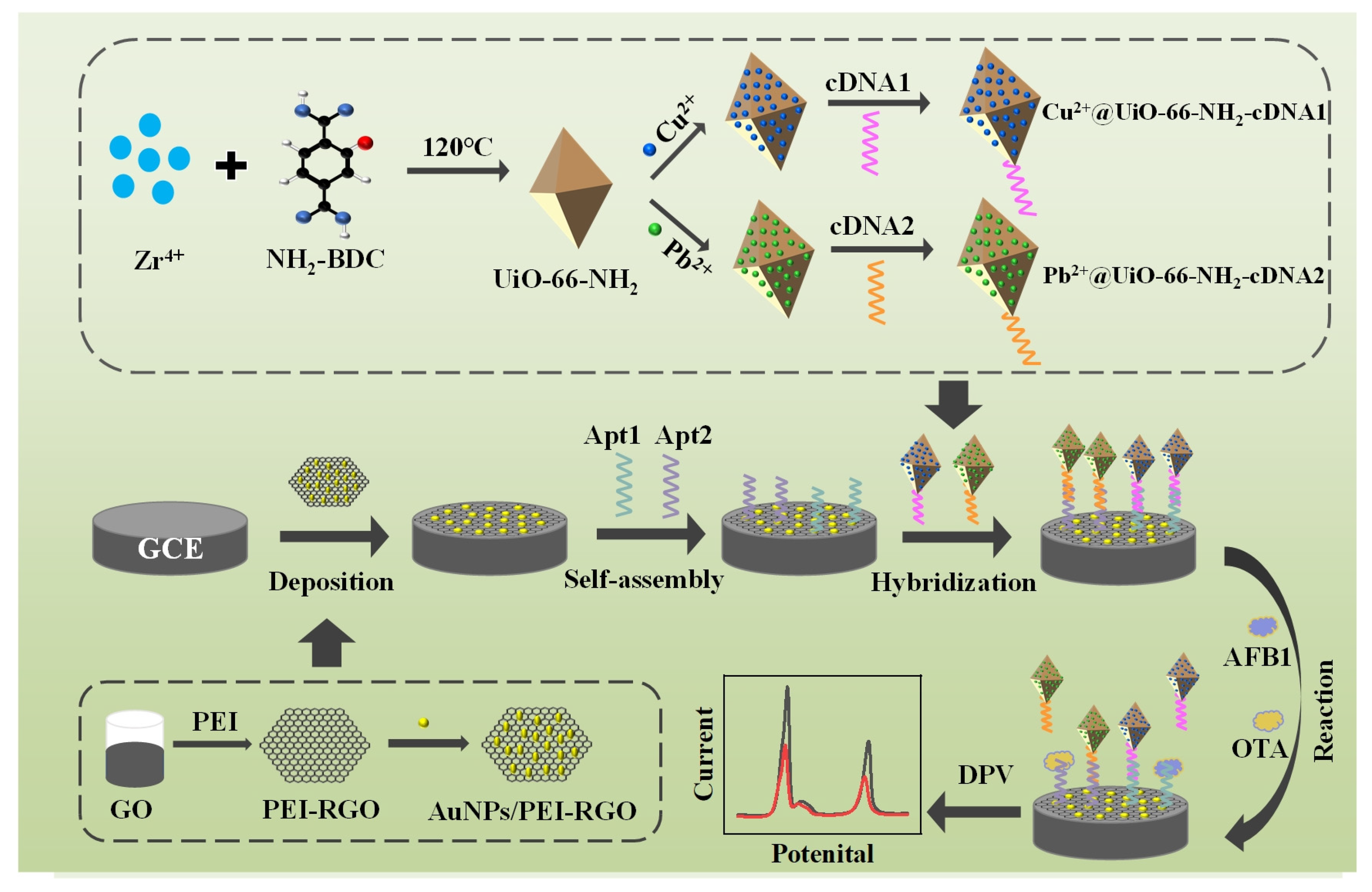
3.1. Principle of the Prepared Electrochemical Aptasensor for AFB1 and OTA Detection
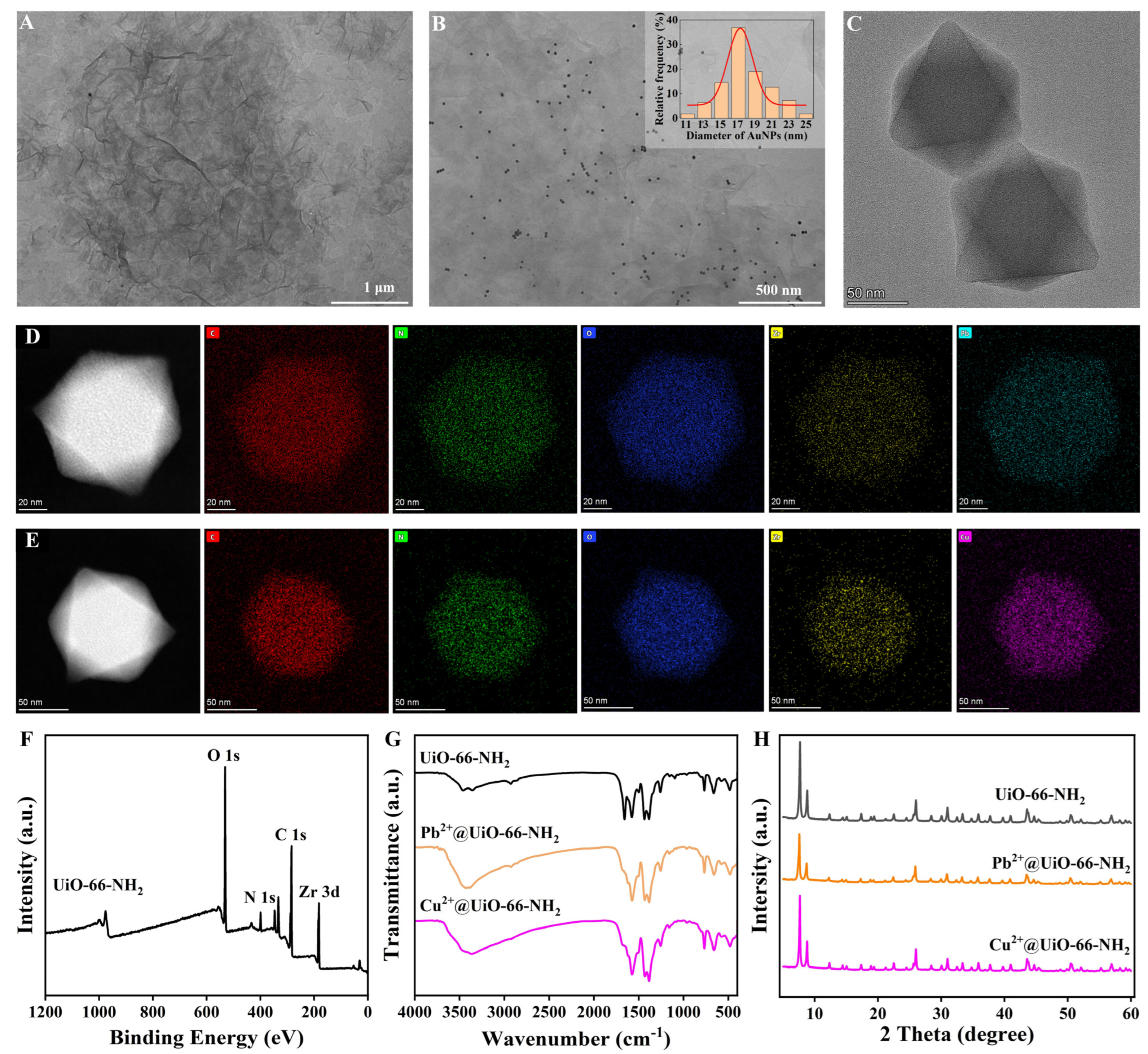
3.2. Characterization of the Synthesized Materials
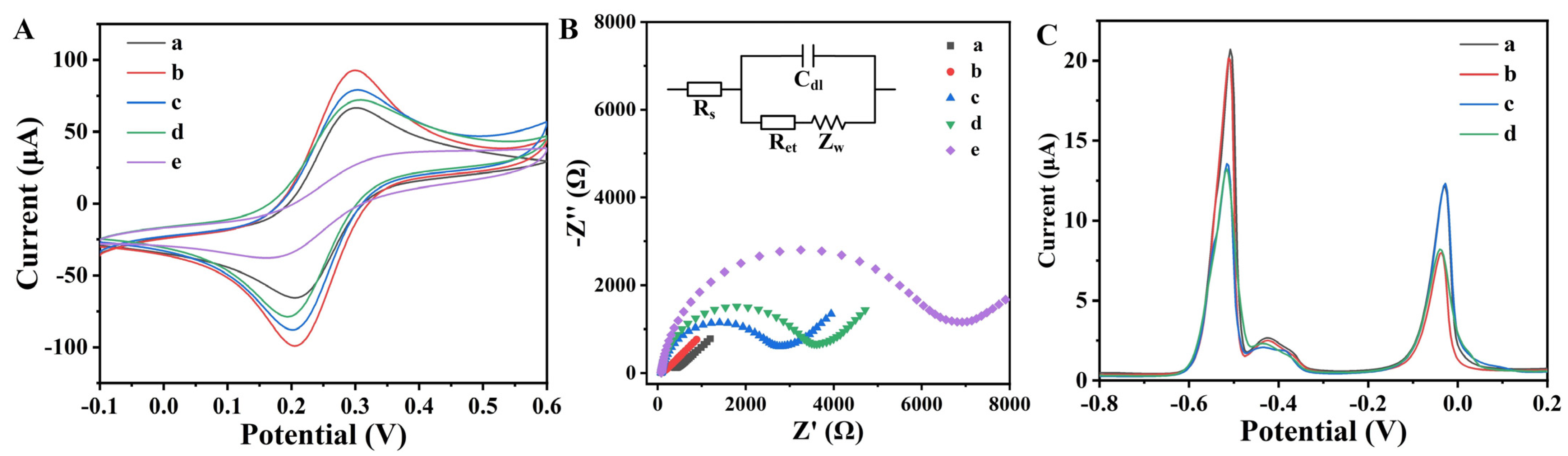
3.3. Electrochemical Characterization
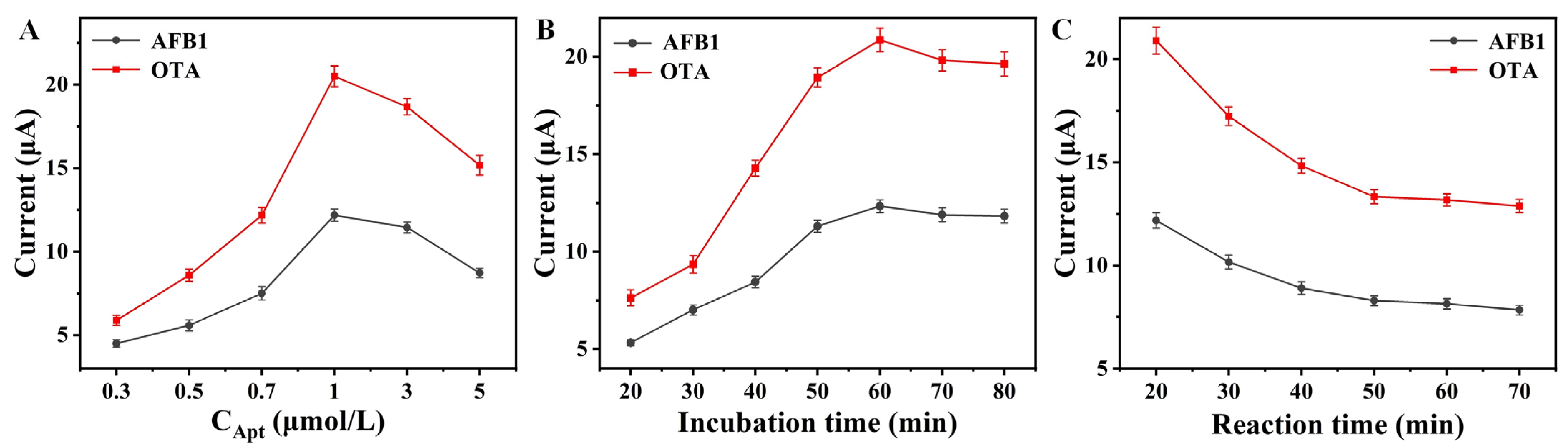
3.4. Optimization of the Aptasensor Performance
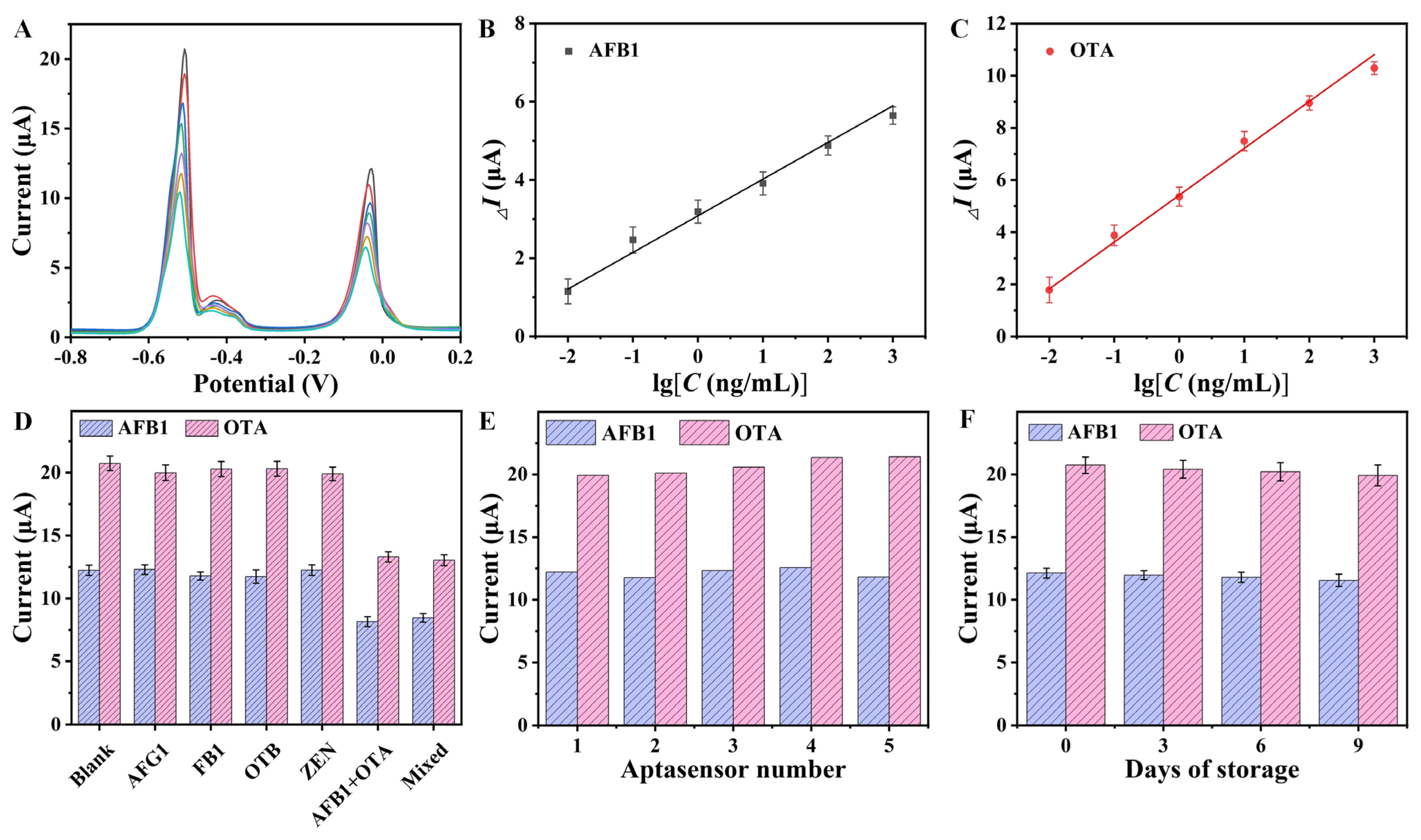
3.5. Analytical Performance of the Aptasensor
3.6. Determination of AFB1 and OTA in Cereal Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campagnollo, F.B.; Ganev, K.C.; Khaneghah, A.M.; Portela, J.B.; Cruz, A.G.; Granato, D.; Corassin, C.H.; Oliveira, C.A.F.; Sant’Ana, A.S. The occurrence and effect of unit operations for dairy products processing on the fate of aflatoxin M1: A review. Food Control 2016, 68, 310–329. [Google Scholar] [CrossRef]
- Milani, J.; Maleki, G. Effects of processing on mycotoxin stability in cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Joubrane, K.; Mnayer, D.; El Khoury, A.; El Khoury, A.; Awad, E. Co-Occurrence of Aflatoxin B1 and Ochratoxin A in Lebanese Stored Wheat. J. Food Prot. 2020, 83, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, A.; Lippolis, V.; Pascale, M. Overview of Recent Liquid Chromatography Mass Spectrometry-Based Methods for Natural Toxins Detection in Food Products. Toxins 2022, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Y.; Pike, A.; Tan, L.L. Recent Advances in Conventional Methods and Electrochemical Aptasensors for Mycotoxin Detection. Foods 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Oplatowska-Stachowiak, M.; Sajic, N.; Xu, Y.; Haughey, S.A.; Mooney, M.H.; Gong, Y.Y.; Verheijen, R.; Elliott, C.T. Fast and sensitive aflatoxin B1 and total aflatoxins ELISAs for analysis of peanuts, maize and feed ingredients. Food Control 2016, 63, 239–245. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Wang, Y.; Liu, J. Nanozyme and aptamer- based immunosorbent assay for aflatoxin B1. J. Hazard. Mater. 2020, 399, 123154. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, H.; Wu, Y.; Wang, X.; Shi, Q.; Xiang, D. A Label-Free Electrochemical Aptasensor Based on Zn/Fe Bimetallic MOF Derived Nanoporous Carbon for Ultra-Sensitive and Selective Determination of Paraquat in Vegetables. Foods 2022, 11, 2405. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X. Aptamer-Based Technology for Food Analysis. Appl. Biochem. Biotechnol. 2015, 175, 603–624. [Google Scholar] [CrossRef]
- Nan, M.-N.; Bi, Y.; Xue, H.-L.; Long, H.-T.; Xue, S.-L.; Pu, L.-M.; Prusky, D. Modification performance and electrochemical characteristics of different groups of modified aptamers applied for label-free electrochemical impedimetric sensors. Food Chem. 2021, 337, 127761. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, W.; Wen, Q.; Xu, D.; Ren, J.; Lin, Q. Aptamer-engineered nanomaterials to aid in mycotoxin determination. Food Control 2022, 135, 108661. [Google Scholar] [CrossRef]
- Feng, L.-N.; Bian, Z.-P.; Peng, J.; Jiang, F.; Yang, G.-H.; Zhu, Y.-D.; Yang, D.; Jiang, L.-P.; Zhu, J.-J. Ultrasensitive Multianalyte Electrochemical Immunoassay Based on Metal Ion Functionalized Titanium Phosphate Nanospheres. Anal. Chem. 2012, 84, 7810–7815. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection based on metal ions doped nanoscale MOFs as signal tracers and RecJf exonuclease-assisted targets recycling amplification. Talanta 2016, 161, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. A novel aptamer- metal ions- nanoscale MOF based electrochemical biocodes for multiple antibiotics detection and signal amplification. Sens. Actuators B Chem. 2017, 242, 1201–1209. [Google Scholar] [CrossRef]
- Wang, J.-C.; Hu, Y.-H.; Chen, G.-J.; Dong, Y.-B. Cu(ii)/Cu(0)@UiO-66-NH2: Base metal@MOFs as heterogeneous catalysts for olefin oxidation and reduction. Chem. Commun. 2016, 52, 13116–13119. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, W.; Shi, J.; Zou, X.; Li, Z.; Zhu, Y. Microfabricated interdigitated Au electrode for voltammetric determination of lead and cadmium in Chinese mitten crab (Eriocheir sinensis). Food Chem. 2016, 201, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yiwei, X.; Wen, Z.; Xiaowei, H.; Jiyong, S.; Xiaobo, Z.; Yanxiao, L.; Xueping, C.; Tahir, H.E.; Zhihua, L. A Self-assembled L-Cysteine and Electrodeposited Gold Nanoparticles-reduced Graphene Oxide Modified Electrode for Adsorptive Stripping Determination of Copper. Electroanalysis 2018, 30, 194–203. [Google Scholar] [CrossRef]
- Ruscito, A.; Smith, M.; Goudreau, D.N.; DeRosa, M.C. Current Status and Future Prospects for Aptamer-Based Mycotoxin Detection. J. AOAC Int. 2016, 99, 865–877. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Qiao, W.; He, B.; Ren, W.; Zhao, R.; Suo, Z.; Yan, H.; Xu, Y.; Wei, M.; Jin, H. Colloidal Au sphere and nanoflower-based immunochromatographic strips for sensitive detection of zearalenone in cereals. Anal. Methods 2022, 14, 3831–3839. [Google Scholar] [CrossRef]
- Wang, B.; He, B.; Xie, L.; Cao, X.; Liang, Z.; Wei, M.; Jin, H.; Ren, W.; Suo, Z.; Xu, Y. A novel detection strategy for nitrofuran metabolite residues: Dual-mode competitive-type electrochemical immunosensor based on polyethyleneimine reduced graphene oxide/gold nanorods nanocomposite and silica-based multifunctional immunoprobe. Sci. Total Environ. 2022, 853, 158676. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Feng, Y.; Suo, D.; Xiao, Z.; Wang, S.; Liang, Y.; Fan, X. Simultaneous Determination of 11 Mycotoxins in Maize via Multiple-Impurity Adsorption Combined with Liquid Chromatography–Tandem Mass Spectrometry. Foods 2022, 11, 3624. [Google Scholar] [CrossRef] [PubMed]
- Peñas-Garzón, M.; Sampaio, M.J.; Wang, Y.L.; Bedia, J.; Rodriguez, J.J.; Belver, C.; Silva, C.G.; Faria, J.L. Solar photocatalytic degradation of parabens using UiO-66-NH2. Sep. Purif. Technol. 2022, 286, 120467. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, C.; He, Y.; Yang, C.; Li, X.; Zheng, J.; Wang, S. Facile preparation of magnetic Zr-MOF for adsorption of Pb(II) and Cr(VI) from water: Adsorption characteristics and mechanisms. Chem. Eng. J. 2021, 415, 128923. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, K.; Magagnin, L.; Wu, X.; Jiang, Z.; Wang, W. Selective adsorption of Pb (II) and Cu (II) on mercapto-functionalized aerogels: Experiments, DFT studies and LCA analysis. J. Clean. Prod. 2023, 393, 136126. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, X.; Zhang, W.; Jiang, H.; Pu, Y.; Cao, J.; Jiang, W. Zirconium(IV)-based metal-organic framework for determination of imidacloprid and thiamethoxam pesticides from fruits by UPLC-MS/MS. Food Chem. 2021, 344, 128650. [Google Scholar] [CrossRef] [PubMed]
- Klingler, R.J.; Kochi, J.K. Electron-transfer kinetics from cyclic voltammetry. Quantitative description of electrochemical reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- Zamarchi, F.; Silva, T.R.; Winiarski, J.P.; Santana, E.R.; Vieira, I.C. Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs. Chemosensors 2022, 10, 357. [Google Scholar] [CrossRef]
- da Silva, V.J.; Baumgarten, L.G.; Dreyer, J.P.; Santana, E.R.; Spinelli, A.; Winiarski, J.P.; Vieira, I.C. Heparin-stabilized gold nanoparticles embedded in graphene for the electrochemical determination of esculetin. Anal. Methods 2024, 16, 2256–2266. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, R.; Zhu, J.; Lu, X.; Li, Y.; Qiu, S.; Jia, L.; Jiao, X.; Song, S.; Fan, C.; et al. Electrochemical DNA Biosensor Based on a Tetrahedral Nanostructure Probe for the Detection of Avian Influenza A (H7N9) Virus. ACS Appl. Mater. Interfaces 2015, 7, 8834–8842. [Google Scholar] [CrossRef]
- Pu, X.; Hu, Y.; Niu, M.; Liu, H.; Li, C.; Ma, W.; Gu, Y. An antifouling electrochemical aptasensor based on a Y-shaped peptide for tetracycline detection in milk. J. Food Compos. Anal. 2024, 132, 106349. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; An, K.; Ren, C.; Lu, X.; Hao, N.; Liu, Q.; Li, H.; Huang, X.; Wang, K. Fabrication of magnetically assembled aptasensing device for label-free determination of aflatoxin B1 based on EIS. Biosens. Bioelectron. 2018, 108, 69–75. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Zhu, C.; Shen, X.; Liu, Y.; You, T. Sensitivity programmable ratiometric electrochemical aptasensor based on signal engineering for the detection of aflatoxin B1 in peanut. J. Hazard. Mater. 2020, 387, 122001. [Google Scholar] [CrossRef] [PubMed]
- Yagati, A.K.; Chavan, S.G.; Baek, C.; Lee, M.-H.; Min, J. Label-Free Impedance Sensing of Aflatoxin B1 with Polyaniline Nanofibers/Au Nanoparticle Electrode Array. Sensors 2018, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Hayat, A.; Catanante, G.; Ocaña, C.; Marty, J.-L. A label free aptasensor for Ochratoxin A detection in cocoa beans: An application to chocolate industries. Anal. Chim. Acta 2015, 889, 106–112. [Google Scholar] [CrossRef]
- Li, D.-l.; Zhang, X.; Ma, Y.; Deng, Y.; Hu, R.; Yang, Y. Preparation of an OTA aptasensor based on a metal–organic framework. Anal. Methods 2018, 10, 3273–3279. [Google Scholar] [CrossRef]
- Argoubi, W.; Algethami, F.K.; Raouafi, N. Enhanced sensitivity in electrochemical detection of ochratoxin A within food samples using ferrocene- and aptamer-tethered gold nanoparticles on disposable electrodes. RSC Adv. 2024, 14, 8007–8015. [Google Scholar] [CrossRef]
| Electrochemical Sensor | Analytes | Linear Ranges (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|---|
| Aptamer/Fe3O4@Au/SPCE | AFB1 | 0.02–50 | 0.015 | [33] |
| Fc-aptamer/cDNA/AuNPs/THI-rGO/GCE | AFB1 | 0.05–20 | 0.016 | [34] |
| Ab/Au-PANI/ITO | AFB1 | 0.1–100 | 0.05 | [35] |
| Aptamer/diazonium/SPCE | OTA | 0.15–2.5 | 0.15 | [36] |
| Cd-MOF@Au/aptamer/cDNA/AuNPs-MoS2/GCE | OTA | 0.05–100 | 0.01 | [37] |
| Apt/FcL/AuNPs/GCE | OTA | 0.5–70 | 0.011 | [38] |
| The prepared aptasensor | AFB1 OTA | 0.01–1000 | 0.0062 0.0037 | This work |
| Sample | Spiked (ng/g) | Detected by the Aptasensor (ng/g) | Recovery | Detected by HPLC-MS (ng/g) | ||||
|---|---|---|---|---|---|---|---|---|
| AFB1 | OTA | AFB1 | OTA | AFB1 | OTA | AFB1 | OTA | |
| Corn | 1 | 1 | 1.04 ± 0.05 | 0.97 ± 0.04 | 103.7% | 96.9% | 1.02 ± 0.05 | 1.05 ± 0.04 |
| 50 | 50 | 52.06 ± 2.25 | 46.94 ± 1.90 | 104.1% | 93.9% | 47.10 ± 1.78 | 51.12 ± 2.23 | |
| Wheat | 5 | 5 | 4.72 ± 0.18 | 5.16 ± 0.24 | 94.4% | 103.2% | 5.22 ± 0.23 | 4.79 ± 0.19 |
| 100 | 100 | 93.90 ± 3.41 | 95.01 ± 4.21 | 93.9% | 95.0% | 91.06 ± 3.90 | 94.35 ± 4.15 | |
| Rice | 25 | 25 | 24.34 ± 1.17 | 25.59 ± 0.95 | 97.4% | 102.4% | 25.07 ± 1.05 | 24.80 ± 0.92 |
| 500 | 500 | 462.53 ± 18.72 | 472.96 ± 21.07 | 92.5% | 94.6% | 483.57 ± 16.35 | 467.32 ± 19.16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Jia, X.; Yang, S.; Cao, M.; He, B.; Ren, W.; Suo, Z. Simultaneous Determination of Aflatoxin B1 and Ochratoxin A in Cereals by a Novel Electrochemical Aptasensor Using Metal–Organic Framework as Signal Carrier. Foods 2024, 13, 2177. https://doi.org/10.3390/foods13142177
Xu Y, Jia X, Yang S, Cao M, He B, Ren W, Suo Z. Simultaneous Determination of Aflatoxin B1 and Ochratoxin A in Cereals by a Novel Electrochemical Aptasensor Using Metal–Organic Framework as Signal Carrier. Foods. 2024; 13(14):2177. https://doi.org/10.3390/foods13142177
Chicago/Turabian StyleXu, Yiwei, Xupeng Jia, Sennan Yang, Mengrui Cao, Baoshan He, Wenjie Ren, and Zhiguang Suo. 2024. "Simultaneous Determination of Aflatoxin B1 and Ochratoxin A in Cereals by a Novel Electrochemical Aptasensor Using Metal–Organic Framework as Signal Carrier" Foods 13, no. 14: 2177. https://doi.org/10.3390/foods13142177
APA StyleXu, Y., Jia, X., Yang, S., Cao, M., He, B., Ren, W., & Suo, Z. (2024). Simultaneous Determination of Aflatoxin B1 and Ochratoxin A in Cereals by a Novel Electrochemical Aptasensor Using Metal–Organic Framework as Signal Carrier. Foods, 13(14), 2177. https://doi.org/10.3390/foods13142177






