Comprehensive Amelioration of Metabolic Dysfunction through Administration of Lactiplantibacillus plantarum APsulloc 331261 (GTB1™) in High-Fat-Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Strain
2.2. Animal Experiment
2.3. Glucose Tolerance Test and Insulin Tolerance Test
2.4. Plasma Analyses
2.5. Histological Analysis
2.6. Hepatic Triglyceride (TG) Quantification
2.7. Quantitative PCR
2.8. Immunoblotting
2.9. Cecal Microbiota Analysis and Short-Chain Fatty Acid Measurement
2.10. Statistical Analyses
3. Results
3.1. GTB1™ Treatment Alleviates High-Fat-Diet-Induced Metabolic Dysfunction in HFD-Fed Mice
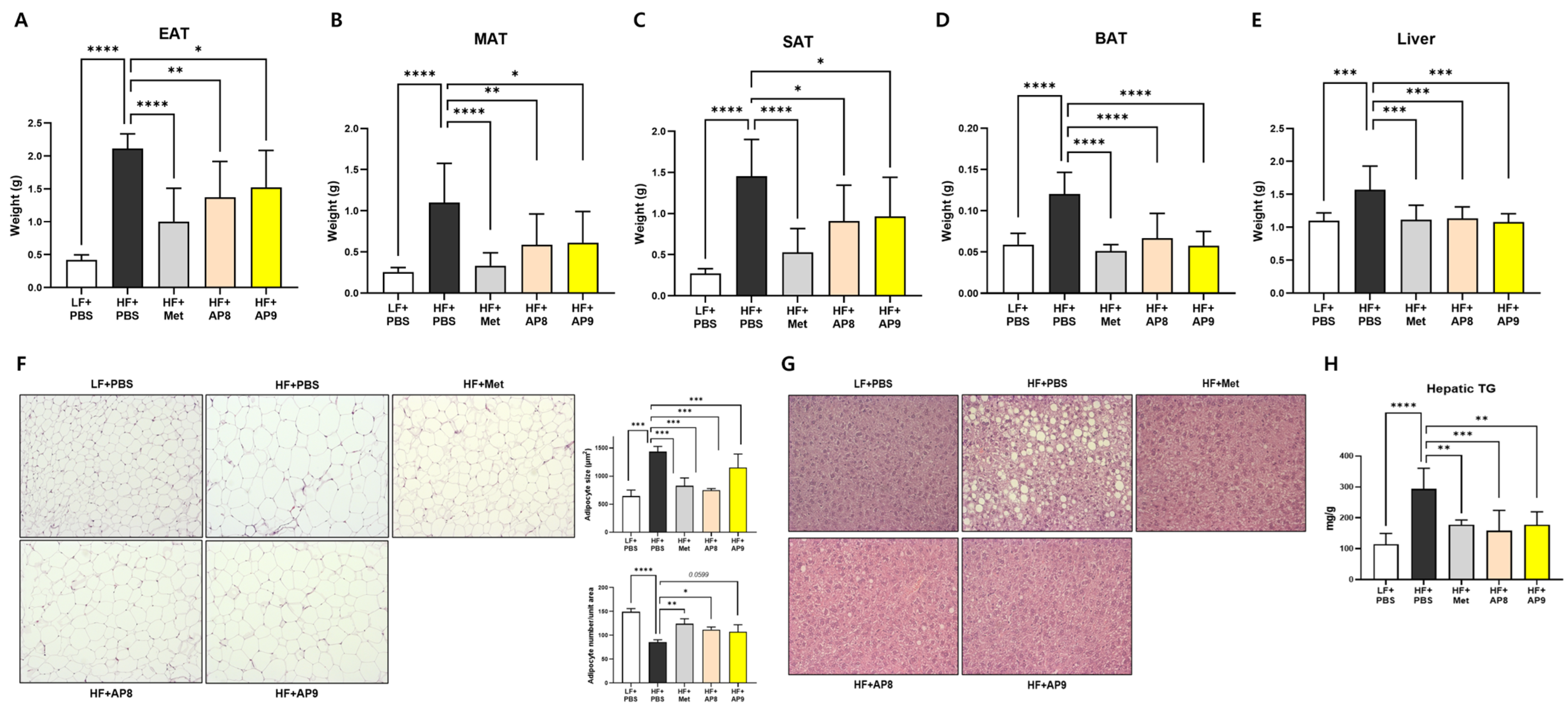
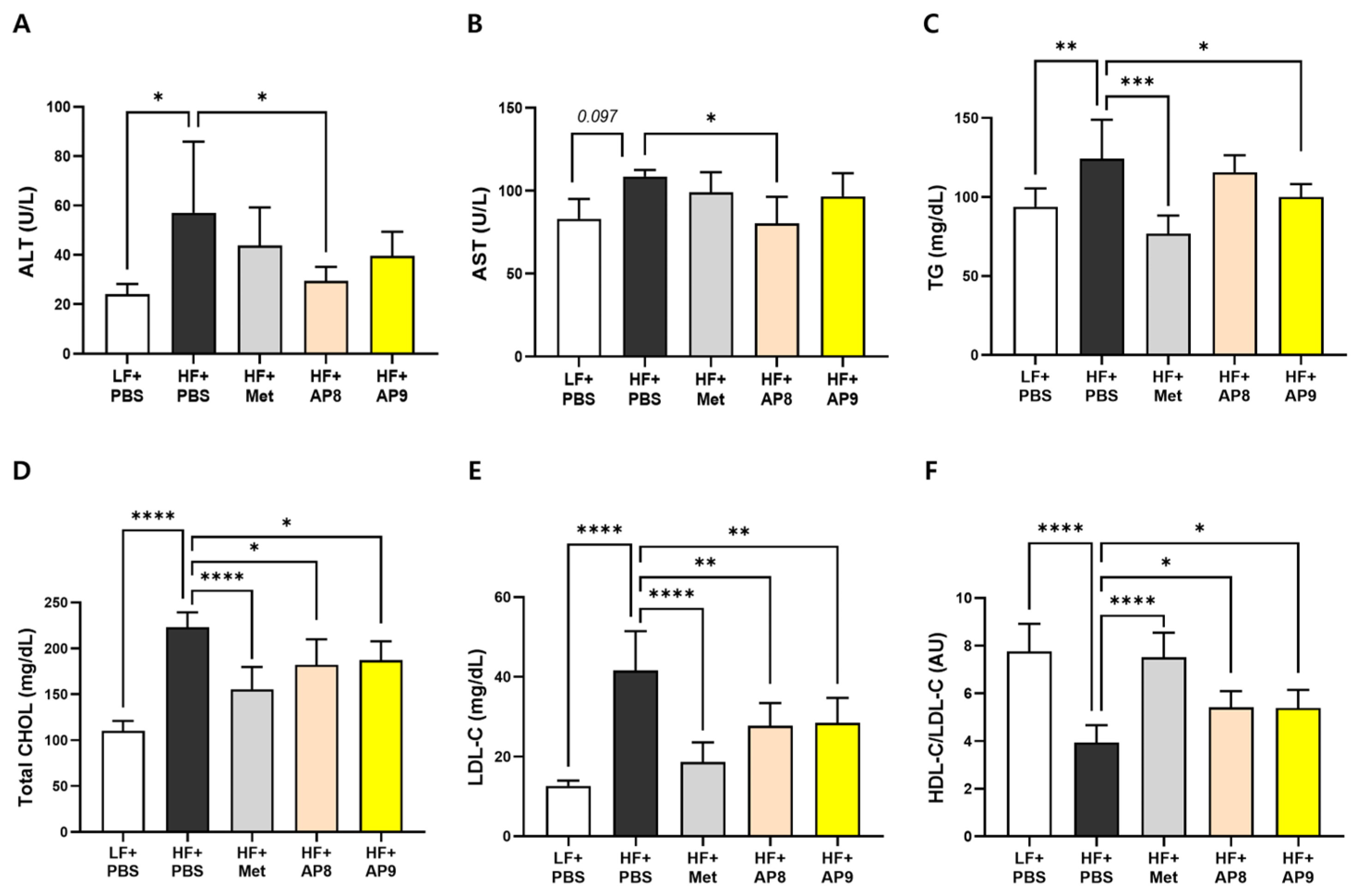
3.2. GTB1™ Treatment Suppresses Tissue Adiposity and Improves Plasma Lipid Profile in HFD-Fed Mice
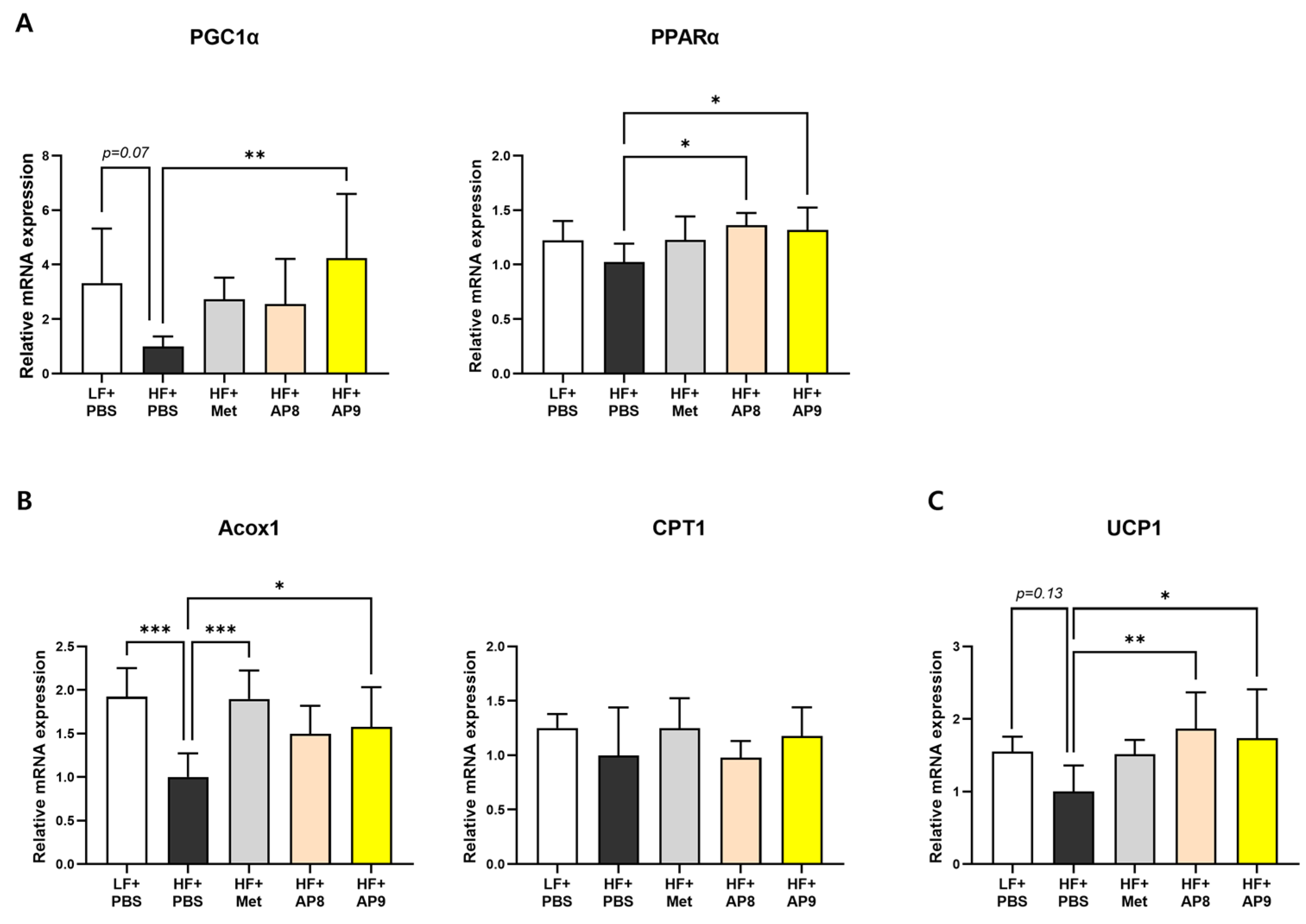
3.3. GTB1™ Treatment Ameliorates Dysregulated Lipid Metabolism in Adipose Tissues of HFD-Fed Mice
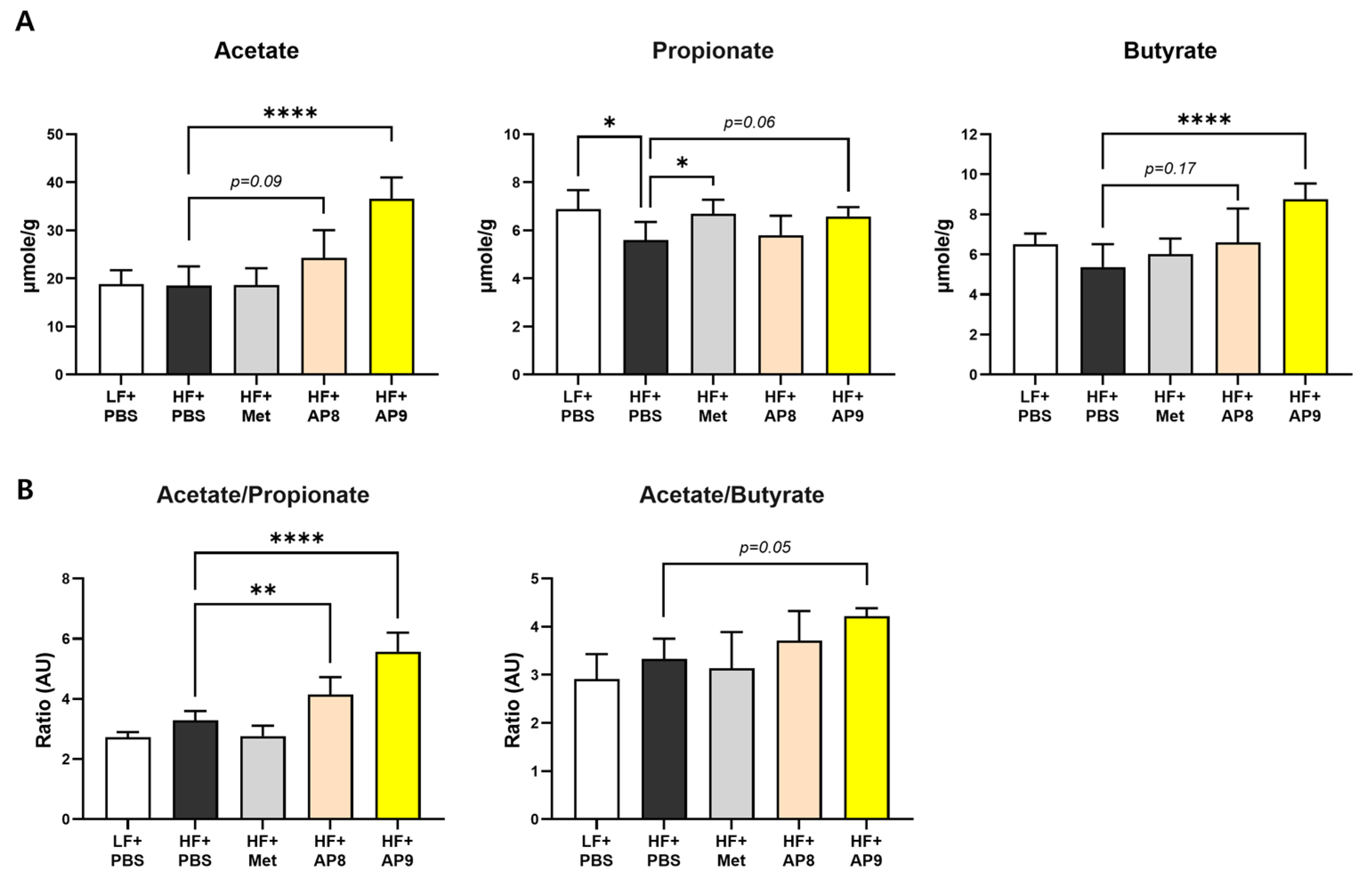
3.4. GTB1™ Treatment Augments Short-Chain Fatty Acid Production and Modulates Microbiota Composition in HFD-Fed Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, Insulin Resistance, and the Metabolic Syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 May 2024).
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Munafò, A.; Frara, S.; Perico, N.; Di Mauro, R.; Cortinovis, M.; Burgaletto, C.; Cantarella, G.; Remuzzi, G.; Giustina, A.; Bernardini, R. In Search of an Ideal Drug for Safer Treatment of Obesity: The False Promise of Pseudoephedrine. Rev. Endocr. Metab. Disord. 2021, 22, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of Obesity: An Update on the Available Medications and Drugs under Investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.-S.; Angoorani, P.; Soroush, A.-R.; Atlasi, R.; Hasani-Ranjbar, S.; Mortazavian, A.M.; Larijani, B. Probiotics Supplementation for the Obesity Management; A Systematic Review of Animal Studies and Clinical Trials. J. Funct. Foods 2019, 52, 228–242. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, B.; Lee, C.; Joung, H.; Kim, B.-K.; Choi, I.S.; Hyun, C.-K. Comprehensive Amelioration of High-Fat Diet-Induced Metabolic Dysfunctions through Activation of the PGC-1α Pathway by Probiotics Treatment in Mice. PLoS ONE 2020, 15, e0228932. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.C.; Wareham, D.W. Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Stenotrophomonas maltophilia. Int. J. Antimicrob. Agents 2010, 36, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, D.; Su, B.; Wei, J.; Găman, M.; Macit, M.S.; Nascimento, I.J.B.D.; Guimaraes, N.S. The Effect of Green Tea Supplementation on Obesity: A Systematic Review and Dose–Response Meta-analysis of Randomized Controlled Trials. Phytother. Res. 2020, 34, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, I.; Marcobal, Á.; Muñoz, R. Tannase Activity by Lactic Acid Bacteria Isolated from Grape Must and Wine. Int. J. Food Microbiol. 2004, 96, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ankolekar, C.; Johnson, D.; Da Silva Pinto, M.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory Potential of Tea Polyphenolics and Influence of Extraction Time Against Helicobacter pylori and Lack of Inhibition of Beneficial Lactic Acid Bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Arellano, K.; Vazquez, J.; Park, H.; Lim, J.; Ji, Y.; Kang, H.-J.; Cho, D.; Jeong, H.W.; Holzapfel, W.H. Safety Evaluation and Whole-Genome Annotation of Lactobacillus plantarum Strains from Different Sources with Special Focus on Isolates from Green Tea. Probiotics Antimicrob. Proteins 2019, 12, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, M.; Campión, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-De-Solórzano, C. Adiposoft: Automated Software for the Analysis of White Adipose Tissue Cellularity in Histological Sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, J.; Kim, M.-S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.-K. Protective Effects of Bacillus Probiotics against High-Fat Diet-Induced Metabolic Disorders in Mice. PLoS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef]
- Ji, Y.; Park, S.; Chung, Y.; Kim, B.; Park, H.; Huang, E.; Jeong, D.; Jung, H.-Y.; Kim, B.; Hyun, C.-K.; et al. Amelioration of Obesity-Related Biomarkers by Lactobacillus Sakei CJLS03 in a High-Fat Diet-Induced Obese Murine Model. Sci. Rep. 2019, 9, 6821. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef]
- Beau, A.; Benoit, B.; Barz, M.L.; Meugnier, E.; Penhoat, A.; Calzada, C.; Pinteur, C.; Loizon, E.; Chanon, S.; Vieille-Marchiset, A.; et al. Inhibition of Intestinal FXR Activity as a Possible Mechanism for the Beneficial Effects of a Probiotic Mix Supplementation on Lipid Metabolism Alterations and Weight Gain in Mice Fed a High Fat Diet. Gut Microbes 2023, 15, 2281015. [Google Scholar] [CrossRef] [PubMed]
- Fabersani, E.; Marquez, A.; Russo, M.; Ross, R.; Torres, S.; Fontana, C.; Puglisi, E.; Medina, R.; Gauffin-Cano, P. Lactic Acid Bacteria Strains Differently Modulate Gut Microbiota and Metabolic and Immunological Parameters in High-Fat Diet-Fed Mice. Front. Nutr. 2021, 8, 718564. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ling, Q.; Wu, L.; Wang, X.; Wang, Z.; Chen, J.; Zheng, Z.; Zhou, Z.; Jia, L.; Li, L.; et al. Akkermansia muciniphila Inhibits Nonalcoholic Steatohepatitis by Orchestrating TLR2-Activated γδT17 Cell and Macrophage Polarization. Gut Microbes 2023, 15, 2221485. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-Promoting Role of Lactiplantibacillus plantarum Isolated from Fermented Foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mah, M.; Ritchie, R.H.; De Blasio, M.J. The Adiponectin Signalling Pathway—A Therapeutic Target for the Cardiac Complications of Type 2 Diabetes? Pharmacol. Ther. 2022, 232, 108008. [Google Scholar] [CrossRef]
- Townsend, L.K.; Steinberg, G.R. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. [Google Scholar] [CrossRef]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose Tissue Fibrosis, Hypertrophy, and Hyperplasia: Correlations with Diabetes in Human Obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Jia, G.; Jia, Y.; Sowers, J.R. Contribution of Maladaptive Adipose Tissue Expansion to Development of Cardiovascular Disease. Compr. Physiol. 2016, 7, 253–262. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 (PGC-1) Family in Physiological and Pathophysiological Process and Diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in Obesity-Induced T2DM, Dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2016, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut Microbiota as a Potential Target of Metabolic Syndrome: The Role of Probiotics and Prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2017, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Besten, G.D.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity via a PPARγ-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Lee, Y.; Lee, C.; Jung, E.S.; Kang, H.; Holzapfel, W.H. Comprehensive Amelioration of Metabolic Dysfunction through Administration of Lactiplantibacillus plantarum APsulloc 331261 (GTB1™) in High-Fat-Diet-Fed Mice. Foods 2024, 13, 2227. https://doi.org/10.3390/foods13142227
Kim B, Lee Y, Lee C, Jung ES, Kang H, Holzapfel WH. Comprehensive Amelioration of Metabolic Dysfunction through Administration of Lactiplantibacillus plantarum APsulloc 331261 (GTB1™) in High-Fat-Diet-Fed Mice. Foods. 2024; 13(14):2227. https://doi.org/10.3390/foods13142227
Chicago/Turabian StyleKim, Bobae, Yuri Lee, Chungho Lee, Eun Sung Jung, Hyeji Kang, and Wilhelm H. Holzapfel. 2024. "Comprehensive Amelioration of Metabolic Dysfunction through Administration of Lactiplantibacillus plantarum APsulloc 331261 (GTB1™) in High-Fat-Diet-Fed Mice" Foods 13, no. 14: 2227. https://doi.org/10.3390/foods13142227




