Emerging Chemical, Biochemical, and Non-Thermal Physical Treatments in the Production of Hypoallergenic Plant Protein Ingredients
Abstract
1. Introduction
2. Chemical Processes to Reduce Food Allergenicity
2.1. Glycation and Glycosylation
2.1.1. Gluten Proteins
2.1.2. Soybean Proteins
2.1.3. Faba Bean Proteins
2.1.4. Peanut Proteins
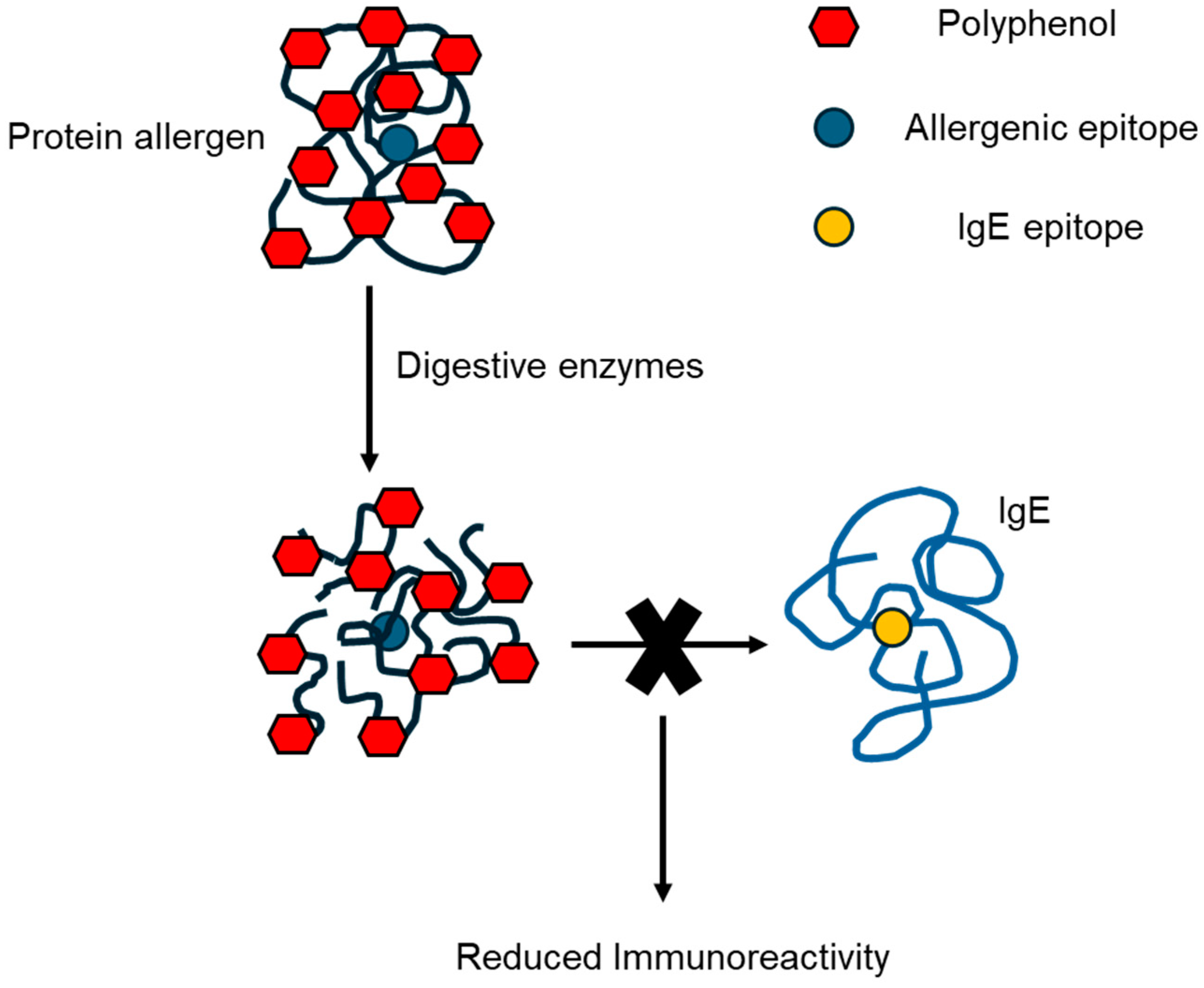
2.2. Polyphenol Complexation
2.2.1. Gluten Proteins
2.2.2. Soybean Proteins
2.2.3. Faba Bean Proteins
2.2.4. Peanut Proteins
3. Biochemical Processes to Reduce Food Allergenicity
3.1. Fermentation through Microbial Activity
3.1.1. Gluten Proteins
3.1.2. Soybean Proteins
3.1.3. Faba Bean Proteins
3.1.4. Peanut Proteins
3.2. Enzymatic Catalysis
3.2.1. Gluten Proteins
3.2.2. Soybean Proteins
3.2.3. Faba Bean Proteins
3.2.4. Peanut Proteins
4. Emerging Technologies to Increase the Effectiveness of Chemical and Biochemical Treatments
4.1. Physical Treatments
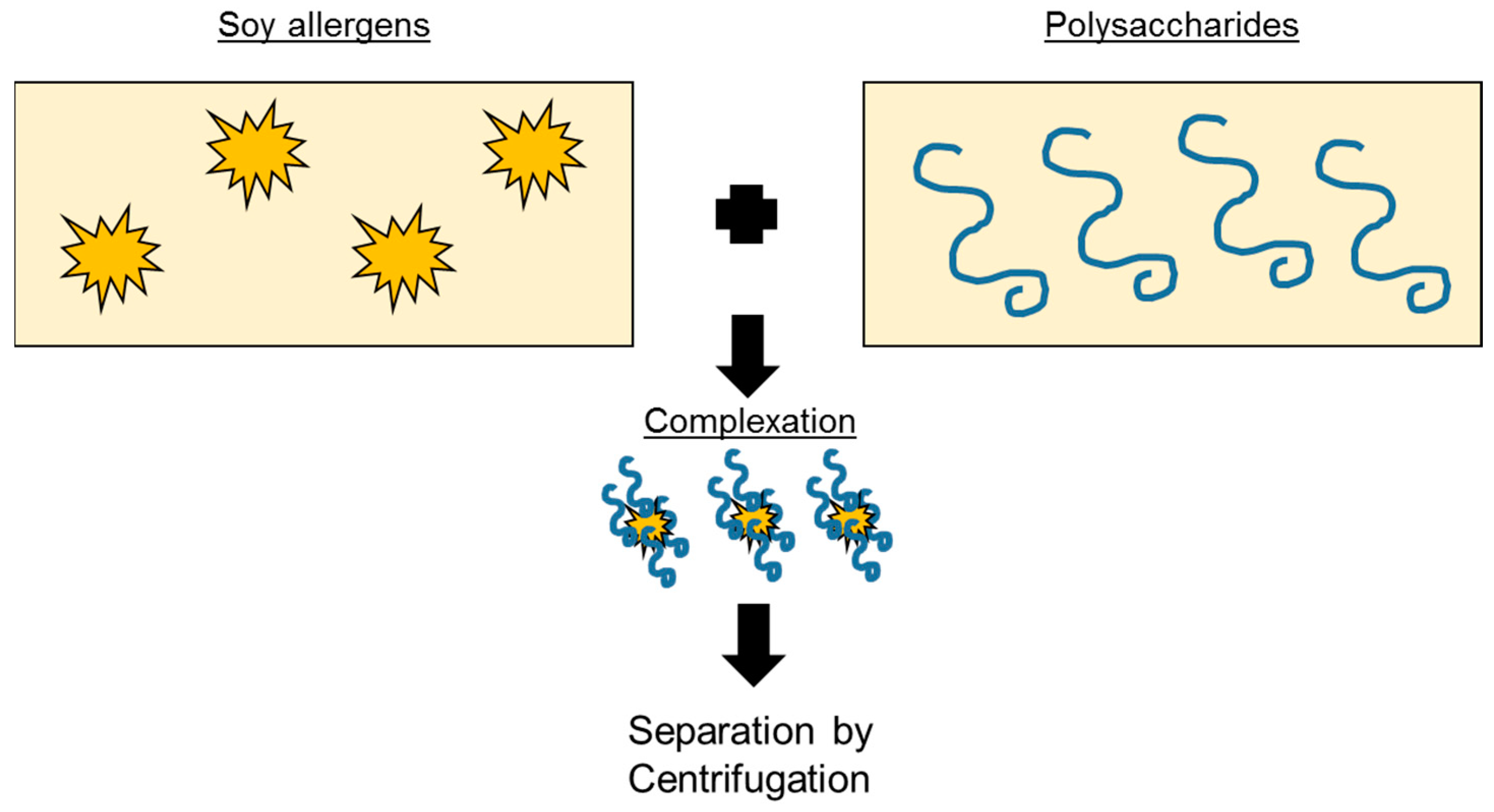
4.2. Colloidal Complexation to Bind Allergens
5. Challenges and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pater, L.; Kollen, C.; Damen, F.W.M.; Zandstra, E.H.; Fogliano, V.; Steenbekkers, B.L.P.A. The perception of 8- to 10-year-old Dutch children towards plant-based meat analogues. Appetite 2022, 178, 106264. [Google Scholar] [CrossRef] [PubMed]
- Sloan, A.E. What to Watch for as Plant-Based Food Market Grows. Food Technol. Mag. 2021, 75, 7. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2021/august/columns/consumer-trends-plant-based-food-market (accessed on 4 July 2023).
- Ha, V.; Sievenpiper, J.L.; De Souza, R.J.; Jayalath, V.H.; Mirrahimi, A.; Agarwal, A.; Chiavaroli, L.; Mejia, S.B.; Sacks, F.M.; Di Buono, M.; et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic review and meta-analysis of randomized controlled trials. Can. Med. Assoc. J. 2014, 186, E252–E262. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hu, F.B.; Ros, E.; Sabaté, J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J. Nutr. 2008, 138, 1746S–1751S. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef]
- Mills, E.N.; Jenkins, J.; Marigheto, N.; Belton, P.S.; Gunning, A.P.; Morris, V.J. Allergens of the cupin superfamily. Biochem. Soc. Trans. 2002, 30, 925–929. [Google Scholar] [CrossRef]
- Mylne, J.S.; Hara-Nishimura, I.; Rosengren, K.J. Seed storage albumins: Biosynthesis, trafficking and structures. Funct. Plant Biol. 2014, 41, 671–677. [Google Scholar] [CrossRef]
- Krause, S.; Reese, G.; Randow, S.; Zennaro, D.; Quaratino, D.; Palazzo, P.; Ciardiello, M.A.; Petersen, A.; Becker, W.-M.; Mari, A. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J. Allergy Clin. Immunol. 2009, 124, 771–778. [Google Scholar]
- Palladino, C.; Breiteneder, H. Peanut allergens. Mol. Immunol. 2018, 100, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monge, R.; Lombardero, M.; García-Sellés, F.J.; Barber, D.; Salcedo, G. Lipid-transfer proteins are relevant allergens in fruit allergy. J. Allergy Clin. Immunol. 1999, 103, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; Ortolani, C.; Baroglio, C.; Pravettoni, V.; Ispano, M.; Giuffrida, M.G.; Fortunato, D.; Farioli, L.; Monza, M.; Napolitano, L.; et al. Complete amino acid sequence determination of the major allergen of peach (Prunus persica) Pru p1. Biol. Chem. 1999, 380, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; Pravettoni, V.; Farioli, L.; Ispano, M.; Fortunato, D.; Monza, M.; Giuffrida, M.G.; Rivolta, F.; Scibola, E.; Ansaloni, R.; et al. Clinical role of a lipid transfer protein that acts as a new apple-specific allergen. J. Allergy Clin. Immunol. 1999, 104, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; D’Ambrosio, F.P.; Pravettoni, V.; Farioli, L.; Giuffrida, G.; Monza, M.; Ansaloni, R.; Fortunato, D.; Scibola, E.; Rivolta, F.; et al. Evidence for a lipid transfer protein as the major allergen of apricot. J. Allergy Clin. Immunol. 2000, 105, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Van Ree, R. Clinical importance of non-specific lipid transfer as food allergens. Biochem. Soc. Trans. 2002, 30, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Pi, X.; Wan, Y.; Yang, Y.; Li, R.; Wu, X.; Xie, M.; Li, X.; Fu, G. Research progress in peanut allergens and their allergenicity reduction. Trends Food Sci. Technol. 2019, 93, 212–220. [Google Scholar] [CrossRef]
- Song, Y.; Yang, S.; Li, J. Effect of Maillard reaction conditions on the solubility and molecular properties of wheat gluten–maltose conjugates. Food Sci. Nutr. 2020, 8, 5898–5906. [Google Scholar] [CrossRef]
- Song, Y.; Huang, D.; Guo, W.; Gao, Y.; Xue, F.; Xiong, X.; Li, C. Physicochemical and structural properties of gluten-konjac glucomannan conjugates prepared by Maillard reaction. Polymers 2023, 15, 631. [Google Scholar] [CrossRef]
- Gao, L.; Ma, W.; Chen, J.; Wang, K.E.; Li, J.; Wang, S.; Bekes, F.; Appels, R.; Yan, Y. Characterization and comparative analysis of wheat high molecular weight glutenin subunits by SDS-PAGE, RP-HPLC, HPCE, and MALDI-TOF-MS. J. Agric. Food Chem. 2010, 58, 2777–2786. [Google Scholar] [CrossRef]
- Laurière, M.; Bouchez, I.; Doyen, C.; Eynard, L. Identification of glycosylated forms of wheat storage proteins using two-dimensional electrophoresis and blotting. Electrophoresis 1996, 17, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Song, T.W.; Hong, J.Y.; Lee, K.E.; Kim, M.N.; Kim, Y.H.; Lee, S.Y.; Kim, K.W.; Sohn, M.H.; Kim, K.E. IgE reactivity to carbohydrate moieties of glycoproteins in wheat allergy. Allergy Asthma Proc. 2015, 36, 192–199. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Wu, S.; Dong, L.; Hu, Y.; Wang, J.; Zhang, Y.; Wang, S. Methylglyoxal Decoration of Glutenin during Heat Processing Could Alleviate the Resulting Allergic Reaction in Mice. Nutrients 2020, 12, 2844. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Silván, J.M.; Moreno, F.J.; Olano, A.; Del Castillo, M.D. In vitro glycation and antigenicity of soy proteins. Food Res. Int. 2007, 40, 153–160. [Google Scholar] [CrossRef]
- Bu, G.; Zhu, T.; Chen, F.; Zhang, N.; Liu, K.; Zhang, L.; Yang, H. Effects of saccharide on the structure and antigenicity of β-conglycinin in soybean protein isolate by glycation. Eur. Food Res. Technol. 2015, 240, 285–293. [Google Scholar] [CrossRef]
- Walter, J.; Greenberg, Y.; Sriramarao, P.; Ismail, B.P. Limited hydrolysis combined with controlled Maillard-induced glycation does not reduce immunoreactivity of soy protein for all sera tested. Food Chem. 2016, 213, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; He, M. Location of destroyed antigenic sites of Gly m Bd 60 K after three processing technologies. Food Res. Int. 2020, 134, 109199. [Google Scholar] [CrossRef]
- Chung, S.Y.; Champagne, E.T. Association of end-product adducts with increased IgE binding of roasted peanuts. J. Agric. Food Chem. 2001, 49, 3911–3916. [Google Scholar] [CrossRef]
- Alavi, F.; Chen, L.; Wang, Z.; Emam-Djomeh, Z. Consequences of heating under alkaline pH alone or in the presence of maltodextrin on solubility, emulsifying and foaming properties of faba bean protein. Food Hydrocoll. 2021, 112, 106335. [Google Scholar] [CrossRef]
- Xu, Y.; Pitkänen, L.; Maina, N.H.; Coda, R.; Katina, K.; Tenkanen, M. Interactions between fava bean protein and dextrans produced by Leuconostoc pseudomesenteroides DSM 20193 and Weissella cibaria Sj 1b. Carbohydr. Polym. 2018, 190, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Vissers, Y.M.; Blanc, F.; Skov, P.S.; Johnson, P.E.; Rigby, N.M.; Przybylski-Nicaise, L.; Bernard, H.; Wal, J.-M.; Ballmer-Weber, B.; Zuidmeer-Jongejan, L.; et al. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS ONE 2011, 6, e23998. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Rao, H.; Xue, W. Advances in research on the detection of peanut allergens, desensitized process and therapy. Sci. Technol. Food Ind. 2017, 38, 306–311. [Google Scholar]
- Zhang, W.; Zhu, Q.; Zhang, T.; Cai, Q.; Chen, Q. Thermal processing effects on peanut allergen Ara h 2 allergenicity in mice and its antigenic epitope structure. Food Chem. 2016, 212, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-D.; Huang, L.; Meng, L.; Lin, Y.-F.; Xu, X.; Dong, M.-S. Soy protein isolate-(-)-epigallocatechin gallate conjugate: Covalent binding sites identification and IgE binding ability evaluation. Food Chem. 2020, 333, 127400. [Google Scholar] [CrossRef] [PubMed]
- Bansode, R.R.; Randolph, P.D.; Plundrich, N.J.; Lila, M.A.; Williams, L.L. Peanut protein-polyphenol aggregate complexation suppresses allergic sensitization to peanut by reducing peanut-specific IgE in C3H/HeJ mice. Food Chem. 2019, 299, 125025. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Perez-Gregorio, R.; Mateus, N.; De Freitas, V. The interaction between tannins and gliadin derived peptides in a celiac disease perspective. RSC Adv. 2015, 5, 32151–32158. [Google Scholar] [CrossRef]
- Pérot, M.; Lupi, R.; Guyot, S.; Delayre-Orthez, C.; Gadonna-Widehem, P.; Thébaudin, J.-Y.; Bodinier, M.; Larré, C. Polyphenol interactions mitigate the immunogenicity and allergenicity of gliadins. J. Agric. Food Chem. 2017, 65, 6442–6451. [Google Scholar] [CrossRef] [PubMed]
- Plundrich, N.J.; Kulis, M.; White, B.L.; Grace, M.H.; Guo, R.; Burks, A.W.; Davis, J.P.; Lila, M.A. Novel strategy to create hypoallergenic peanut protein–polyphenol edible matrices for oral immunotherapy. J. Agric. Food Chem. 2014, 62, 7010–7021. [Google Scholar] [CrossRef]
- Van Buiten, C.B.; Yennawar, N.H.; Pacheco, C.N.; Hatzakis, E.; Elias, R.J. Physicochemical interactions with (−)-epigallocatechin-3-gallate drive structural modification of celiac-associated peptide α 2-gliadin (57–89) at physiological conditions. Food Funct. 2019, 10, 2997–3007. [Google Scholar] [CrossRef]
- Van Buiten, C.B.; Lambert, J.D.; Elias, R.J. Green tea polyphenols mitigate gliadin-mediated inflammation and permeability in vitro. Mol. Nutr. Food Res. 2018, 62, 1700879. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ye, L.; He, K.; Zhang, T.; Sun, F.; Mei, T.; Wu, X. A new method to reduce allergenicity by improving the functional properties of soybean 7S protein through covalent modification with polyphenols. Food Chem. 2022, 373, 131589. [Google Scholar] [CrossRef] [PubMed]
- Kosińska, A.; Karamać, M.; Penkacik, K.; Urbalewicz, A.; Amarowicz, R. Interactions between tannins and proteins isolated from broad bean seeds (Vicia faba Major) yield soluble and non-soluble complexes. Eur. Food Res. Technol. 2011, 233, 213–222. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kato, Y.; Champagne, E.T. Polyphenol oxidase/caffeic acid may reduce the allergenic properties of peanut allergens. J. Sci. Food Agric. 2005, 85, 2631–2637. [Google Scholar] [CrossRef]
- He, W.; Zhang, T.; Velickovic, T.C.; Li, S.; Lyu, Y.; Wang, L.; Yi, J.; Liu, Z.; He, Z.; Wu, X. Covalent conjugation with (−)-epigallo-catechin 3-gallate and chlorogenic acid changes allergenicity and functional properties of Ara h1 from peanut. Food Chem. 2020, 331, 127355. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Sun, Y.; Cheng, J.; Fu, G.; Guo, M. A review on polyphenols and their potential application to reduce food allergenicity. Crit. Rev. Food Sci. Nutr. 2022, 23, 10014–10031. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Cheng, J.-H.; Sun, D.-W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Pi, X.; Fu, G.; Dong, B.; Yang, Y.; Wan, Y.; Xie, M. Effects of fermentation with Bacillus natto on the allergenicity of peanut. LWT 2021, 141, 110862. [Google Scholar] [CrossRef]
- Tan, J.; Taitz, J.; Sun, S.M.; Langford, L.; Ni, D.; Macia, L. Your regulatory T cells are what you eat: How diet and gut microbiota affect regulatory T cell development. Front. Nutr. 2022, 9, 878382. [Google Scholar] [CrossRef]
- Costa, J.; Bavaro, S.L.; Benedé, S.; Diaz-Perales, A.; Bueno-Diaz, C.; Gelencser, E.; Klueber, J.; Larré, C.; Lozano-Ojalvo, D.; Lupi, R.; et al. Are Physicochemical Properties Shaping the Allergenic Potency of Plant Allergens? Clin. Rev. Allergy Immunol. 2022, 62, 37–63. [Google Scholar] [CrossRef]
- Caminero, A.; Herrán, A.R.; Nistal, E.; Pérez-Andrés, J.; Vaquero, L.; Vivas, S.; Ruiz de Morales, J.M.G.; Albillos, S.M.; Casqueiro, J. Diversity of the cultivable human gut microbiome involved in gluten metabolism: Isolation of microorganisms with potential interest for coeliac disease. FEMS Microbiol. Ecol. 2014, 88, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; Lavermicocca, P.; De Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef]
- Gerez, C.L.; Dallagnol, A.; Rollán, G.; de Valdez, G.F. A combination of two lactic acid bacteria improves the hydrolysis of gliadin during wheat dough fermentation. Food Microbiol. 2012, 32, 427–430. [Google Scholar] [CrossRef]
- El Mecherfi, K.-E.; Lupi, R.; Cherkaoui, M.; Albuquerque, M.A.C.; Todorov, S.D.; Tranquet, O.; Klingebiel, C.; Rogniaux, H.; Denery-Papini, S.; Onno, B.; et al. Fermentation of gluten by Lactococcus lactis LLGKC18 reduces its antigenicity and allergenicity. Probiotics Antimicrob. Proteins 2022, 14, 779–791. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J. Cell. Biochem. 2010, 109, 801–807. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, X.; Ding, X.; Dong, H.; Wang, W. Effects of high-intensity ultrasound pretreatment on structure, properties, and enzymolysis of soy protein isolate. Molecules 2019, 24, 3637. [Google Scholar] [CrossRef]
- Lucas, I.; Petermeier, H.; Becker, T.; Jekle, M. Definition of network types—Prediction of dough mechanical behaviour under shear by gluten microstructure. Sci. Rep. 2019, 9, 4700. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Huang, J.; Xing, G.; Zhang, Q.; Li, W.; Dong, M. Changes in soy protein immunoglobulin E reactivity, protein degradation, and conformation through fermentation with Lactobacillus plantarum strains. LWT 2019, 99, 156–165. [Google Scholar] [CrossRef]
- Liu, B.; Teng, D.; Yang, Y.; Wang, X.; Wang, J. Development of a competitive ELISA for the detection of soybean α subunit of β-conglycinin. Process Biochem. 2012, 47, 280–287. [Google Scholar] [CrossRef]
- Biscola, V.; de Olmos, A.R.; Choiset, Y.; Rabesona, H.; Garro, M.S.; Mozzi, F.; Chobert, J.-M.; Drouet, M.; Haertlé, T.; de Franco, M.B.D.G. Soymilk fermentation by Enterococcus faecalis VB43 leads to reduction in the immunoreactivity of allergenic proteins β-conglycinin (7S) and glycinin (11S). Benef. Microbes 2017, 8, 635–643. [Google Scholar] [CrossRef]
- Xia, J.; Zu, Q.; Yang, A.; Wu, Z.; Li, X.; Tong, P.; Yuan, J.; Wu, Y.; Fan, Q.; Chen, H. Allergenicity reduction and rheology property of Lactobacillus-fermented soymilk. J. Sci. Food Agric. 2019, 99, 6841–6849. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-H.; Cho, S.-J. Changes in allergenic and antinutritional protein profiles of soybean meal during solid-state fermentation with Bacillus subtilis. LWT 2016, 70, 208–212. [Google Scholar] [CrossRef]
- Amnuaycheewa, P.; de Mejia, E.G. Purification, characterisation, and quantification of the soy allergen profilin (Gly m 3) in soy products. Food Chem. 2010, 119, 1671–1680. [Google Scholar] [CrossRef]
- Sozer, N.; Melama, L.; Silbir, S.; Rizzello, C.G.; Flander, L.; Poutanen, K. Lactic acid fermentation as a pre-treatment process for faba bean flour and its effect on textural, structural and nutritional properties of protein-enriched gluten-free faba bean breads. Foods 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Pontonio, E.; Montemurro, M.; Rizzello, C.G. Fermentation as strategy for improving nutritional, functional, technological, and sensory properties of legumes. In Legumes Research-Volume 2; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Chandra-Hioe, M.V.; Wong, C.H.M.; Arcot, J. The Potential use of fermented chickpea and faba bean flour as food ingredients. Plant Foods Hum. Nutr. 2016, 71, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Effect of Microbial Fermentation on the Allergens in Peanut Protein; Henan University of Technology: Zhengzhou, China, 2014. [Google Scholar]
- Won, T.J.; Kim, B.; Song, D.S.; Lim, Y.T.; Oh, E.S.; Lee, D.I.; Park, E.S.; Min, H.; Park, S.Y.; Hwang, K.W. Modulation of Th1/Th2 balance by Lactobacillus strains isolated from Kimchi via stimulation of macrophage cell line J774A.1 in vitro. J Food Sci. 2011, 76, H55–H61. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Changes in n-Hexanal content of peanut milk fermented with lactic acid bacteria. Food Sci. Biotechnol. 2001, 10, 387–390. [Google Scholar]
- Ahmed, I.; Chen, H.; Li, J.; Wang, B.; Li, Z.; Huang, G. Enzymatic crosslinking and food allergenicity: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5856–5879. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Roncoroni, L.; Hils, M.; Pasternack, R.; Barisani, D.; Terrani, C.; Vaira, V.; Ferrero, S.; Bardella, M.T. Immunological effects of transglutaminase-treated gluten in coeliac disease. Hum. Immunol. 2012, 73, 992–997. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; Bonavita, R.; Maurano, F.; Bergamo, P.; Siciliano, R.A.; Rossi, M. Biochemical modifications of gliadins induced by microbial transglutaminase on wheat flour. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 5166–5174. [Google Scholar] [CrossRef]
- Lombardi, E.; Bergamo, P.; Maurano, F.; Bozzella, G.; Luongo, D.; Mazzarella, G.; Rotondi Aufiero, V.; Iaquinto, G.; Rossi, M. Selective inhibition of the gliadin-specific, cell-mediated immune response by transamidation with microbial transglutaminase. J. Leukocycte Biol. 2013, 93, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Nunes, F.M.; Guedes, S.; Domingues, P.; Silva, A.M.; Carrillo, J.M.; Rodriguez-Quijano, M.; Branlard, G.; Igrejas, G. Efficient chemo-enzymatic gluten detoxification: Reducing toxic epitopes for celiac patients improving functional properties. Sci. Rep. 2015, 5, 18041. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, J.; Łącka, A.; Bryszewska, M. The use of transglutaminase in the reduction of immunoreactivity of wheat flour. Food Agric. Immunol. 2006, 17, 105–113. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; De Giulio, B.; Senger, S.; Rossi, M.; Malorni, A.; Siciliano, R.A. Identification of transglutaminase-mediated deamidation sites in a recombinant α-gliadin by advanced mass-spectrometric methodologies. Protein Sci. 2003, 12, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, B. Immunoreactivity of wheat proteins modified by hydrolysis and polymerisation. Eur. Food Res. Technol. 2016, 242, 1025–1040. [Google Scholar] [CrossRef]
- Agyare, K.K.; Addo, K.; Xiong, Y.L. Emulsifying and foaming properties of transglutaminase-treated wheat gluten hydrolysate as influenced by pH, temperature and salt. Food Hydrocoll. 2009, 23, 72–81. [Google Scholar] [CrossRef]
- Walter, T.; Wieser, H.; Koehler, P. Degradation of Gluten in Wheat Bran and Bread Drink by Means of a Proline-Specific Peptidase. J. Nutr. Food Sci. 2014, 4, 1000293. [Google Scholar] [CrossRef]
- Wolf, C.; Siegel, J.B.; Tinberg, C.; Camarca, A.; Gianfrani, C.; Paski, S.; Guan, R.; Montelione, G.; Baker, D.; Pultz, I.S. Engineering of Kuma030: A Gliadin Peptidase That Rapidly Degrades Immunogenic Gliadin Peptides in Gastric Conditions. J. Am. Chem. Soc. 2015, 137, 13106–13113. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Giosafatto, C.V.L.; Rui, X.; Dong, M.; Mariniello, L. Microbial transglutaminase-mediated polymerization in the presence of lactic acid bacteria affects antigenicity of soy protein component present in bio-tofu. J. Funct. Foods 2019, 53, 292–298. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, H.; Yang, A.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Effect of microbial transglutaminase cross-linking on the quality characteristics and potential allergenicity of tofu. Food Funct. 2019, 10, 5485–5497. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Yuan, D.; Zhao, X.; Cui, S.; Hu, J.; Wang, J. Reduction of the allergenic protein in soybean meal by enzymatic hydrolysis. Food Agric. Immunol. 2014, 25, 301–310. [Google Scholar] [CrossRef]
- Lee, H.W.; Keum, E.H.; Lee, S.J.; Sung, D.E.; Chung, D.H.; Lee, S.I.; Oh, S. Allergenicity of proteolytic hydrolysates of the soybean 11S globulin. J. Food Sci. 2007, 72, C168–C172. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.; Ahn, K.M.; Lim, S.; Oh, S. Allergenicity of an enzymatic hydrolysate of soybean 2S protein. J. Sci. Food Agric. 2014, 94, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Damodaran, S.; Heinonen, M. Effects of microbial transglutaminase treatment on physiochemical properties and emulsifying functionality of faba bean protein isolate. LWT 2019, 99, 396–403. [Google Scholar] [CrossRef]
- Nivala, O.; Mäkinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring colloidal oat and faba bean protein particles via enzymatic modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Han, J.; Swallow, K.; Tian, Z.; Jarpa-Parra, M.; Chen, L. Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem. 2019, 96, 725–741. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Toschi, T.G.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, C.; Xue, W.; Wang, Z. Crosslinked recombinant-Ara h 1 catalyzed by microbial transglutaminase: Preparation, structural characterization and allergic assessment. Foods 2020, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Hefle, S.L.; Taylor, S.L.; De Jong, G.A.H. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: A comparative in vitro study and partial characterization of digestion-resistant peptides. Mol. Nutr. Food Res. 2010, 54, 1711–1721. [Google Scholar] [CrossRef]
- Cabanillas, B.; Pedrosa, M.M.; Rodriguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of enzymatic hydrolysis on the allergenicity of roasted peanut protein extract. Int. Arch. Allergy Immunol. 2012, 157, 41–50. [Google Scholar] [CrossRef]
- Vanga, S.K.; Singh, A.; Raghavan, V. Review of conventional and novel food processing methods on food allergens. Crit. Rev. Food Sci. Nutr. 2017, 57, 2077–2094. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, J.; Raghavan, V. Critical reviews and recent advances of novel non- thermal processing techniques on the modification of food allergens. Crit. Rev. Food Sci. Nutr. 2021, 61, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, H.; Huang, S.; Yang, M.; Wu, J.; Ci, Z.; He, Y.; Wu, Z.; Han, L.; Zhang, D. Insight into the incredible effects of microwave heating: Driving changes in the structure, properties and functions of macromolecular nutrients in novel food. Front. Nutr. 2022, 9, 941527. [Google Scholar] [CrossRef] [PubMed]
- Lamacchia, C.; Landriscina, L.; D’Agnello, P. Changes in wheat kernel proteins induced by microwave treatment. Food Chem. 2016, 197, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Mahroug, H.; Ribeiro, M.; Rhazi, L.; Bentallah, L.; Zidoune, M.N.; Nunes, F.M.; Igrejas, G. How microwave treatment of gluten affects its toxicity for celiac patients? A study on the effect of microwaves on the structure, conformation, functionality and immunogenicity of gluten. Food Chem. 2019, 297, 124986. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W.; Guo, X. Comparative study of four physical approaches about allergenicity of soybean protein isolate for infant formula. Food Agric. Immunol. 2016, 27, 604–623. [Google Scholar] [CrossRef]
- Vanga, S.K.; Singh, A.; Raghavan, V. Effect of thermal and electric field treatment on the conformation of Ara h 6 peanut protein allergen. Innov. Food Sci. Emerg. Technol. 2015, 30, 79–88. [Google Scholar] [CrossRef]
- Astuti, R.M.; Palupi, N.S.; Suhartono, M.T.; Kusumaningtyas, E.; Lioe, H.N. Effect of processing treatments on the allergenicity of nuts and legumes: A meta-analysis. J. Food Sci. 2023, 88, 28–56. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, Y.; Wang, Z.; Liao, A.-M.; Pan, L.; Zhang, J.; Dong, Y.-Q.; Hu, Z.-Y.; Huang, J.-H.; Ou, X.-Q. Effect of fermentation on structural properties and antioxidant activity of wheat gluten by Bacillus subtilis. Front. Nutr. 2023, 10, 1116982. [Google Scholar] [CrossRef]
- Gentile, L. Protein–polysaccharide interactions and aggregates in food formulations. Curr. Opin. Colloid Interface Sci. 2020, 48, 18–27. [Google Scholar] [CrossRef]
- Wu, C.-L.; Chen, Q.-H.; Li, X.-Y.; Su, J.-h.; He, S.; Liu, J.; Yuan, Y. Formation and characterisation of food protein–polysaccharide thermal complex particles: Effects of pH, temperature and polysaccharide type. Int. J. Food Sci. Technol. 2020, 55, 1368–1374. [Google Scholar] [CrossRef]
- Huang, G.-Q.; Wang, H.-O.; Wang, F.-W.; Du, Y.-L.; Xiao, J.-X. Maillard reaction in protein—Polysaccharide coacervated microcapsules and its effects on microcapsule properties. Int. J. Biol. Macromol. 2020, 155, 1194–1201. [Google Scholar] [CrossRef]
- Chen, H.; Gan, J.; Ji, A.; Song, S.; Yin, L. Development of double network gels based on soy protein isolate and sugar beet pectin induced by thermal treatment and laccase catalysis. Food Chem. 2019, 292, 188–196. [Google Scholar] [CrossRef]


| Processing | Measured Sample Form | Processing Condition | n b | Actual Allergenicity Reduction (%) c | ||
|---|---|---|---|---|---|---|
| Min | Max | Ave | ||||
| Maillard reaction * | Protein | Reaction at 40–100 °C, 20 min–5 h | 4 (40) | 0 | 91.4 | 17.53 |
| Maillard reaction * | Protein | Reaction at 60 °C, 24–72 h | 1 (20) | 11.12 | 54.23 | 26.82 |
| Maillard reaction * | Whole sample | Reaction at 170 °C, 20 min | 2 (20) | 16.89 | 49.16 | 37.98 |
| Cross-linking with polyphenol * | Protein | Reaction at 25–100 °C, 1–24 h | 3 (49) | 0 | 82.07 | 34.06 |
| Proteolytic hydrolysis † | Protein | Temperature 37–60 °C, 15 min–8 h | 7 (70) | 0 | 100 | 55.35 |
| Proteolytic hydrolysis † | Whole sample | Temperature 45–55 °C, 1–2 h | 3 (30) | 19.27 | 99.95 | 75.43 |
| Fermentation † | Protein | Temperature 33–37 °C, 24–48 h | 3 (25) | 0 | 100 | 75.29 |
| Fermentation † | Whole sample | Temperature 30–37 °C, 24–48 h | 4 (47) | 15.16 | 99.15 | 66.74 |
| High hydrostatic pressure (HHP) and proteolytic hydrolysis ‡ | Protein | HHP at 100–600 MPa, 50 °C for 15 min, then hydrolysis at 50 °C for 15 min | 1 (24) | 32.11 | 99.89 | 70.49 |
| Microwaving and cross-linking with polyphenol ‡ | Whole sample | Microwaving at 500 W for 1–3 min | 1 (8) | 0 | 23.96 | 9.69 |
| Maillard reaction and cross-linking with polyphenol ‡ | Whole sample | Roasting at 170 °C for 20 min, Sugar: sucrose or glucose | 1 (16) | 0 | 40.37 | 28.06 |
| Fermentation and proteolytic hydrolysis ‡ | Whole sample | Fermentation at 30–37 °C for 24 h, then hydrolysis at 50 °C for 15 min | 1 (10) | 77.89 | 96.39 | 85.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narciso, J.O.; Gulzar, S.; Soliva-Fortuny, R.; Martín-Belloso, O. Emerging Chemical, Biochemical, and Non-Thermal Physical Treatments in the Production of Hypoallergenic Plant Protein Ingredients. Foods 2024, 13, 2180. https://doi.org/10.3390/foods13142180
Narciso JO, Gulzar S, Soliva-Fortuny R, Martín-Belloso O. Emerging Chemical, Biochemical, and Non-Thermal Physical Treatments in the Production of Hypoallergenic Plant Protein Ingredients. Foods. 2024; 13(14):2180. https://doi.org/10.3390/foods13142180
Chicago/Turabian StyleNarciso, Joan Oñate, Saqib Gulzar, Robert Soliva-Fortuny, and Olga Martín-Belloso. 2024. "Emerging Chemical, Biochemical, and Non-Thermal Physical Treatments in the Production of Hypoallergenic Plant Protein Ingredients" Foods 13, no. 14: 2180. https://doi.org/10.3390/foods13142180
APA StyleNarciso, J. O., Gulzar, S., Soliva-Fortuny, R., & Martín-Belloso, O. (2024). Emerging Chemical, Biochemical, and Non-Thermal Physical Treatments in the Production of Hypoallergenic Plant Protein Ingredients. Foods, 13(14), 2180. https://doi.org/10.3390/foods13142180









