Identification, Bioaccessibility, and Antioxidant Properties of Phenolic Compounds in Carob Syrup
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Materials
2.2. Phenolic Compounds Extraction from Carob Syrups
2.3. In Vitro Digestion of Carob Syrups
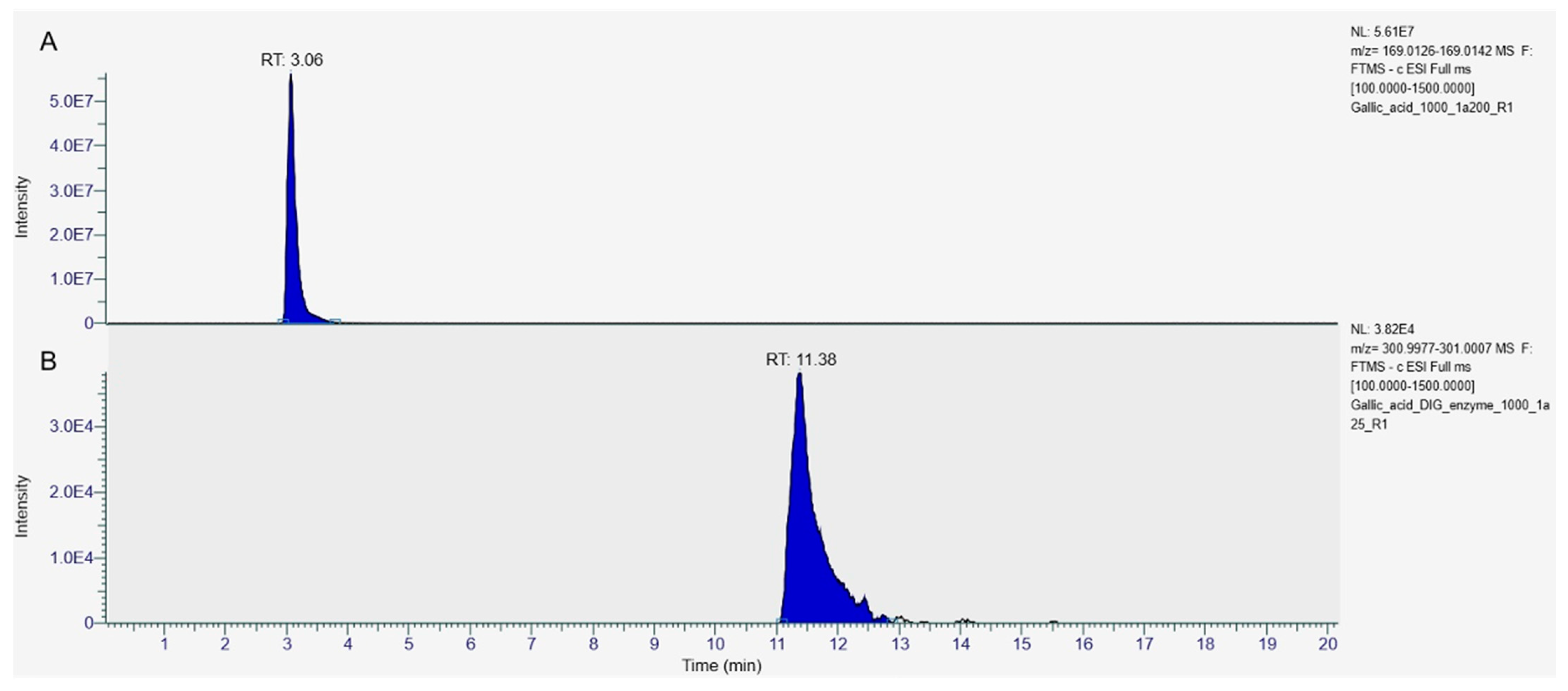
2.4. Identification and Quantification of Phenolic Compounds by High-Performance Mass Spectrometry in Chemical Extracts and In Vitro Digested Samples
2.5. Total Phenolic Compounds Quantification and Anti-Oxidant Activity Assays
2.5.1. Total Phenolic Compounds Quantification
2.5.2. Assessment of the ABTS Radical Scavenging Activity
2.5.3. Determination of the Reducing Ability
2.5.4. Evaluation of the Inhibitory Activity against Fenton Reaction
2.6. Determination of Browning Index
2.7. Statistics
3. Result and Discussion
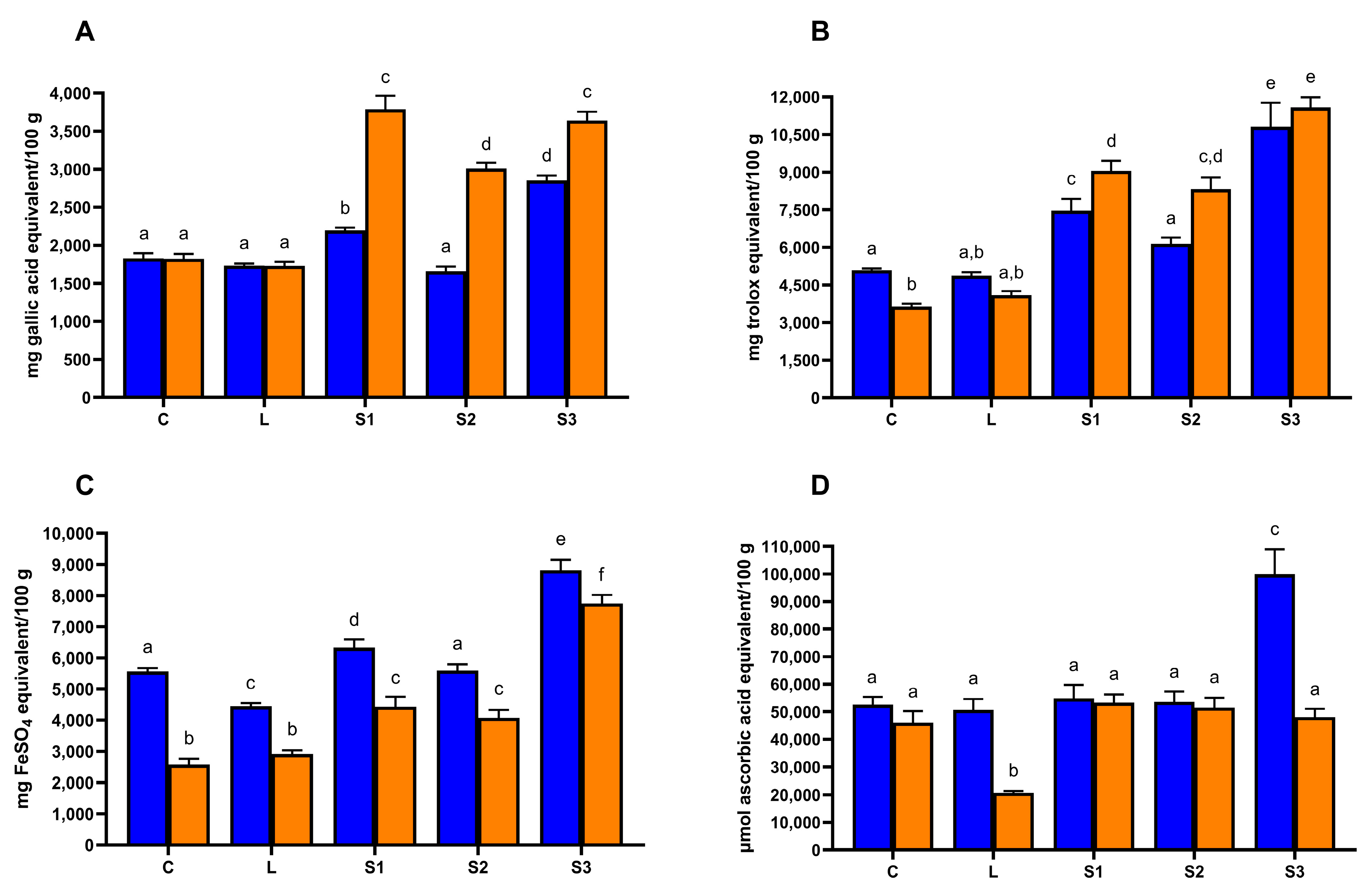
3.1. Total Phenolic Compounds, Anti-Oxidant Properties and Browning Index of Carob Syrups
3.2. Identification of Individual Phenolic Compounds in Carob Syrups by High-Resolution Mass Spectrometry
3.3. Correlation Analysis among the Variables in Carob Syrups
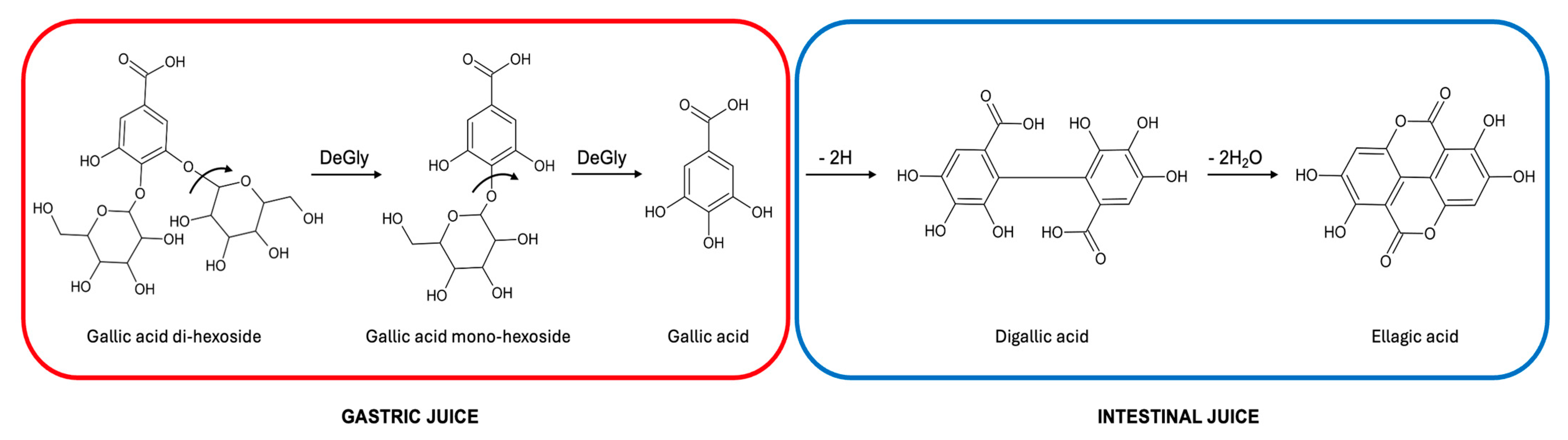
3.4. Effect of In Vitro Gastro–Intestinal Digestion on Total Phenolic Compounds and Anti-Oxidant Properties of Carob Syrups
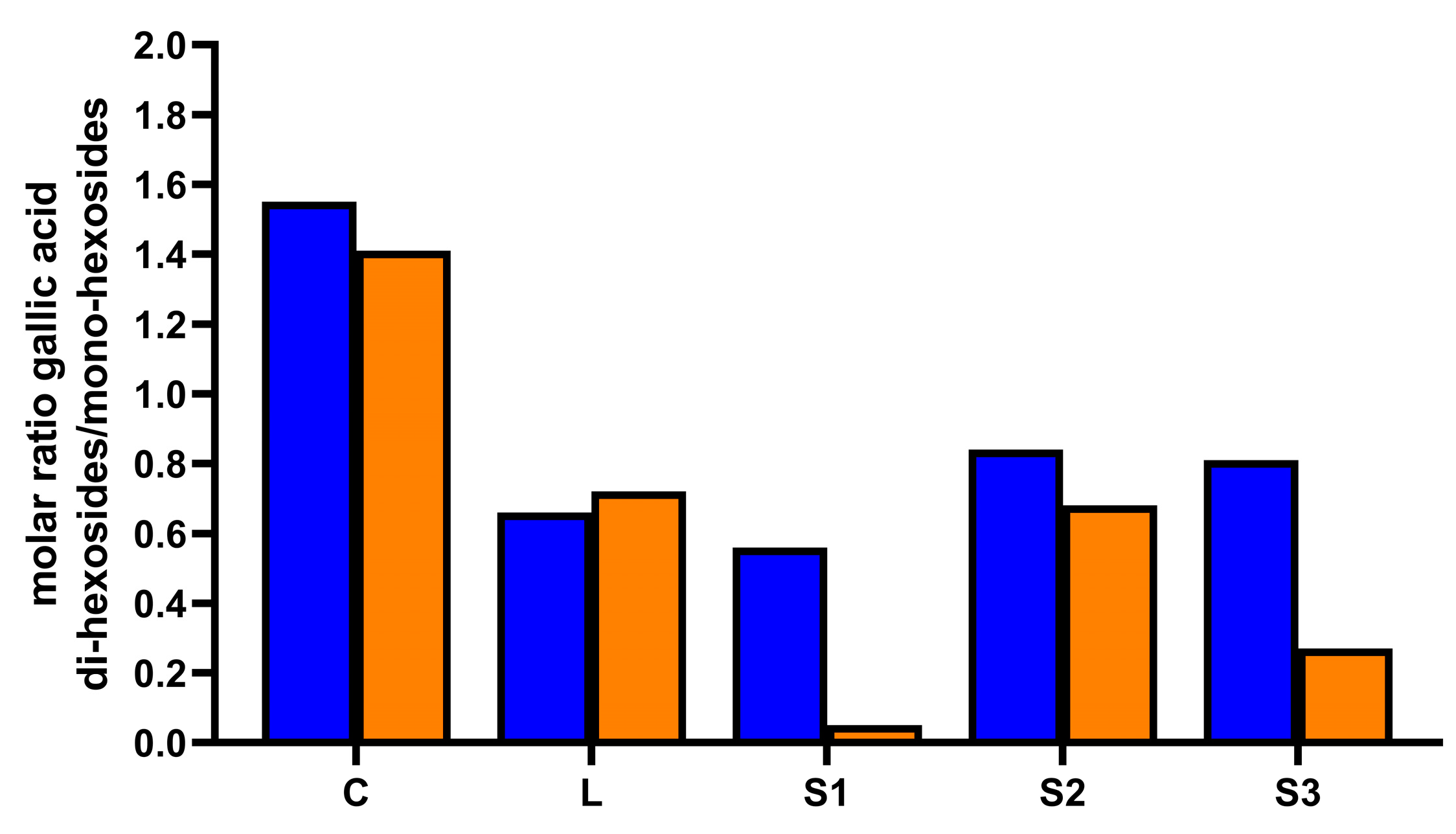
3.5. Bioaccessibility of Individual Phenolic Compounds in Carob Syrups after In Vitro Gastro–Intestinal Digestion
3.6. Correlation Analysis among the Variables in Digested Carob Syrups
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Andrikopoulos, N.K.; Kaliora, A.C. Carob: A sustainable opportunity for metabolic health. Foods 2022, 11, 2154. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications-A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Ikram, A.; Khalid, W.; Wajeeha Zafar, K.; Ali, A.; Afzal, M.F.; Aziz, A.; Faiz ul Rasool, I.; Al-Farga, A.; Aqlan, F.; Koraqi, H. Nutritional, biochemical, and clinical applications of carob: A review. Food Sci. Nutr. 2023, 11, 3641–3654. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Processing of carob kernels to syrup by ultrasound-assisted extraction. Processes 2022, 10, 983. [Google Scholar] [CrossRef]
- Nemet, M.; Vasilic, M.; Tomas, A. Lipid-lowering effects of carob extracts (Ceratonia siliqua): Proposed mechanisms and clinical importance. Front. Pharmacol. 2022, 13, 921123. [Google Scholar] [CrossRef]
- Wang, B.S.; Chang, L.W.; Kang, Z.C.; Chu, H.L.; Tai, H.M.; Huang, M.H. Inhibitory effects of molasses on mutation and nitric oxide production. Food Chem. 2011, 126, 1102–1107. [Google Scholar] [CrossRef]
- Dhaouadi, K.; Belkhir, M.; Akinicho, I.; Raboudi, F.; Pamies, D.; Barrajón, E.; Estevan, C.; Fattouch, S. Sucrose supplementation during traditional carob syrup processing affected its chemical characteristics and biological activities. LWT-Food Sci. Technol. 2014, 57, 1–8. [Google Scholar] [CrossRef]
- Ioannou, G.D.; Savva, I.K.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Phenolic profile, antioxidant activity, and chemometric classification of carob pulp and products. Molecules 2023, 28, 2269. [Google Scholar] [CrossRef]
- Ben Ayache, S.; Behija Saafi, E.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological activities of aqueous extracts from carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules 2020, 25, 3120. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-Benna, J.; Sebai, H.; Marzouki, L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT-Food Sci. Technol. 2020, 117, 108623. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef]
- Dantas, A.M.; Fernandes, F.G.; Magnani, M.; da Silva Campelo Borges, G. Gastrointestinal digestion assays for evaluating the bioaccessibility of phenolic compounds in fruits and their derivates: An overview. Food Res. Int. 2023, 170, 112920. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef]
- Pais, A.C.S.; Coscueta, E.R.; Pintado, M.M.; Silvestre, A.J.D.; Santos, S.A.O. Exploring the bioaccessibility and intestinal absorption of major classes of pure phenolic compounds using in vitro simulated gastrointestinal digestion. Heliyon 2024, 10, e28894. [Google Scholar] [CrossRef]
- Li, C.X.; Wang, F.R.; Zhang, B.; Deng, Z.Y.; Li, H.Y. Stability and antioxidant activity of phenolic compounds during in vitro digestion. J. Food Sci. 2023, 88, 696–716. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Martini, S.; Tagliazucchi, D.; Minelli, G.; Lo Fiego, D.P. Influence of linseed and antioxidant-rich diets in pig nutrition on lipid oxidation during cooking and in vitro digestion of pork. Food Res. Int. 2020, 137, 109528. [Google Scholar] [CrossRef]
- Cattivelli, A.; Di Lorenzo, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Red-skinned onion phenolic compounds stability and bioaccessibility: A comparative study between deep-frying and air-frying. J. Food Compos. Anal. 2023, 115, 105024. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. Bovine milk antioxidant properties: Effect of in vitro digestion and identification of antioxidant compounds. Dairy Sci. Technol. 2016, 96, 657–676. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Schols, H.A.; van Boekel, M.A.J.S.; Smit, G. High molecular weight melanoidins from coffee brew. J. Agric. Food Chem. 2006, 54, 7658–7666. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef]
- Toufeili, I.; Itani, M.; Zeidan, M.; Al Yamani, O.; Kharroubi, S. Nutritional and functional potential of carob syrup versus date and maple syrups. Food Technol. Biotechnol. 2022, 60, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Martinez-Piernas, A.B.; Stavrou, I.J.; Garcia-Reyes, J.F.; Kapnissi-Christodoulou, C.P. HPLC-ESI-HRMS and chemometric analysis of carobs polyphenols–technological and geographical parameters affecting their phenolic composition. J. Food Compos. Anal. 2022, 114, 104744. [Google Scholar] [CrossRef]
- Richane, A.; Rim, B.M.; Wided, M.; Riadh, K.; Khaoula, A.; Nizar, M.; Hanen, B.I. Variability of phenolic compounds and antioxidant activities of ten Ceratonia siliqua L. provenances. Biochem. Syst. Ecol. 2022, 104, 104486. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Bellesia, A. The gastro-intestinal tract as the major site of biological action of dietary melanoidins. Amino Acids 2015, 47, 1077–1089. [Google Scholar] [CrossRef]
- Nunes, F.M.; Coimbra, M.A. Role of hydroxycinnamates in coffee melanoidin formation. Phytochem. Rev. 2010, 9, 171–185. [Google Scholar] [CrossRef]
- Boles, J.S.; Crerar, D.A.; Grissom, G.; Key, T.C. Aqueous thermal degradation of gallic acid. Geochim. Cosmochim. Acta 1988, 52, 341–344. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef]
- Cattivelli, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Influence of cooking methods on onion phenolic compounds bioaccessibility. Foods 2021, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A.L. Impact of roasting on the phenolic and volatile compounds in coffee beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Vilas-Boas, A.M.; Brassesco, M.E.; Quintino, A.C.; Vieira, M.C.; Brandão, T.R.S.; Silva, C.L.M.; Azevedo, M.; Pintado, M. Particle size effect of integral carob flour on bioaccessibility of bioactive compounds during simulated gastrointestinal digestion. Foods 2022, 11, 1272. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef]
- Herrera-Cazares, L.A.; Ramírez-Jiménez, A.K.; Luzardo-Ocampo, I.; Antunes-Ricardo, M.; Loarca-Piña, G.; Wall-Medrano, A.; Gaytán-Martínez, M. Gastrointestinal metabolism of monomeric and polymeric polyphenols from mango (Mangifera indica L.) bagasse under simulated conditions. Food Chem. 2021, 365, 130528. [Google Scholar] [CrossRef]
- Patel, S.S.; Goyal, R.K. Biotransformation of gallotannins from fresh fruit juice of Emblica officinalis in in-vitro system. Res. J. Phytochem. 2013, 7, 18–23. [Google Scholar]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The Influence of in vitro gastrointestinal digestion on the chemical composition and antioxidant and enzyme inhibitory capacities of carob liqueurs obtained with different elaboration techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, G.; Veselinovic, A.; Mitic, Z.; Zivanovic, S. HPLC-DAD study of gallic acid autoxidation in alkaline aqueous solutions and the influence of Mg(II) ion. Acta Facult. Med. Naissensis 2011, 28, 219–224. [Google Scholar]
- Pant, A.F.; Özkasikci, D.; Fürtauer, S.; Reinelt, M. The effect of deprotonation on the reaction kinetics of an oxygen scavenger based on gallic acid. Front. Chem. 2019, 7, 680. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A.; Sabatini, F.; Degano, I. A model iron gall ink: An in-depth study of ageing processes involving gallic acid. Molecules 2022, 27, 8603. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.; Lobo, L.A.; Domingues, R.M.C.P.; Monteiro, M.; Perrone, D. Bioaccessibility and gut metabolism of free and melanoidin-bound phenolic compounds from coffee and bread. Front. Nutr. 2021, 8, 708928. [Google Scholar] [CrossRef]
- Choi, E.H.; Rha, C.S.; Balusamy, S.R.; Kim, D.O.; Shim, S.M. Impact of bioconversion of gallated catechins and flavonol glycosides on bioaccessibility and intestinal cellular uptake of catechins. J. Agric. Food Chem. 2019, 67, 2331–2339. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioaccessibility, bioactivity and cell metabolism of dark chocolate phenolic compounds after in vitro gastro-intestinal digestion. J. Funct. Foods 2018, 49, 424–436. [Google Scholar] [CrossRef]


| Carob Syrup | |||||
|---|---|---|---|---|---|
| Compound | C | L | S1 | S2 | S3 |
| Hydroxybenzoic acids | |||||
| Hydroxybenzoic acid isomer 1 | 0.07 ± 0.02 | 0.13 ± 0.01 | 0.30 ± 0.18 | 0.25 ± 0.07 | 0.10 ± 0.01 |
| Hydroxybenzoic acid isomer 2 | 0.30 ± 0.02 | 0.47 ± 0.03 | 0.48 ± 0.09 | 0.43 ± 0.05 | 0.35 ± 0.01 |
| Hydroxybenzoic acid isomer3 | 0.33 ± 0.01 | 0.19 ± 0.00 | 0.12 ± 0.02 | 0.09 ± 0.01 | 0.14 ± 0.00 |
| Dihydroxybenzoic acid isomer | n.d. | 0.31 ± 0.02 | 0.65 ± 0.27 | 0.58 ± 0.17 | 0.24 ± 0.10 |
| Protocatechuic acid | 0.15 ± 0.01 | 0.20 ± 0.01 | 0.34 ± 0.07 | 0.29 ± 0.05 | 0.22 ± 0.06 |
| Gentisic acid | 0.21 ± 0.01 | 0.51 ± 0.01 | 0.17 ± 0.03 | 0.09 ± 0.04 | 0.14 ± 0.02 |
| Gallic acid | 54.28 ± 5.97 | 85.03 ± 1.24 | 117.73 ± 18.57 | 87.93 ± 9.19 | 104.79 ± 1.73 |
| Malonyl-gallic acid | 0.16 ± 0.02 | 0.26 ± 0.01 | 0.41 ± 0.33 | 0.55 ± 0.31 | 1.38 ± 0.52 |
| Hydroxybenzoic acid-O-hexoside isomer 1 | n.d. | n.d. | 0.19 ± 0.03 | 0.17 ± 0.02 | 0.16 ± 0.01 |
| Hydroxybenzoic acid-O-hexoside isomer 2 | n.d. | 0.05 ± 0.00 | 0.14 ± 0.01 | 0.10 ± 0.03 | 0.10 ± 0.03 |
| Dihydroxybenzoic acid-O-hexoside isomer | 0.27 ± 0.03 | 0.27 ± 0.02 | n.d. | n.d. | n.d. |
| Hydroxy-methoxybenzoic acid-O-hexoside isomer | 5.02 ± 0.50 | 8.66 ± 0.35 | 16.56 ± 2.20 | 9.61 ± 0.25 | 5.88 ± 0.14 |
| Gallic acid-O-hexoside isomer 1 | 2.29 ± 0.08 | 2.21 ± 0.09 | 3.32 ± 0.51 | 8.53 ± 0.05 | 12.21 ± 2.08 |
| Gallic acid-O-hexoside isomer 2 | 1.90 ± 0.21 | 1.97 ± 0.07 | 2.91 ± 0.30 | 5.30 ± 1.06 | 9.74 ± 1.01 |
| Gallic acid-O-hexoside 3 isomer | 4.53 ± 0.25 | 6.02 ± 0.16 | 9.84 ± 0.21 | 13.74 ± 0.52 | 12.55 ± 0.46 |
| Gallic acid-O-hexoside isomer 4 | 4.56 ± 0.63 | 6.27 ± 0.14 | 9.95 ± 0.05 | 15.51 ± 0.14 | 10.40 ± 1.07 |
| Gallic acid-O-hexoside isomer 5 | 0.71 ± 0.08 | 0.39 ± 0.00 | 2.06 ± 0.64 | 2.59 ± 0.11 | 25.78 ± 1.94 |
| Gallic acid-O-glucuronide isomer 1 | 0.67 ± 0.08 | 0.87 ± 0.12 | n.d. | 1.45 ± 0.14 | 1.64 ± 0.06 |
| Gallic acid-O-glucuronide isomer 2 | 0.42 ± 0.08 | 0.45 ± 0.01 | 0.65 ± 0.23 | 0.77 ± 0.07 | 0.86 ± 0.05 |
| Gallic acid-O-glucuronide isomer 3 | 0.50 ± 0.14 | 0.58 ± 0.03 | 0.80 ± 0.27 | 0.66 ± 0.58 | 0.95 ± 0.14 |
| Gallic acid-O-glucuronide isomer 4 | n.d. | 0.5 ± 0.02 | 0.74 ± 0.02 | 0.72 ± 0.03 | 0.77 ± 0.03 |
| Syringic acid-O-hexoside isomer | 1.43 ± 0.19 | 2.06 ± 0.13 | 3.23 ± 0.55 | 2.93 ± 0.04 | 2.84 ± 0.03 |
| Vanillic acid-O-hexoside-pentoside isomer 1 | 6.87 ± 0.20 | 7.45 ± 0.39 | 13.11 ± 2.22 | 20.09 ± 0.41 | 25.08 ± 1.50 |
| Vanillic acid-O-hexoside-pentoside isomer 2 | n.d. | 1.30 ± 0.05 | n.d. | n.d. | n.d. |
| Gallic acid-O-hexoside-O-hexoside isomer 1 | 12.77 ± 1.38 | 7.47 ± 0.29 | 8.98 ± 0.71 | 22.05 ± 1.66 | 31.38 ± 1.57 |
| Gallic acid-O-hexoside-O-hexoside isomer 2 | 8.58 ± 0.96 | 3.45 ± 0.23 | 6.10 ± 0.27 | 15.49 ± 0.43 | 24.17 ± 1.60 |
| Gallic acid-O-hexoside-O-hexoside-O-pentoside | 0.29 ± 0.11 | 0.21 ± 0.01 | 0.61 ± 0.15 | 0.87 ± 0.09 | 1.50 ± 0.62 |
| Total hydroxybenzoic acids | 106.30 ± 10.99 | 137.37 ± 3.44 | 199.37 ± 27.94 | 210.79 ± 15.53 | 497.76 ± 28.35 |
| Hydroxycinnamic acids | |||||
| Hydroxycinnamic acid isomer 1 | n.d. | n.d. | 0.17 ± 0.07 | 0.18 ± 0.06 | 0.10 ± 0.02 |
| Hydroxycinnamic acid isomer 2 | n.d. | n.d. | 0.16 ± 0.08 | 0.14 ± 0.07 | 0.06 ± 0.02 |
| p-Coumaric acid | 0.27 ± 0.01 | 0.83 ± 0.04 | 1.03 ± 0.41 | 1.07 ± 0.41 | 0.86 ± 0.53 |
| Ferulic acid | n.d. | 0.14 ± 0.01 | 0.13 ± 0.10 | 0.29 ± 0.15 | 0.17 ± 0.07 |
| Caffeoyl-hexose isomer 1 | n.d. | 0.06 ± 0.00 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.02 |
| Caffeoyl-hexose isomer 2 | n.d. | 0.14 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 |
| Caffeoyl-hexose isomer 3 | n.d. | 0.18 ± 0.00 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 |
| Ferulic acid-O-hexoside isomer 1 | n.d. | 0.22 ± 0.01 | 0.39 ± 0.06 | 0.43 ± 0.05 | 0.29 ± 0.08 |
| Ferulic acid-O-hexoside isomer 2 | n.d. | 0.18 ± 0.02 | 0.24 ± 0.07 | 0.14± 0.01 | 0.11 ± 0.04 |
| Ferulic acid-O-hexoside isomer 3 | n.d. | 0.60 ± 0.04 | 0.23 ± 0.04 | 0.18± 0.02 | 0.18 ± 0.01 |
| Ferulic acid-O-hexoside isomer 4 | n.d. | 0.52 ± 0.06 | 0.20 ± 0.03 | 0.16 ± 0.02 | 0.16 ± 0.01 |
| Dimethoxy-hydroxycinnamic acid-O-hexoside isomer | n.d. | 0.24 ± 0.05 | 0.68 ± 0.13 | 0.93 ± 0.12 | 0.78 ± 0.04 |
| Coumaric acid-O-hexoside-pentoside | n.d. | n.d. | 0.60 ± 0.10 | n.d. | n.d. |
| Total hydroxycinnamic acids | 0.27 ± 0.01 | 3.11 ± 0.24 | 4.03 ± 1.11 | 11.87 ± 1.75 | 2.83 ± 0.84 |
| Flavanols | |||||
| Epicatechin | n.d. | n.d. | n.d. | 0.04 ± 0.00 | 0.44 ± 0.04 |
| Catechin | n.d. | n.d. | 0.04 ± 0.06 | 0.01 ± 0.00 | 0.09 ± 0.02 |
| Epigallocatechin | n.d. | n.d. | 0.25 ± 0.44 | 0.04 ± 0.00 | 0.66 ± 0.09 |
| Gallocatechin | n.d. | n.d. | 0.10 ± 0.11 | 0.03 ± 0.03 | 0.18 ± 0.05 |
| Epicatechin-3-O-gallate | n.d. | n.d. | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.18 ± 0.02 |
| Epigallocatechin-3-O-gallate | n.d. | 0.00 ± 0.01 | 0.04 ± 0.06 | 0.02 ± 0.00 | 0.11 ± 0.01 |
| Epigallocatechin gallate isomer | n.d. | n.d. | n.d. | 0.02 ± 0.00 | 0.23 ± 0.01 |
| Procyanidin-type B dimer isomer | n.d. | n.d. | 0.16 ± 0.27 | n.d. | 0.50 ± 0.02 |
| Total flavanols | n.d. | 0.01 ± 0.01 | 0.61 ± 0.94 | 0.19 ± 0.05 | 2.38 ± 0.25 |
| Flavanones | |||||
| Naringenin isomer | n.d. | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.01 |
| Naringenin | n.d. | n.d. | 0.03 ± 0.04 | 0.04 ± 0.1 | 0.09 ± 0.2 |
| Tetra-hydroxyflavanone isomer | n.d. | n.d. | 0.02 ± 0.03 | 0.02 ± 0.00 | 0.07 ± 0.01 |
| Naringenin-O-hexoside isomer 1 | n.d. | n.d. | 0.08 ± 0.07 | 0.06 ± 0.02 | 0.16 ± 0.01 |
| Naringenin-O-hexoside isomer 2 | n.d. | n.d. | 0.11 ± 0.10 | 0.08 ± 0.01 | 0.19 ± 0.02 |
| Naringenin-O-hexoside isomer 3 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.32 ± 0.26 | 0.27 ± 0.02 | 0.69 ± 0.08 |
| Tetra-hydroxyflavanone-O-hexoside isomer 1 | n.d. | 0.06 ± 0.01 | 0.17 ± 0.05 | 0.30 ± 0.01 | 0.58 ± 0.04 |
| Tetra-hydroxyflavanone-O-hexoside isomer 2 | n.d. | n.d. | 0.09 ± 0.05 | 0.06 ± 0.01 | 0.19 ± 0.02 |
| Tetra-hydroxyflavanone-O-hexoside isomer 3 | n.d. | 0.01 ± 0.00 | 0.06 ± 0.08 | 0.04 ± 0.01 | 0.19 ± 0.02 |
| Total flavanones | 0.02 ± 0.00 | 0.09 ± 0.01 | 0.90 ± 0.68 | 0.89 ± 0.07 | 2.20 ± 0.23 |
| Flavones | |||||
| Luteolin | n.d. | n.d. | 0.12 ± 0.17 | 0.18 ± 0.02 | 0.35 ± 0.03 |
| Tri-hydroxy-methoxyflavone isomer | n.d. | n.d. | 0.01 ± 0.00 | 0.11 ± 0.01 | 0.09 ± 0.01 |
| Apigenin-7-O-glucoside | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.26 ± 0.29 | 0.22 ± 0.2 | 0.66 ± 0.7 |
| Luteolin-O-rhamnoside | n.d. | 0.01 ± 0.00 | 0.38 ± 0.30 | 0.44 ± 0.05 | 0.57 ± 0.42 |
| Luteolin-7-O-glucoside | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.25 ± 0.27 | 0.25 ± 0.00 | 0.58 ± 0.02 |
| Total flavones | 0.02 ± 0.00 | 0.06 ± 0.00 | 1.03 ± 1.03 | 5.51 ± 0.58 | 7.69 ± 0.75 |
| Flavonols | |||||
| Quercetin | n.d. | n.d. | 0.19 ± 0.13 | 0.25 ± 0.01 | 0.37 ± 0.03 |
| Methyl-quercetin isomer | n.d. | n.d. | 0.03 ± 0.01 | 0.13 ± 0.02 | 0.06 ± 0.01 |
| Isorhamnetin | n.d. | n.d. | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Myricetin | n.d. | n.d. | 0.06 ± 0.06 | 0.10 ± 0.00 | 0.13 ± 0.01 |
| Quercetin-3-O-pentoside | n.d. | n.d. | 0.17 ± 0.17 | 0.30 ± 0.03 | 0.40 ± 0.03 |
| Quercetin-3-O-pentoside isomer 1 | n.d. | n.d. | 0.24 ± 0.29 | 0.40 ± 0.08 | 0.59 ± 0.03 |
| Quercetin-3-O-pentoside isomer 2 | n.d. | n.d. | 0.22 ± 0.25 | 0.43 ± 0.04 | 0.64 ± 0.12 |
| Quercetin-3-O-rhamnoside | 0.01 ± 0.00 | 0.06 ± 0.05 | 4.14 ± 0.39 | 12.62 ± 0.65 | 19.12 ± 1.16 |
| Myricetin-O-rhamnoside | 0.03 ± 0.04 | 0.10 ± 0.02 | 0.95 ± 0.08 | 3.99 ± 0.33 | 3.18 ± 0.21 |
| Quercetin-3-O-glucoside | 0.02 ± 0.00 | n.d. | 0.79 ± 0.82 | 0.99 ± 0.08 | 1.96 ± 0.21 |
| Quercetin glucoside isomer | n.d. | n.d. | 0.06 ± 0.06 | 0.04 ± 0.03 | 0.13 ± 0.01 |
| Total flavonols | 0.06 ± 0.04 | 0.17 ± 0.06 | 6.87 ± 2.29 | 19.27 ± 1.28 | 26.62 ± 1.82 |
| Others | |||||
| Dihydroxyphenylacetic acid isomer | n.d. | 2.76 ± 0.36 | 2.54 ± 0.88 | 2.19 ± 0.99 | 1.52 ± 0.19 |
| Ellagic acid | 3.46 ± 0.81 | 2.64 ± 0.10 | 4.33 ± 0.24 | 27.56 ± 0.35 | 12.31 ± 0.03 |
| Total others | 3.46 ± 0.81 | 5.40 ± 0.47 | 6.87 ± 1.12 | 29.75 ± 1.34 | 13.83 ± 0.22 |
| Total phenolic by MS | 110.13 ± 11.87 | 146.21 ± 4.24 | 219.68 ± 35.11 | 265.73 ± 19.31 | 323.46 ± 18.77 |
| Compound | Carob Syrup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | L | S1 | S2 | S3 | ||||||
| After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | |
| Hydroxybenzoic acids | ||||||||||
| Hydroxybenzoic acid isomer 1 | 0.09 ± 0.00 | 118.1 | 0.12 ± 0.00 | 86.3 | 0.14 ± 0.01 | 48.1 | 0.06 ± 0.01 | 24.6 | 0.07 ± 0.00 | 75.1 |
| Hydroxybenzoic acid isomer 2 | 0.31 ± 0.02 | 105.3 | 0.43 ± 0.01 | 91.1 | 0.57 ± 0.01 | 118.7 | 0.50 ± 0.01 | 115.4 | 0.49 ± 0.00 | 142.0 |
| Hydroxybenzoic acid isomer 3 | 0.44 ± 0.01 | 131.1 | 0.28 ± 0.00 | 147.4 | 0.14 ± 0.00 | 121.0 | 0.12 ± 0.01 | 127.4 | 0.19 ± 0.01 | 135.3 |
| Dihydroxybenzoic acid isomer | 0.04 ± 0.00 | n.f. | 0.13 ± 0.01 | 40.7 | 0.09 ± 0.01 | 13.3 | 0.07 ± 0.01 | 12.3 | 0.07 ± 0.01 | 29.9 |
| Protocatechuic acid | 0.16 ± 0.01 | 106.9 | 0.20 ± 0.00 | 97.9 | 1.40 ± 0.09 | 416.1 | 0.45 ± 0.00 | 157.3 | 0.68 ± 0.02 | 307.1 |
| Gentisic acid | 0.22 ± 0.01 | 105.0 | 0.47 ± 0.01 | 92.7 | 0.17 ± 0.01 | 98.9 | 0.10 ± 0.00 | 108.4 | 0.15 ± 0.02 | 112.3 |
| Gallic acid | 19.32 ± 0.62 | 35.6 | 18.23 ± 0.76 | 21.4 | 0.61 ± 0.03 | 0.5 | 19.70 ± 0.54 | 22.4 | 0.98 ± 0.03 | 0.9 |
| Malonyl-gallic acid | 0.15 ± 0.00 | 95.7 | 0.28 ± 0.12 | 106.9 | 0.08 ± 0.00 | 20.6 | 0.23 ± 0.01 | 42.6 | 0.12 ± 0.00 | 8.5 |
| Hydroxybenzoic acid-O-hexoside isomer 1 | n.d. | n.d. | n.d. | n.d. | 0.28 ± 0.01 | 145.6 | 0.24 ± 0.00 | 146.1 | 0.30 ± 0.01 | 191.6 |
| Hydroxybenzoic acid-O-hexoside isomer 2 | n.d. | n.d. | 0.14 ± 0.02 | 275.3 | 0.16 ± 0.01 | 116.2 | 0.14 ± 0.01 | 134.7 | 0.15 ± 0.00 | 153.7 |
| Dihydroxybenzoic acid-O-hexoside isomer | 0.40 ± 0.01 | 147.9 | 0.28 ± 0.01 | 102.2 | n.d. | n.d. | n.d. | n.d. | 0.44 ± 0.02 | n.f. |
| Hydroxy-methoxybenzoic acid-O-hexoside isomer | 4.90 ± 0.08 | 97.6 | 7.40 ± 0.46 | 85.4 | 16.19 ± 0.78 | 97.8 | 8.22 ± 0.20 | 85.5 | 6.68 ± 0.07 | 113.7 |
| Gallic acid-O-hexoside isomer 1 | 1.59 ± 0.03 | 69.2 | 1.07 ± 0.02 | 48.6 | 0.05 ± 0.01 | 1.5 | 4.47 ± 0.03 | 52.4 | 1.28 ± 0.06 | 10.5 |
| Gallic acid-O-hexoside isomer 2 | 1.13 ± 0.03 | 59.3 | 0.82 ±0.08 | 41.6 | 0.04 ± 0.00 | 1.2 | 3.35 ± 0.12 | 63.2 | 0.87 ± 0.08 | 8.9 |
| Gallic acid-O-hexoside isomer 3 | 3.40 ± 0.01 | 75.0 | 3.00 ±0.06 | 49.9 | 1.48 ± 0.07 | 15.1 | 6.42 ± 0.11 | 46.7 | 4.93 ± 0.03 | 39.2 |
| Gallic acid-O-hexoside isomer 4 | 2.70 ± 0.09 | 59.3 | 2.52 ±0.11 | 40.2 | 0.07 ± 0.01 | 0.7 | 5.93 ± 0.25 | 38.2 | 0.60 ± 0.03 | 5.8 |
| Gallic acid-O-hexoside isomer 5 | 0.57 ± 0.01 | 80.0 | 0.31 ±0.03 | 78.9 | 1.33 ± 0.06 | 64.4 | 2.22 ± 0.06 | 85.7 | 2.35 ± 0.11 | 9.1 |
| Gallic acid-O-glucuronide isomer 1 | 0.45 ± 0.02 | 67.2 | 0.48 ± 0.02 | 54.7 | 0.02 ± 0.00 | n.f. | 0.71 ± 0.02 | 49.0 | 0.11 ± 0.01 | 6.8 |
| Gallic acid-O-glucuronide isomer 2 | 0.26 ± 0.02 | 62.7 | 0.23 ± 0.01 | 50.8 | 0.02 ± 0.00 | 3.6 | 0.34 ± 0.02 | 44.8 | 0.06 ± 0.01 | 7.2 |
| Gallic acid-O-glucuronide isomer 3 | 0.34 ± 0.02 | 67.4 | 0.28 ± 0.02 | 47.5 | 0.02 ± 0.00 | 2.0 | 0.36 ± 0.01 | 54.5 | 0.08 ± 0.00 | 8.4 |
| Gallic acid-O-glucuronide isomer 4 | 0.23 ± 0.01 | n.f. | 0.25 ± 0.01 | 42.4 | 0.02 ± 0.00 | 2.9 | 0.23 ± 0.01 | 31.9 | 0.06 ± 0.00 | 7.8 |
| Syringic acid-O-hexoside isomer | 1.65 ± 0.03 | 115.7 | 1.99 ± 0.09 | 96.6 | 3.20 ± 0.26 | 99.1 | 2.58 ± 0.03 | 88.1 | 2.88 ± 0.10 | 101.5 |
| Vanillic acid-O-hexoside-pentoside isomer 1 | 5.65 ± 0.17 | 82.2 | 6.31 ± 0.31 | 84.7 | 11.17 ± 0.81 | 85.2 | 15.25 ± 0.16 | 75.9 | 18.50 ± 0.45 | 73.78 |
| Vanillic acid-O-hexoside-pentoside isomer 2 | 0.89 ± 0.03 | n.f. | 1.05 ± 0.08 | 80.4 | 0.91 ± 0.01 | n.f. | 2.34 ± 0.08 | n.f. | 3.18 ± 0.09 | n.f. |
| Gallic acid-O-hexoside-O-hexoside isomer 1 | 8.11 ± 0.56 | 63.5 | 3.52 ± 0.10 | 47.1 | 0.06 ± 0.00 | 0.7 | 8.73 ± 0.02 | 39.6 | 1.16 ± 0.11 | 3.7 |
| Gallic acid-O-hexoside-O-hexoside isomer 2 | 4.86 ± 0.27 | 56.7 | 1.88 ± 0.02 | 54.6 | 0.08 ± 0.00 | 1.3 | 6.17 ± 0.02 | 39.8 | 1.48 ± 0.08 | 6.1 |
| Gallic acid-O-hexoside-O-hexoside-O-pentoside | 0.21 ± 0.01 | 72.7 | 0.12 ± 0.01 | 59.4 | n.d. | 0.8 | 0.37 ± 0.00 | 42.1 | 0.12 ± 0.00 | 7.8 |
| Total hydroxybenzoic acids | 58.06 ± 2.05 | 54.6 | 51.78 ± 2.38 | 37.7 | 38.30 ± 2.18 | 19.2 | 89.30 ± 1.74 | 42.4 | 47.99 ± 1.35 | 17.6 |
| Hydroxycinnamic acids | ||||||||||
| Hydroxycinnamic acid isomer 1 | 0.05 ± 0.00 | n.f. | 0.06 ± 0.00 | n.f. | 0.26 ± 0.02 | 154.1 | 0.20 ± 0.00 | 109.1 | 0.10 ± 0.00 | 106.6 |
| Hydroxycinnamic acid isomer 2 | 0.04 ± 0.00 | n.f. | 0.07 ± 0.00 | n.f. | 0.23 ± 0.00 | 137.6 | 0.11 ± 0.00 | 81.6 | 0.07 ± 0.00 | 116.2 |
| p-Coumaric acid | 0.56 ± 0.05 | 205.2 | 1.20 ± 0.05 | 143.2 | 1.75 ± 0.11 | 169.0 | 2.34 ± 0.04 | 218.8 | 1.27 ± 0.04 | 146.6 |
| Ferulic acid | 0.09 ± 0.00 | n.f. | 0.28 ± 0.12 | 200.3 | 0.08 ± 0.00 | 62.3 | 0.23 ± 0.01 | 82.2 | 0.12 ± 0.00 | 68.2 |
| Caffeoyl-hexose isomer 1 | n.d. | n.d. | 0.05 ± 0.00 | 77.6 | 0.07 ± 0.00 | 90.5 | 0.08 ± 0.00 | 89.4 | 0.07 ± 0.00 | 87.9 |
| Caffeoyl-hexose isomer 2 | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 | 18.1 | n.d. | 16.4 | n.d. | 7.3 |
| Caffeoyl-hexose isomer 3 | n.d. | n.d. | n.d. | n.d. | 0.03 ± 0.00 | 51.5 | 0.02 ± 0.00 | 68.8 | 0.02 ± 0.00 | 80.5 |
| Ferulic acid-O-hexoside isomer 1 | n.d. | n.d. | 0.27 ± 0.00 | 123.9 | 0.38 ± 0.04 | 97.7 | 0.46 ± 0.01 | 107.7 | 0.25 ± 0.01 | 88.7 |
| Ferulic acid-O-hexoside isomer 2 | n.d. | n.d. | 0.19 ± 0.00 | 105.1 | 0.27 ± 0.01 | 110.5 | 0.17 ± 0.00 | 119.6 | 0.11 ± 0.00 | 99.0 |
| Ferulic acid-O-hexoside isomer 3 | n.d. | n.d. | 0.16 ± 0.01 | 27.1 | 0.02 ± 0.00 | 9.4 | 0.07 ± 0.01 | 40.9 | 0.04 ± 0.00 | 22.8 |
| Ferulic acid-O-hexoside isomer 4 | n.d. | n.d. | 0.16 ± 0.02 | 30.2 | 0.02 ± 0.00 | 8.6 | 0.06 ± 0.00 | 38.3 | 0.04 ± 0.00 | 23.1 |
| Dimethoxy-hydroxycinnamic acid-O-hexoside isomer | n.d. | n.d. | 2.26 ± 0.02 | 957.2 | 0.42 ± 0.01 | 62.2 | 0.89 ± 0.01 | 96.2 | 0.78 ± 0.00 | 99.6 |
| Coumaric acid-O-hexoside-pentoside | 0.19 ± 0.01 | n.f. | 0.16 ± 0.01 | n.f. | 0.57 ± 0.03 | 94.7 | 0.35 ± 0.01 | n.f. | 0.28 ± 0.01 | n.f. |
| Total hydroxycinnamic acids | 0.92 ± 0.06 | 338.3 | 4.84 ± 0.23 | 155.7 | 4.11 ± 0.22 | 101.8 | 5.00 ± 0.10 | 136.7 | 3.15 ± 0.07 | 111.4 |
| Flavanols | ||||||||||
| Epicatechin | n.d. | n.d. | n.d. | n.d. | 0.02 ± 0.01 | n.f. | 0.01 ± 0.00 | 14.8 | 0.01 ± 0.00 | 2.9 |
| Catechin | n.d. | n.d. | n.d. | n.d. | 0.02 ± 0.01 | 68.2 | n.d. | 28.1 | 0.03 ± 0.00 | 36.4 |
| Epigallocatechin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Gallocatechin | n.d. | n.d. | n.d. | n.d. | 0.03 ± 0.00 | 32.0 | n.d. | n.d. | n.d. | n.d. |
| Epicatechin-3-O-gallate | n.d. | n.d. | n.d. | n.d. | n.d. | 14.0 | n.d. | 7.6 | n.d. | 0.9 |
| Epigallocatechin-3-O-gallate | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Epigallocatechin gallate isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Procyanidin-type B dimer isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total flavanols | n.d. | n.d. | n.d. | n.d. | 0.07 ± 0.02 | 12.2 | 0.01 ± 0.00 | 5.1 | 0.05 ± 0.00 | 1.9 |
| Flavanones | ||||||||||
| Naringenin isomer | n.d. | 79.4 | n.d. | 3.4 | 0.01 ± 0.00 | 52.8 | 0.02 ± 0.00 | 95.1 | 0.04 ± 0.00 | 81.7 |
| Naringenin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 | 23.9 | 0.02 ± 0.00 | 24.2 |
| Tetra-hydroxyflavanone isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 | 71.0 | 0.02 ± 0.00 | 27.4 |
| Naringenin-O-hexoside isomer 1 | n.d. | n.d. | 0.01 ± 0.00 | n.f. | n.d. | 5.8 | 0.04 ± 0.00 | 69.8 | 0.07 ± 0.01 | 46.1 |
| Naringenin-O-hexoside isomer 2 | n.d. | n.d. | 0.01 ± 0.00 | n.d. | 0.01 ± 0.00 | 8.0 | 0.07 ± 0.00 | 76.9 | 0.11 ± 0.00 | 60.5 |
| Naringenin-O-hexoside isomer 3 | 0.01 ± 0.00 | 74.6 | 0.01 ± 0.00 | 117.1 | 0.11 ± 0.00 | 34.4 | 0.23 ± 0.00 | 82.7 | 0.42 ± 0.01 | 60.9 |
| Tetra-hydroxyflavanone-O-hexoside isomer 1 | n.d. | n.d. | 0.04 ± 0.01 | 69.1 | 0.19 ± 0.00 | 110.9 | 0.30 ± 0.00 | 102.1 | 0.52 ± 0.01 | 89.7 |
| Tetra-hydroxyflavanone-O-hexoside isomer 2 | 0.01 ± 0.00 | n.d. | n.d. | n.d. | 0.02 ± 0.00 | 24.4 | 0.05 ± 0.00 | 82.4 | 0.06 ± 0.00 | 32.8 |
| Tetra-hydroxyflavanone-O-hexoside isomer 3 | n.d. | n.d. | n.d. | 67.4 | 0.00 ± 0.00 | 1.5 | 0.01 ± 0.00 | 33.4 | 0.02 ± 0.00 | 13.3 |
| Total flavanones | 0.03 ± 0.00 | 159.6 | 0.08 ± 0.01 | 90.3 | 0.35 ± 0.00 | 38.7 | 0.74 ± 0.00 | 83.1 | 1.29 ± 0.03 | 58.9 |
| Flavones | ||||||||||
| Luteolin | n.d. | 36.6 | n.d. | 53.8 | n.d. | 37.3 | 0.15 ± 0.00 | 85.7 | 0.15 ± 0.00 | 44.6 |
| Tri-hydroxy-methoxyflavone isomer | n.d. | n.d. | n.d. | n.d. | n.d. | 18.7 | 0.07 ± 0.00 | 65.87 | 0.06 ± 0.00 | 66.74 |
| Apigenin-7-O-glucoside | 0.01 ± 0.00 | 135.9 | 0.03 ± 0.00 | 108.1 | 0.10 ± 0.01 | 33.6 | 0.24 ± 0.00 | 108.1 | 0.62 ± 0.01 | 94.2 |
| Luteolin-O-rhamnoside | n.d. | n.d. | n.d. | 43.9 | 0.03 ± 0.00 | 20.5 | 0.23 ± 0.01 | 53.8 | 0.30 ± 0.01 | 52.8 |
| Luteolin-7-O-glucoside | 0.01 ± 0.00 | 63.5 | 0.02 ± 0.00 | 78.5 | 0.08 ± 0.00 | n.d. | 0.26 ± 0.00 | 105.8 | 0.49 ± 0.01 | 83.7 |
| Total flavones | 0.01 ± 0.00 | 78.5 | 0.05 ± 0.00 | 84.6 | 0.21 ± 0.01 | 20.7 | 0.96 ± 0.02 | 80.5 | 1.62 ± 0.03 | 72.2 |
| Flavonols | ||||||||||
| Quercetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.9 | n.d. | n.d. |
| Methyl-quercetin isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.09 ± 0.00 | 72.8 | 0.02 ± 0.00 | 34.2 |
| Isorhamnetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myricetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-O-pentoside | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 | 4.2 | 0.14 ± 0.00 | 47.2 | 0.14 ± 0.00 | 35.0 |
| Quercetin-3-O-pentoside isomer 1 | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 | 2.7 | 0.20 ± 0.00 | 51.0 | 0.26 ± 0.01 | 44.0 |
| Quercetin-3-O-pentoside isomer 2 | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 | 4.8 | 0.21 ± 0.01 | 48.9 | 0.20 ± 0.01 | 31.4 |
| Quercetin-3-O-rhamnoside | 0.01 ± 0.00 | 72.8 | 0.05 ± 0.00 | 76.0 | 0.41 ± 0.02 | 9.8 | 6.93 ± 0.08 | 54.9 | 6.55 ± 0.08 | 34.2 |
| Myricetin-O-rhamnoside | n.d. | n.d. | 0.02 ± 0.00 | 21.9 | n.d. | n.d. | 0.48 ± 0.03 | 12.1 | 0.03 ± 0.00 | 1.0 |
| Quercetin-3-O-glucoside | n.d. | n.d. | n.d. | n.d. | 0.02 ± 0.00 | 2.5 | 0.43 ± 0.00 | 43.2 | 0.30 ± 0.00 | 15.2 |
| Quercetin glucoside isomer | n.d. | n.d. | n.d. | n.d. | 0.00 ± 0.00 | n.d. | 0.04 ± 0.00 | 83.6 | 0.05 ± 0.00 | 40.0 |
| Total flavonols | 0.02 ± 0.01 | 29.3 | 0.07 ± 0.01 | 42.7 | 0.46 ± 0.02 | 6.7 | 8.53 ± 0.14 | 44.3 | 7.55 ± 0.11 | 28.4 |
| Others | ||||||||||
| Dihydroxyphenylacetic acid isomer | 0.67 ± 0.00 | n.f. | 1.64 ± 0.15 | 59.6 | 6.85 ± 0.21 | 270.3 | 2.38 ± 0.03 | 109.0 | 1.96 ± 0.09 | 129.0 |
| Ellagic acid | 15.83 ± 1.98 | 457.9 | 23.18 ± 0.75 | 876.7 | 67.67 ± 1.03 | 1561.5 | 78.08 ± 2.74 | 283.3 | 236.38 ± 5.3 | 1920.4 |
| Total others | 16.49 ± 1.99 | 477.1 | 24.83 ± 0.89 | 459.5 | 74.53 ± 1.24 | 1085.0 | 80.46 ± 2.77 | 270.5 | 238.33 ± 5.4 | 1723.9 |
| Total phenolic by MS | 75.55 ± 4.11 | 68.6 | 81.70 ± 3.52 | 55.8 | 118.24 ± 3.72 | 53.8 | 185.95 ± 4.78 | 69.9 | 301.61 ± 7.07 | 93.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannini, M.; Cattivelli, A.; Nissen, L.; Conte, A.; Gianotti, A.; Tagliazucchi, D. Identification, Bioaccessibility, and Antioxidant Properties of Phenolic Compounds in Carob Syrup. Foods 2024, 13, 2196. https://doi.org/10.3390/foods13142196
Zannini M, Cattivelli A, Nissen L, Conte A, Gianotti A, Tagliazucchi D. Identification, Bioaccessibility, and Antioxidant Properties of Phenolic Compounds in Carob Syrup. Foods. 2024; 13(14):2196. https://doi.org/10.3390/foods13142196
Chicago/Turabian StyleZannini, Melissa, Alice Cattivelli, Lorenzo Nissen, Angela Conte, Andrea Gianotti, and Davide Tagliazucchi. 2024. "Identification, Bioaccessibility, and Antioxidant Properties of Phenolic Compounds in Carob Syrup" Foods 13, no. 14: 2196. https://doi.org/10.3390/foods13142196
APA StyleZannini, M., Cattivelli, A., Nissen, L., Conte, A., Gianotti, A., & Tagliazucchi, D. (2024). Identification, Bioaccessibility, and Antioxidant Properties of Phenolic Compounds in Carob Syrup. Foods, 13(14), 2196. https://doi.org/10.3390/foods13142196











