Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Hawthorn Pomace Soluble Dietary Fiber
2.3. Structural Characterization of Hawthorn Pomace Soluble Dietary Fiber
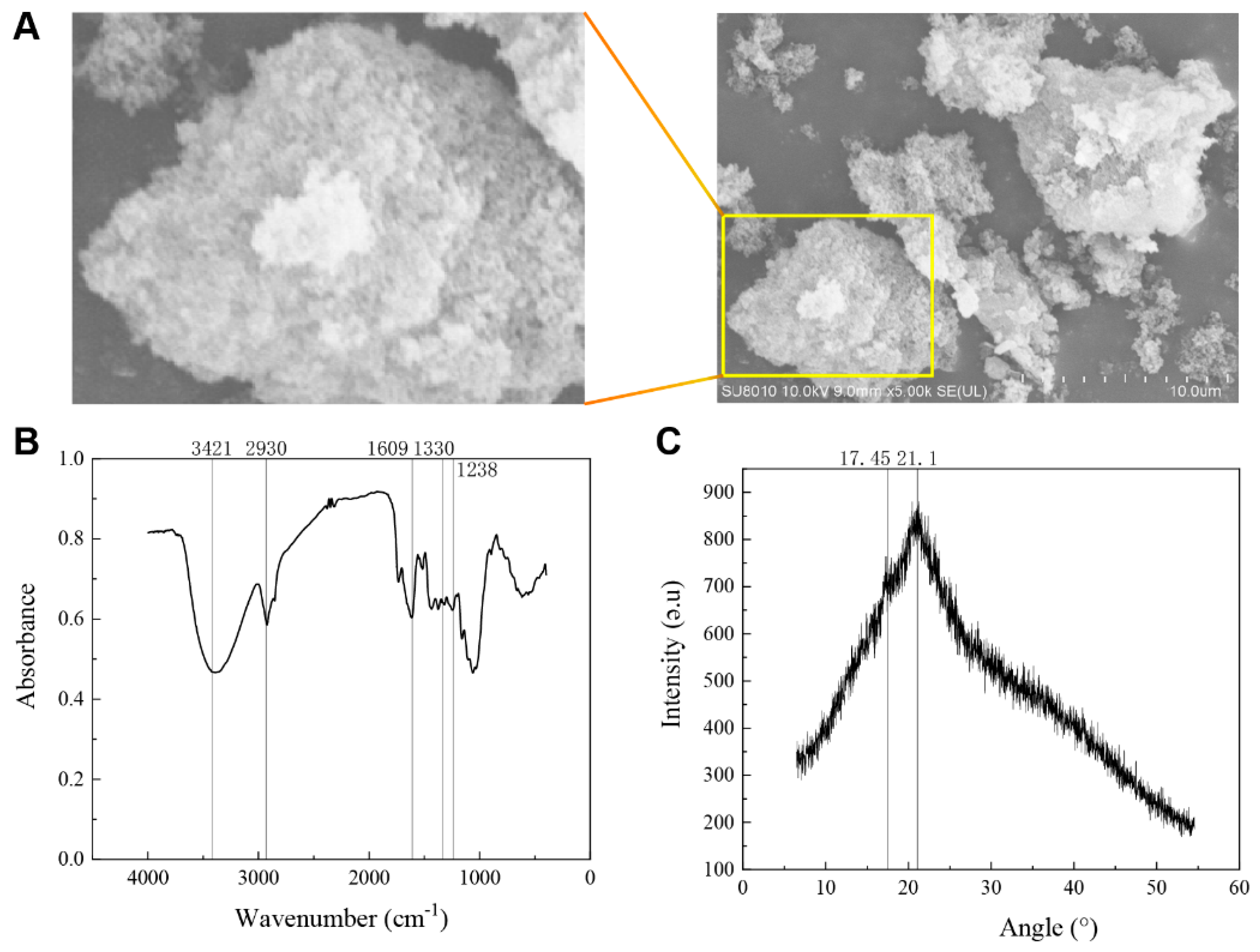
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Observation of Hawthorn Pomace Soluble Dietary Fiber
2.3.3. X-ray Diffraction Analysis
2.3.4. Determination of Hawthorn Pomace Soluble Dietary Fiber Molecular Weight
2.3.5. Composition of Monosaccharide
2.4. Animal Treatment
2.5. Evaluation of Defecation Function of Constipation Mice
2.6. Histopathology
2.7. Physiological Index Measurement
2.8. Western Blot Analysis
2.9. Determination of Short-Chain Fatty Acids in Colon Contents
2.10. High Throughput Sequencing of 16S rRNA of Mouse Intestinal Flora
2.11. Statistical Analysis
3. Results
3.1. Hawthorn Pomace Soluble Dietary Fiber Structure and Composition Analysis
3.2. Hawthorn Pomace Soluble Dietary Fiber Improves the Physiological Condition of Mice
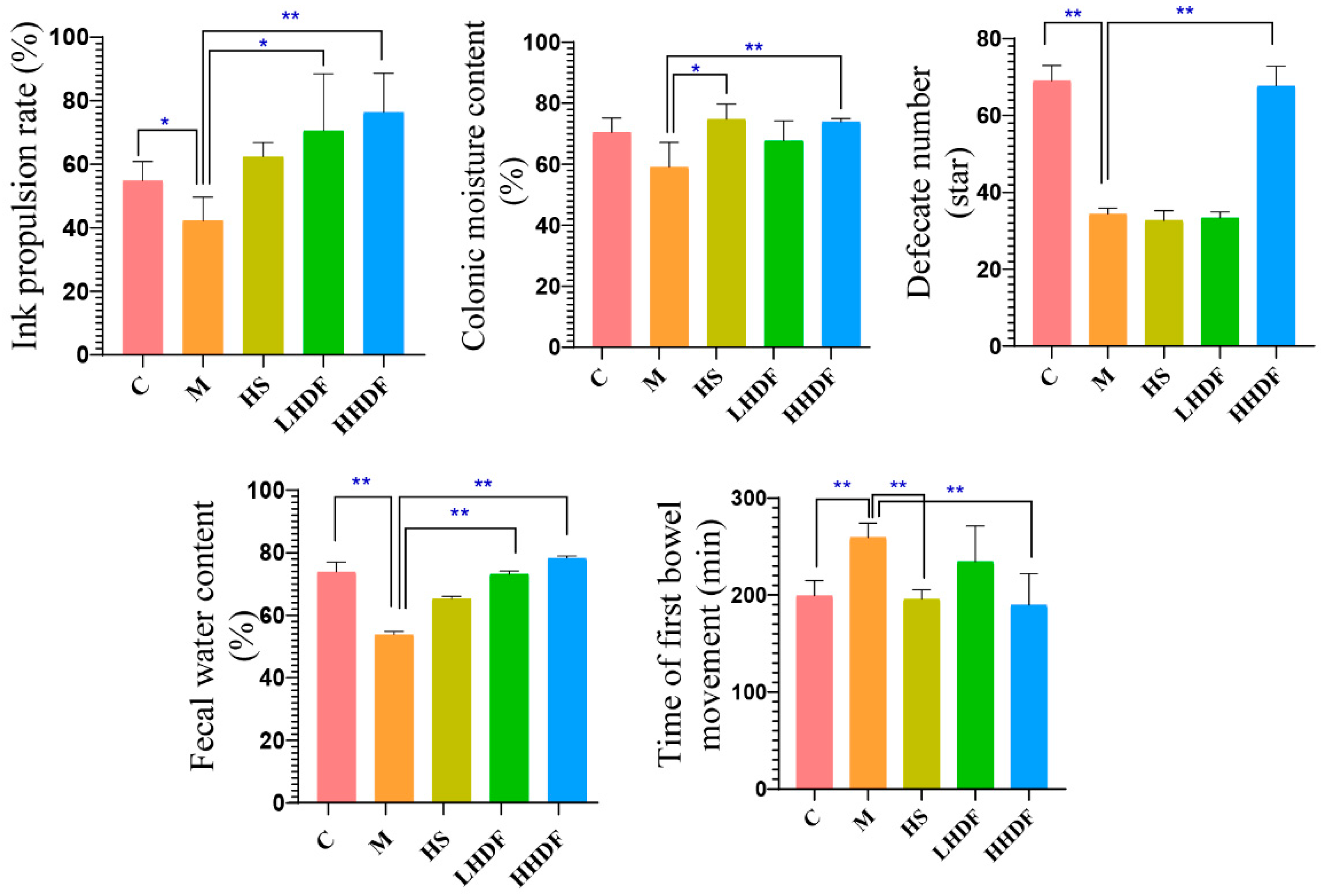
3.3. Hawthorn Pomace Soluble Dietary Fiber Improves Defecation Indexes of Mice
3.4. Hawthorn Pomace Soluble Dietary Fiber Improves Gastrointestinal Hormone Levels in Mice with Constipation
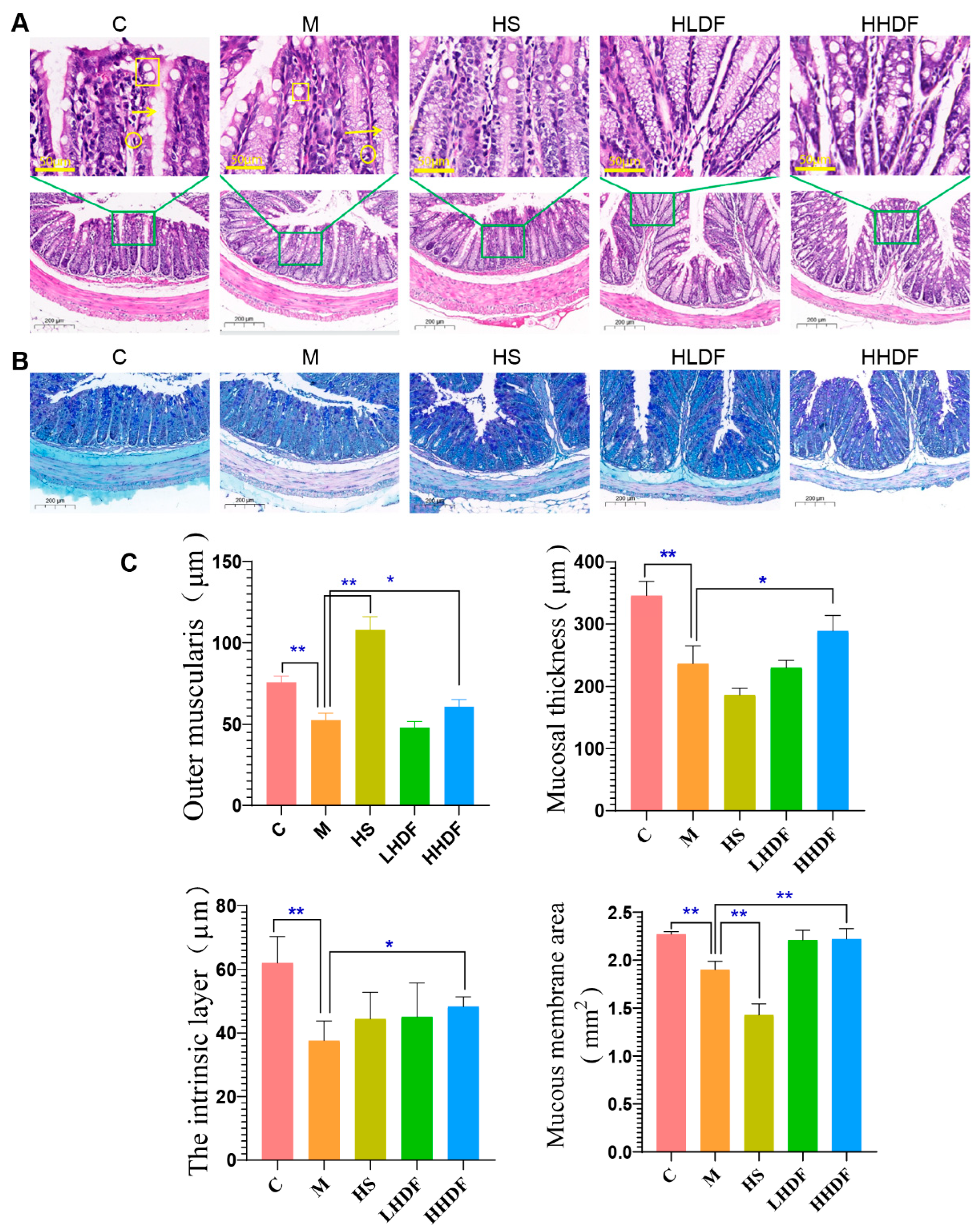
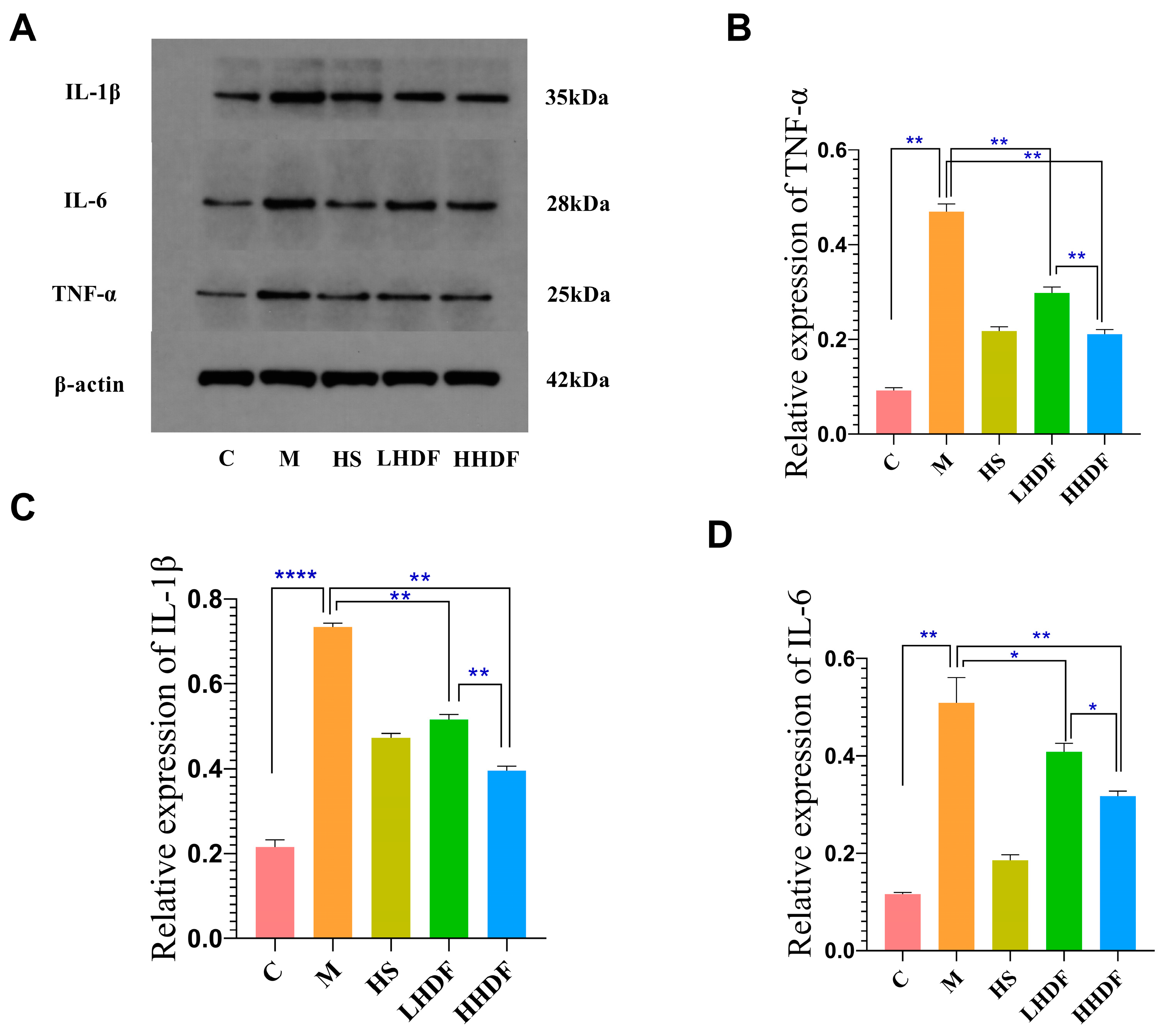
3.5. Hawthorn Pomace Soluble Dietary Fiber Reduces Inflammation of Colon Tissue
3.6. Hawthorn Pomace Soluble Dietary Fiber Regulates AQP Water Pathway Protein Expression to Relieve Constipation in Mice
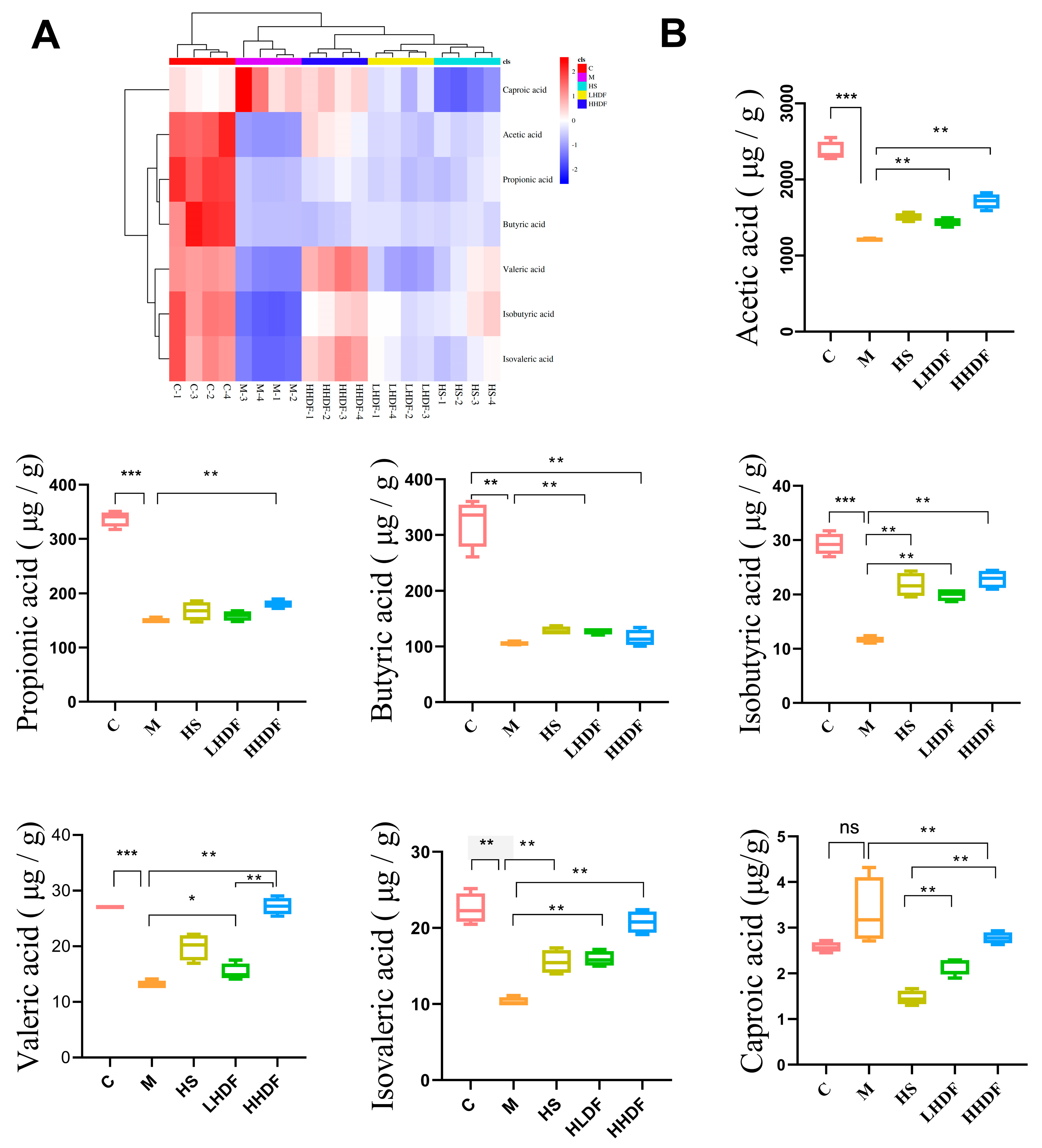
3.7. Hawthorn Pomace Soluble Dietary Fiber Improves the Composition of Short-Chain Fatty Acids in the Colon Contents of Mice with Constipation
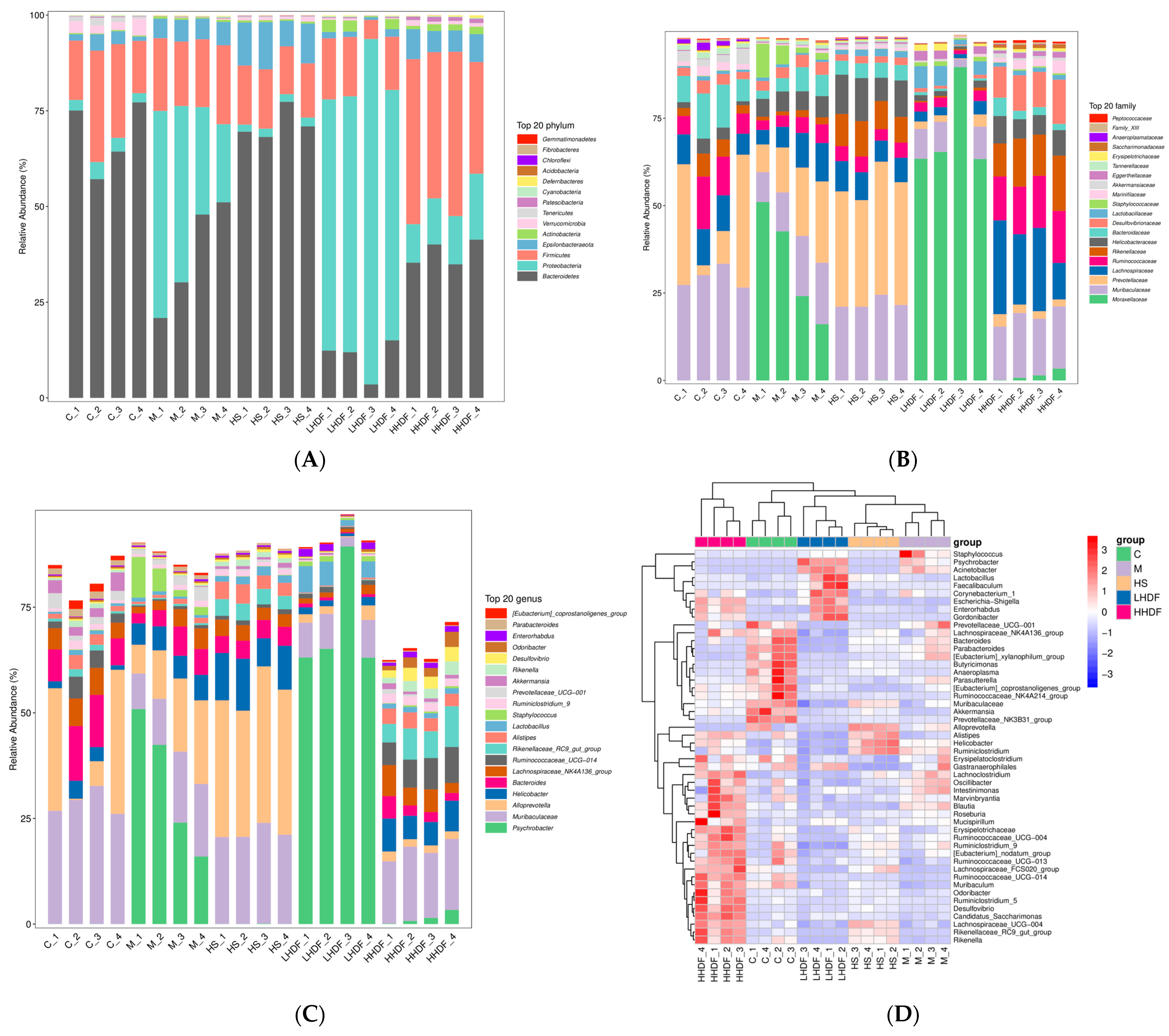
3.8. Hawthorn Pomace Soluble Dietary Fiber Improves the Intestinal Flora and Relieves Constipation in Mice
3.8.1. Expression of Alpha Diversity
3.8.2. Beta Cluster, Beta Diversity, and Linear Discriminant Analysis Effect Size Analysis
3.8.3. Analysis of Species Difference and Marker Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adil, E.B.; Brian, E.L. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology 2020, 158, 1232–1249. [Google Scholar]
- Lee, C.S.; Tan, P.L.; Eor, J.Y.; Choi, D.H.; Park, M.; Seo, S.K.; Yoon, S.; Yang, S.; Kim, S.H. Prophylactic use of probiotic chocolate modulates intestinal physiological functions in constipated rats. J. Sci. Food Agric. 2019, 99, 3045–3056. [Google Scholar] [CrossRef]
- Yang, L.; Wan, Y.; Li, W.W.; Liu, C.; Li, H.; Dong, Z.L.; Zhu, K.; Jiang, S.; Shang, E.; Qian, D.; et al. Targeting intestinal flora and its metabolism to explore the laxative effects of rhubarb. Appl. Microbiol. Biotechnol. 2022, 106, 1615–1631. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yang, H.B.; Zheng, J.P.; Jiang, N.; Sun, G.J.; Bao, X.K.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef]
- Hu, T.G.; Wen, P.; Fu, H.Z.; Lin, G.Y.; Liao, S.T.; Zou, Y.X. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.D.; Ye, S.M.; Xu, Z.M.; Su, H.H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Quigley, E.M.M. American College of gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109, S2–S26. [Google Scholar] [CrossRef] [PubMed]
- Werth, B. Epidemiology of constipation in adults: Why estimates of prevalence differ. J. Epidemiol. Res. 2019, 5, 37–49. [Google Scholar] [CrossRef][Green Version]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Tomoko, Y.; Hitoshi, T.; Teruyoshi, M.; Junji, T. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1, 1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201e04. [Google Scholar]
- Zhang, X.Y.; Tian, H.L.; Gu, L.L.; Nie, Y.Z.; Ding, C.; Ge, X.L.; Yang, B.; Gong, J.; Li, N. Long-term follow-up of the effects of fecal microbiota transplantation in combination with soluble dietary fiber as a therapeutic regimen in slow transit constipation. Sci. China Life Sci. 2018, 61, 779–786. [Google Scholar] [CrossRef]
- Li, Y.; Tong, W.D.; Qian, Y. Effect of Physical Activity on the Association Between Dietary Fiber and Constipation: Evidence from the National Health and Nutrition Examination Survey 2005–2010. Neurogastroenterol. Motil. 2021, 27, 97–107. [Google Scholar] [CrossRef]
- Yang, F.; Yang, J.; Ruan, Z.Y.; Wang, Z.M. Fermentation of dietary fibers modified by an enzymatic-ultrasonic treatment and evaluation of their impact on gut microbiota in mice. J. Food Process Preserv. 2021, 45, e15337. [Google Scholar] [CrossRef]
- Xie, J.H.; Song, Q.Q.; Yu, Q.; Chen, Y.; Hong, Y.Z.; Shen, M.Y. Dietary polysaccharide from Mung bean [Vigna radiate (Linn.) Wilczek] skin modulates gut microbiota and short-chain fatty acids in mice. Int. J. Food Sci. Technol. 2022, 57, 2581–2589. [Google Scholar] [CrossRef]
- Dutta, A.; Das, M. Deciphering the role of aquaporins in metabolic diseases: A mini review. Am. J. Med. Sci. 2022, 364, 148–162. [Google Scholar] [CrossRef]
- Deng, Z.T.; Fu, Z.T.; Yan, W.; Nie, K.; Ding, L.L.; Ma, D.; Huang, H.; Li, T.; Xie, J.; Fu, L. The different effects of Chinese Herb Solid Drink and lactulose on gut microbiota in rats with slow transit constipation induced by compound diphenoxylate. Food Res. Int. 2021, 143, 110273. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.R.; Jiang, C.X.; Liu, X.L.; Zheng, X.Q.; Wei, X.Y. Effect and mechanism of soluble dietary fiber from corn bran on loperamide-induced constipation in mice. Food Ind. Technol. 2024, 45, 1–17. [Google Scholar]
- Lv, Q.H.; Liu, L.Z.; Zhu, Z. Study on the improvement of constipation in mice by composite dietary fiber and its mechanism of action. J. Food Saf. Qual. Test. 2024, 15, 260–270. [Google Scholar]
- Li, T.; Fu, S.Y.; Huang, X.; Zhang, X.S.; Cui, Y.M.; Zhang, Z.Y.; Ma, Y.; Zhang, X.; Yu, Q.; Yang, S.; et al. Biological properties and potential application of Hawthorn and its major functional components: A review. J. Funct. Foods 2022, 90, 104988. [Google Scholar] [CrossRef]
- Sun, L.P.; Chi, B.Q.; Xia, M.F.; Ma, Z.; Zhang, H.B.; Jiang, H.Q.; Zhang, F.; Tian, Z. LC–MS-based lipidomic analysis of liver tissue sample from spontaneously hypertensive rats treated with extract Hawthorn fruits. Front. Pharmacol. 2022, 13, 963280. [Google Scholar] [CrossRef]
- Song, J.X.; Wu, Y.; Ma, X.J.; Feng, L.J.; Wang, Z.G.; Jiang, G.Q.; Tong, H. Structural characterization and α-glycosidase inhibitory activity of a novel polysaccharide fraction from Aconitum coreanum. Carbohydr. Polym. 2020, 230, 115586. [Google Scholar] [CrossRef]
- Jiang, H.M.; Dong, J.; Jiang, H.J.; Liang, Q.X.; Zhang, Y.; Liu, Z.H.; Ma, C.; Wang, J.; Kang, W. Effect of Durio zibethinus rind polysaccharide on functional constipation and intestinal microbiota in rats. Food Res. Int. 2020, 136, 109316. [Google Scholar] [CrossRef]
- Deng, M.; Lin, Y.S.; Dong, L.H.; Jia, X.C.; Shen, Y.L.; Liu, L.; Chi, J.; Huang, F.; Zhang, M.; Zhang, R. Physicochemical functional properties of dietary fiber from pummelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars. Food Biosci. 2021, 40, 100890. [Google Scholar] [CrossRef]
- Yang, C.R.; Ma, Y.; Chen, Y.; Xie, J.H.; Hu, X.B.; Yu, Q. Improving the physicochemical and in vitro hypolipidemic properties of soluble dietary fiber in camellia seed residue by a cellulose degrading fungus YC49. Food Funct. 2022, 13, 11321–11333. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Takemura, N.; Sonoyama, K.; Kawagishi, H.; Topping, D.L.; Conlon, M.A.; Morita, T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J. Agric. Food Chem. 2011, 59, 5771–5778. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Q.; Liang, Y.C.; Wang, D.L.; Yan, Z.P.; Yin, H.Z.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Rattanakovit, K.; Patcharatrakul, T. Diagnosis and management of chronic constipation in adults. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Liang, Q.G.; Zhao, Q.C.; Tang, Q.; Ahmed, A.F.; Zhang, Y.; Kang, W. The effect of microbial composition and proteomic on improvement of functional constipation by Chrysanthemum morifolium polysaccharide. Food Chem. Toxicol. 2021, 153, 112305. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Chen, J.-N.; Zhou, X.-J.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y.-L. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.). LWT 2021, 145, 111313. [Google Scholar] [CrossRef]
- Cong, L.; Duan, L.W.; Su, W.P.; Hao, S.H.; Li, D.F. Efficacy of high specific volume polysaccharide—A new type of dietary fiber–on molecular mechanism of intestinal water metabolism in rats with constipation. Med. Sci. Monit. 2019, 25, 5028–5035. [Google Scholar] [CrossRef]
- Asano, H.; Tomita, T.; Nakamura, K.; Yamasaki, T.; Okugawa, T.; Kondo, T.; Kono, T.; Tozawa, K.; Ohda, Y.; Oshima, T.; et al. Prevalence of gastric motility disorders in patients with functional dyspepsia. Neurogastroenterol. Motil. 2017, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Yik, Y.I.; Farmer, P.J.; King, S.K.; Chow, C.W.; Hutson, J.M.; Southwell, B.R. Gender differences in reduced substance P (SP) in children with slow-transit constipation. Pediatr. Surg. Int. 2011, 27, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.D.; Qu, C.; Lin, G.S.; Zhang, Z.B.; Xie, J.H.; Chen, H.B.; Liang, J.; Li, C.; Wang, H.; Su, Z. Haracter and laxative activity of polysaccharides isolated from Dendrobium officinale. J. Funct. Foods 2017, 34, 106–117. [Google Scholar] [CrossRef]
- Huang, L.S.; Zhu, Q.; Qu, X.; Qin, H.L. Microbial treatment in chronic constipation. Sci. China Life Sci. 2018, 61, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Mao, W.J.; Chen, Y.; Guo, S.D.; Li, H.Y.; Qi, X.H.; Chen, Y.-L.; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Ma, H.T.; Xiong, H.Y.; Zhu, X.L.; Ji, C.L.; Xue, J.A.; Li, R.Z.; Ge, B.; Cui, H. Polysaccharide from spirulina platensis ameliorates diphenoxylate-induced constipation symptoms in mice. Int. J. Biol. Macromol. 2019, 33, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Q.; Ji, Y.; Chen, X.; Su, A.X.; Ma, G.X.; Chen, G.T.; Hu, Q.; Zhao, L. Effects of a β-type glycosidic polysaccharide from Flammulina velutipes on anti-inflammation and gut microbiota modulation in colitis mice. Food Funct. 2020, 11, 4259–4274. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.G.; Zhao, Q.C.; Hao, X.T.; Wang, J.M.; Ma, C.Y.; Xi, X.F.; Kang, W. The effect of Flammulina velutipes polysaccharide on immunization analyzed by intestinal flora and proteomics. Front. Nutr. 2022, 9, 35. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, J.W.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Choi, Y.S.; Lee, Y.J.; Lee, H.S.; Hong, J.T.; Hwang, D.Y. Anti-inflammatory response and muscarinic cholinergic regulation during the laxative effect of Asparagus cochinchinensis in loperamide-induced constipation of SD rats. Int. J. Mol. Sci. 2019, 20, 946. [Google Scholar] [CrossRef]
- Thiagarajah, J.R.; Chang, J.; Goettel, J.A.; Verkman, A.S.; Lencer, W.I. Lencer, Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl. Acad. Sci. USA 2017, 114, 568–573. [Google Scholar] [CrossRef]
- Li, X.M.; Liu, Y.; Guan, W.; Xia, Y.G.; Zhou, Y.Y.; Yang, B.Y.; Kuang, H. Physicochemical properties and laxative effects of polysaccharides from Anemarrhena asphodeloides Bge. in loperamide-induced rats. J. Ethnopharmacol. 2019, 240, 111961. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.D.; Li, R.; Zhang, T.; Li, M.; Mou, H.J. Study on the ability of partially hydrolyzed guar gum to modulate the gut microbiota and relieve constipation. J. Food Biochem. 2019, 43, e12715. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Boushra, D.; Lukas, V.O.; Bram, V.; Kristin, V. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar]
- Kristjánsson, G.; Venge, P.; Wanders, A.; Lööf, L.; Hällgren, R. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: Studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut 2004, 53, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wen, Y.; Du, L.J.; Wang, X.X.; Tang, S.Y.; Kong, P.F.; Huang, W.-G.; Tang, X.-G. Effects of maren pills on the intestinal microflora and short-chain fatty acid profile in drug-induced slow transit constipation model rats. Front. Pharmacol. 2022, 13, 804723. [Google Scholar] [CrossRef]
- Yang, G.; Dong, L.J.; Zhou, Q. Study on the moisturizing and defecating effects of dietary fiber reinforced freeze-dried hawthorn fruit on mice. Food Res. Dev. 2016, 37, 8–10. [Google Scholar]
- Lee, H.B.; Kim, Y.S.; Park, H.Y. Pectic polysaccharides: Targeting gut microbiota in obesity and intestinal health. Carbohydr. Polym. 2022, 287, 119363. [Google Scholar] [CrossRef]
- Hu, H.B.; Liang, H.P.; Wu, Y. Isolation, purification and structural characterization of polysaccharide from Acanthopanax brachypus. Carbohydr. Polym. 2015, 127, 94–100. [Google Scholar] [CrossRef]
- Liu, H.C.; Fan, H.X.; Zhang, J.; Zhang, S.S.; Zhao, W.T.; Liu, T.T.; Wang, D. Isolation, purification, structural characteristic and antioxidative property of polysaccharides from A. cepa L. var. agrogatum Don. Food Sci. Hum. Wellness 2020, 9, 71–79. [Google Scholar] [CrossRef]
- Ye, S.X.; Shah, B.R.; Li, J.; Liang, H.S.; Zhan, F.C.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]




| Distribution Name | Mn (Daltons) | Mw (Daltons) | MP (Daltons) | Mz (Daltons) | Polydispersity |
|---|---|---|---|---|---|
| 1 | 14,787 | 17,973 | 13,587 | 22,127 | 1.215433 |
| 2 | 2744 | 3381 | 1558 | 4219 | 1.232293 |
| Monosaccharide Composition | Monosaccharide Content/(μg/mg) |
|---|---|
| d-mannose | 2.07 ± 0.08 |
| l-rhamnose monohydrate | 24.67 ± 1.02 |
| l-arabinose | 113.22 ± 3.12 |
| d-galactose | 39.45 ± 1.13 |
| Glucose | 42.83 ± 1.32 |
| d-xylose | 7.20 ± 0.23 |
| d-mannose | 3.12 ± 0.16 |
| d-galacturonic acid | 306.07 ± 6.35 |
| d-glucuronic acid | 1.55 ± 0.05 |
| Total | 540.18 ± 6.58 |
| Groups | MTL (pg/mL) | GAS (pg/mL) | SP (pg/mL) | VIP (pg/mL) | SS (pg/mL) | NO (μg/mL) | MDA (nmoL/mL) |
|---|---|---|---|---|---|---|---|
| C | 83.72 ± 7.18 | 6.61 ± 0.79 | 108.35 ± 5.63 | 89.54 ± 4.07 | 45.24 ± 3.61 | 8.00 ± 0.09 | 27.61 ± 0.87 |
| M | 52.14 ± 11.49 ** | 4.28 ± 0.09 ** | 96 ± 3.81 * | 31.59 ± 2.72 ** | 102.66 ± 5.37 ** | 9.10 ± 0.28 ** | 53.14 ± 1.59 ** |
| HS | 62.14 ± 4.53 | 6.21 ± 0.44 | 91.98 ± 3.81 | 58.51 ± 3.56 ## | 58.91 ± 3.31 ## | 5.34 ± 2.40 ## | 26.72 ± 1.51 ## |
| LHDF | 72.49 ± 4.25 ## | 5.18 ± 0.26 ## | 135.58 ± 12.50 ## | 44.67 ± 3.95 # | 94.74 ± 5.65 # | 8.44 ± 0.75 # | 46.37 ± 1.84 ## |
| HHDF | 85.85 ± 2.45 ##ss | 6.00 ± 0.88 ##ss | 142.98 ± 14.28 ##s | 73.72 ± 4.24 ##ss | 49.62 ± 2.33 ##ss | 6.20 ± 1.00 ##ss | 27.02 ± 0.95 ##ss |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zu, Q.; Zhang, J.; Liu, S.; Zhang, G.; Chang, X.; Li, X. Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice. Foods 2024, 13, 2220. https://doi.org/10.3390/foods13142220
Zhang H, Zu Q, Zhang J, Liu S, Zhang G, Chang X, Li X. Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice. Foods. 2024; 13(14):2220. https://doi.org/10.3390/foods13142220
Chicago/Turabian StyleZhang, Henghui, Qixin Zu, Jiancai Zhang, Suwen Liu, Guohua Zhang, Xuedong Chang, and Xiaojun Li. 2024. "Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice" Foods 13, no. 14: 2220. https://doi.org/10.3390/foods13142220
APA StyleZhang, H., Zu, Q., Zhang, J., Liu, S., Zhang, G., Chang, X., & Li, X. (2024). Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice. Foods, 13(14), 2220. https://doi.org/10.3390/foods13142220




