Effects of Chlorich®EnergyBoost on Enhancing Physical Performance and Anti-Fatigue Properties in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry
2.3. Bacterial Reverse Mutation Test
2.4. Acute Oral Toxicity Test
2.5. Animals and Experimental Design
2.6. Forelimb Grip Strength Assessment
2.7. Swimming Exercise Endurance Test
2.8. Serum Biochemistry Analysis
2.9. Evaluation of Hepatic and Muscular Glycogen Content
2.10. Statistic Analysis
3. Results
3.1. MALDI-TOF Mass Spectrometry Analysis
3.2. Bacterial Reverse Mutation Test Demonstrating No Cytotoxic Effects
3.3. Acute Oral Toxicity Assessment Indicating No Observable Effects
3.4. General Characteristics of Mice after Four Weeks of Chlorich®EnergyBoost Supplementation
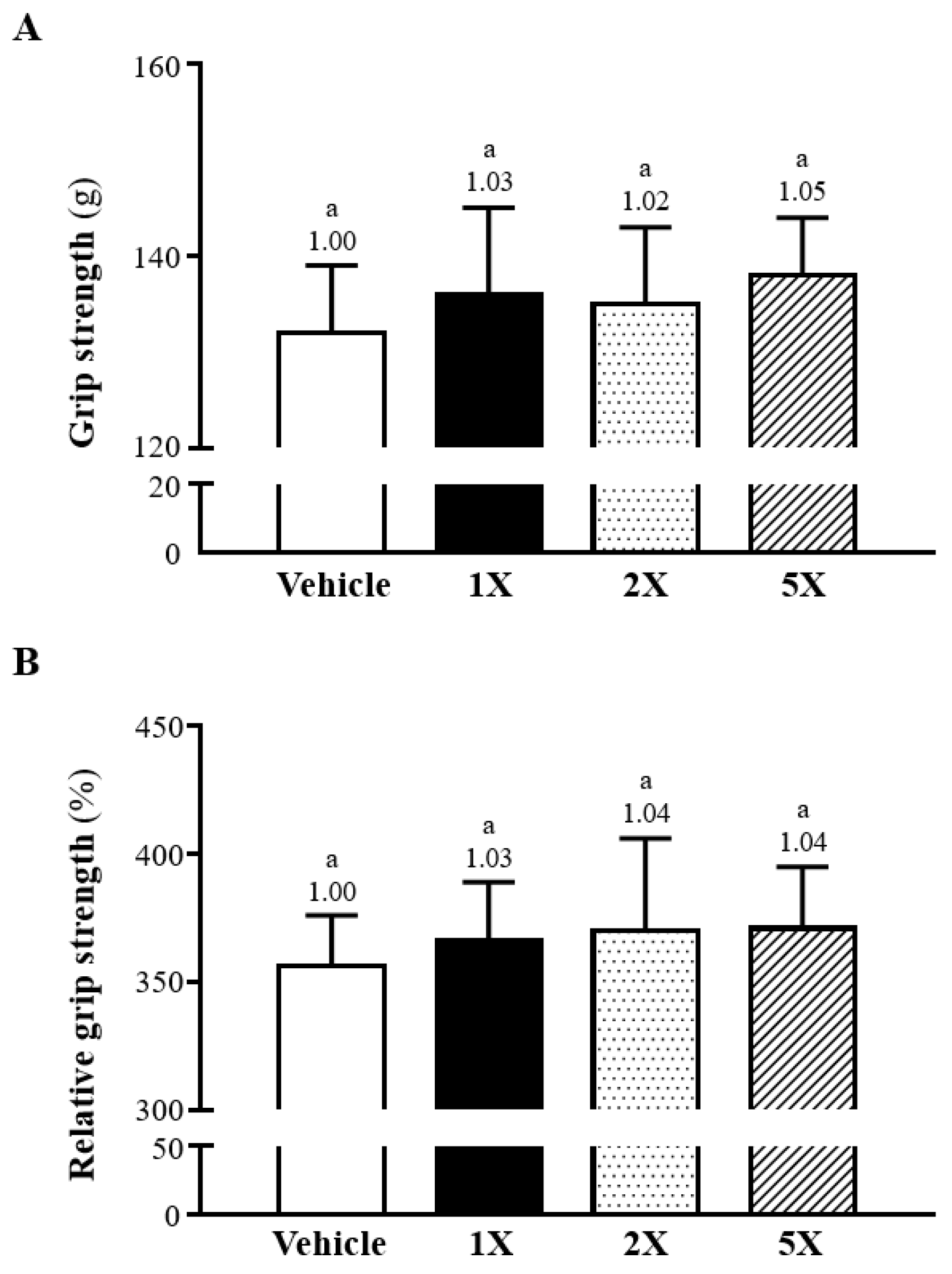
3.5. Effect of Chlorich®EnergyBoost Supplementation on Muscle Strength
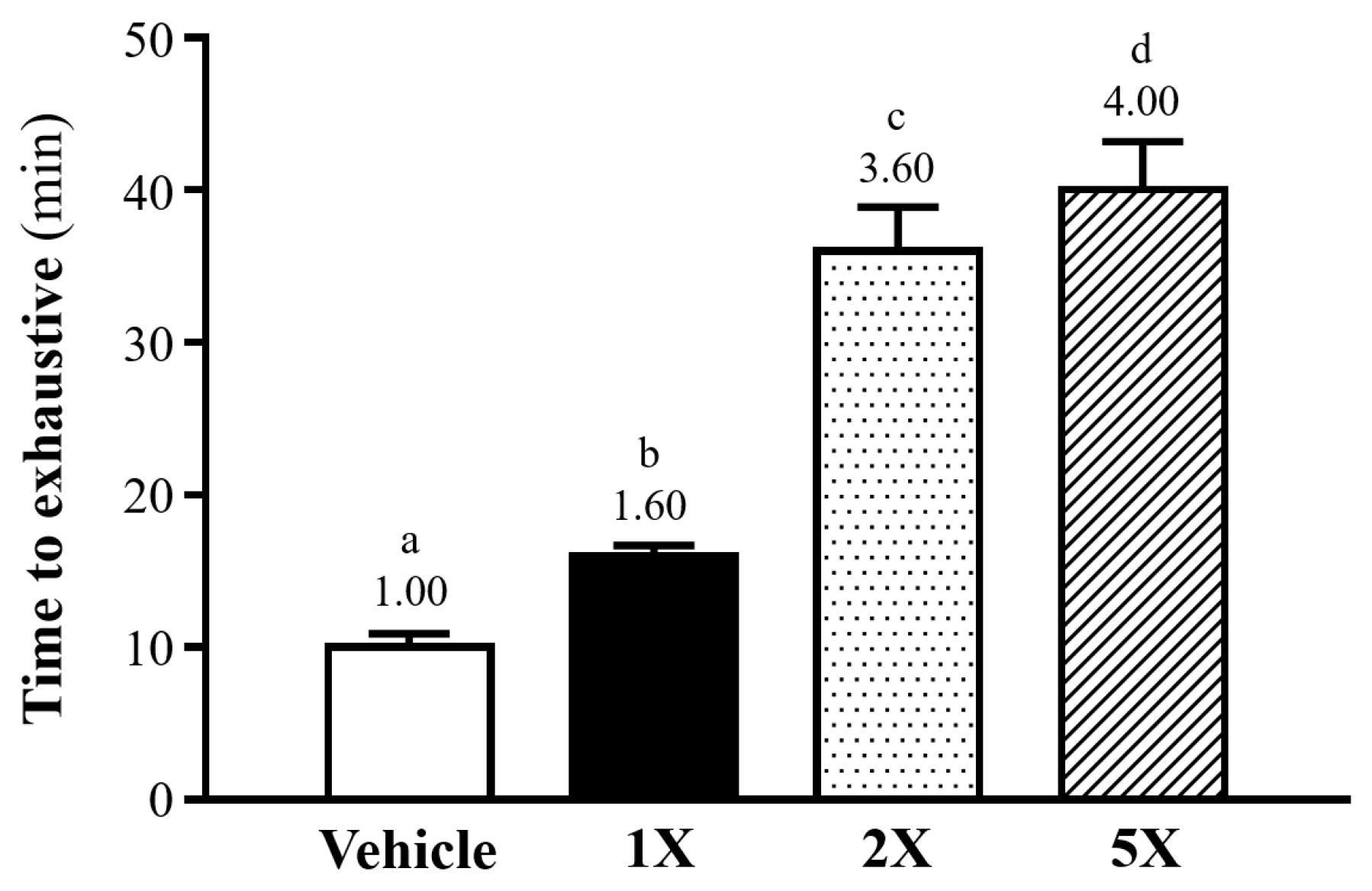
3.6. Enhancement of Exercise Performance by Chlorich®EnergyBoost Supplementation
3.7. Effect of Chlorich®EnergyBoost Supplementation on Serum Lactate Levels Following a 10-Min Swim Test
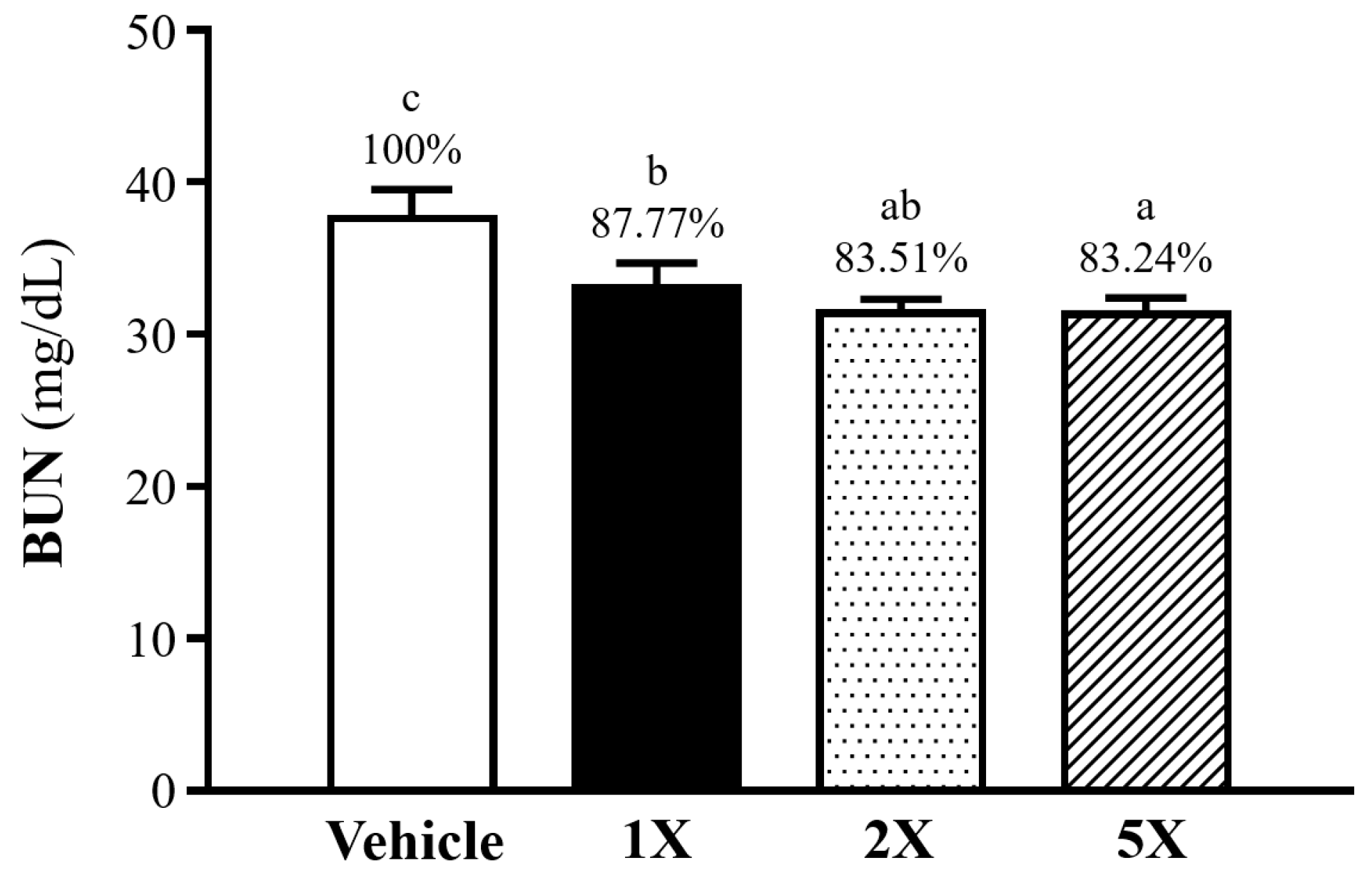
3.8. Effect of Chlorich®EnergyBoost Supplementation on Blood Urea Nitrogen Following a 90-Min Swim Test and 60-Min Rest
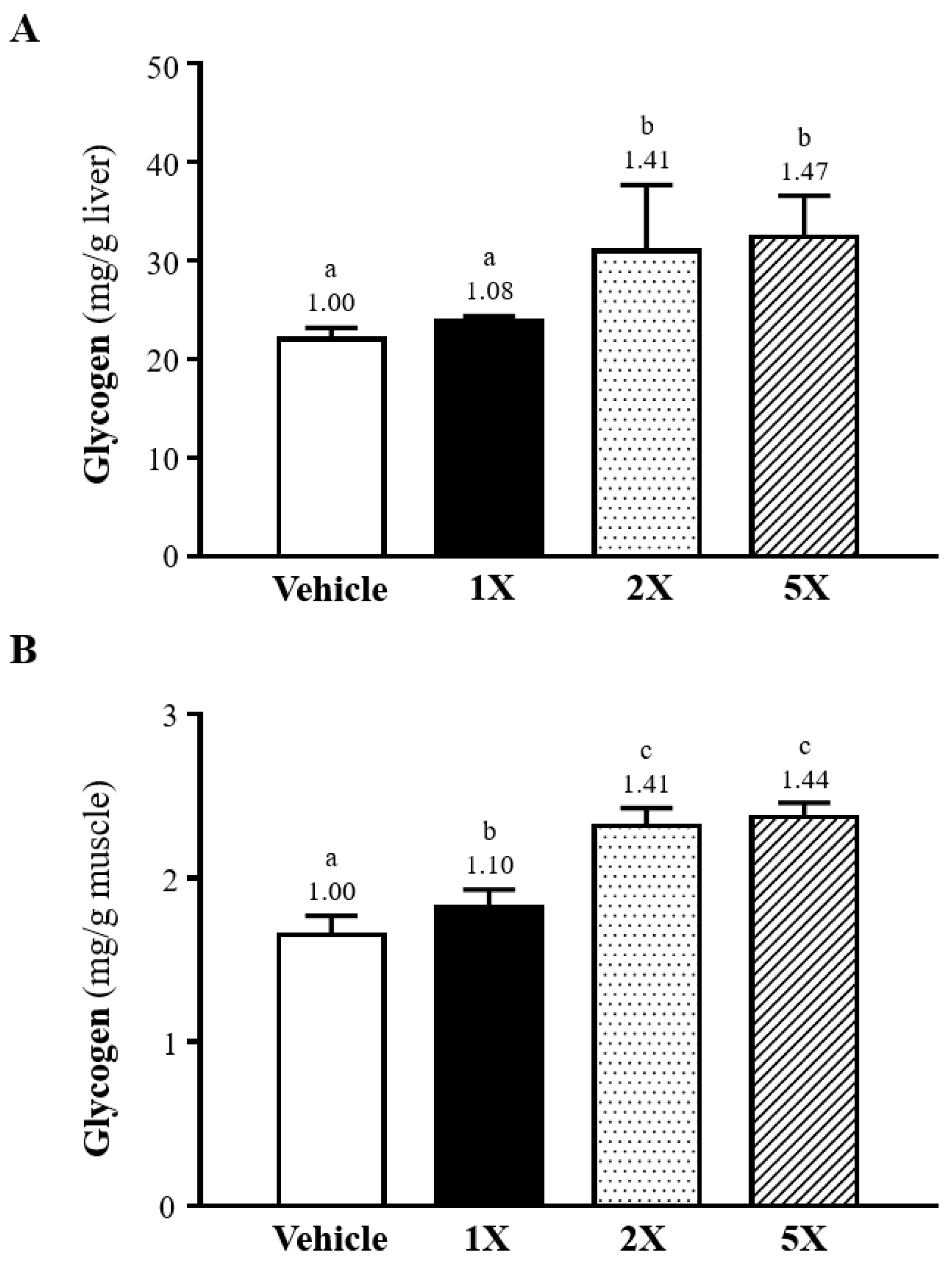
3.9. Effect of Chlorich®EnergyBoost Supplementation on Hepatic and Muscular Glycogen Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2018; pp. 1–46.
- World Health Organization. Global Status Report on Physical Activity 2022; World Health Organization: Geneva, Switzerland, 2022; pp. 1–53.
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; pp. 1–25.
- Santos, A.C.; Willumsen, J.; Meheus, F.; Ilbawi, A.; Bull, F.C. The cost of inaction on physical inactivity to public health-care systems: A population-attributable fraction analysis. Lancet Glob. Health 2023, 11, e32–e39. [Google Scholar] [CrossRef]
- Billones, R.; Liwang, J.K.; Butler, K.; Graves, L.; Saligan, L.N. Dissecting the fatigue experience: A scoping review of fatigue definitions, dimensions, and measures in non-oncologic medical conditions. Brain Behav. Immun. Health 2021, 15, 100266. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 19 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Park, N.H.; Kang, Y.-E.; Ahn, Y.C.; Lee, E.J.; Son, C.G. The demographic features of fatigue in the general population worldwide: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1192121. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Yoshida, N.; Kakuma, T.; Toyomasu, K. Effect of Chlorella Ingestion on Oxidative Stress and Fatigue Symptoms in Healthy Men. Kurume Med. J. 2017, 64, 83–90. [Google Scholar] [CrossRef]
- Wang, T.; Yin, J.; Miller, A.H.; Xiao, C. A systematic review of the association between fatigue and genetic polymorphisms. Brain Behav. Immun. 2017, V62, 230–244. [Google Scholar] [CrossRef]
- Lesley, M.A. Understanding Fatigue in Major Depressive Disorder and Other Medical Disorders. Psychosomatics 2008, V49, 185–190. [Google Scholar] [CrossRef]
- Hege, L.H.; Kristin, V.R.; Jon, H.L.; Vessela, N.K.; Vanessa, D.P.; Sophi, D.F.; Anne-Lise, B.D.; Hege, E. The Genetics and Epigenetics of Fatigue. PM&R 2010, V2, 456–465. [Google Scholar] [CrossRef]
- James, B.H.; Rebecca, E.; Xiaonan, Z.; Nancy, W.G.; Theodore, J.H.; Caterina, R. Fatigue and perceived energy in a sample of older adults over 10 years: A resting state functional connectivity study of neural correlates. Exp. Gerontol. 2024, V188, 112–388. [Google Scholar] [CrossRef]
- Motl, R.W.; Suh, Y.; Weikert, M.; Dlugonski, D.; Balantrapu, S.; Sandroff, B. Fatigue, depression, and physical activity in relapsing-remitting multiple sclerosis: Results from a prospective, 18-month study. Mult. Scler. Relat. Disord. 2012, 1, 43–48. [Google Scholar] [CrossRef]
- Nutrition Business Journal. Supplement Business Report; Informa PLC: London, UK, 2023; pp. 12–88. [Google Scholar]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Lorenzo, K.; Santocildes, G.; Torrella, J.R.; Magalhães, J.; Pagès, T.; Viscor, G.; Torres, J.L.; Ramos-Romero, S. Bioactivity of Macronutrients from Chlorella in Physical Exercise. Nutrients 2023, 15, 2168. [Google Scholar] [CrossRef]
- Prybylski, N.; Toucheteau, C.; El Alaoui, H.; Bridiau, N.; Maugard, T.; Abdelkafi, S.; Fendri, I.; Delattre, C.; Dubessay, P.; Pierre, G.; et al. Handbook of Microalgae-Based Processes and Products: Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds, 1st ed.; Elsevier: Cambridge, MA, USA, 2020; Chapter 20; pp. 533–571. ISBN 978-0-12-818536-0. [Google Scholar]
- Guehaz, K.; Boual, Z.; Abdou, I.; Telli, A.; Belkhalfa, H. Microalgae’s polysaccharides, are they potent antioxidants? Critical review. Arch. Microbiol. 2024, 206, 14. [Google Scholar] [CrossRef] [PubMed]
- Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.M.; Skjånes, K.; Rieder, A. Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris. Foods 2021, 10, 3078. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.N.; Kobayashi, N.; Barnes, A.; Noel, E.A.; Betenbaugh, M.J.; Oyler, G.A. Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PLoS ONE 2014, 9, e92460. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.M.; Keshavarz, S.A.; Nasli-Esfahani, E.; Amiri, F.; Janani, L. The effects of Chlorella supplementation on glycemic control, lipid profile and anthropometric measures on patients with type 2 diabetes mellitus. Eur. J. Nutr 2021, 60, 3131–3141. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Habibian Dehkordi, S.; Engardeh, J.; Mahmoodnia, L.; Khaledifar, A.; Jafari, T. Effect of Chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1892–1901. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, A.P.; Arisseto Bragotto, A.P. Microalgae-based products: Food and public health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Yulia, S. Toxicity assessment of residual biomass of microalgae Chlorella sorokiniana. J. Butlerov Commun. 2019, 59, 92–98. [Google Scholar] [CrossRef]
- Zainul Azlan, N.; Abd Ghafar, N.; Mohd Yusof, Y.A.; Makpol, S. Toxicity study of Chlorella vulgaris water extract on female Sprague Dawley rats by using the Organization for Economic Cooperation and Development (OECD) Guideline 420. J. Appl. Phycol. 2020, 32, 3063–3075. [Google Scholar] [CrossRef]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.S.; Panjaitan, F.C.A.; Chang, Y.-W. Prediction of bioactive peptides from Chlorella sorokiniana proteins using proteomic techniques in combination with bioinformatics analyses. Int. J. Mol. Sci. 2019, 20, 1786. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.; Mendes, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 00144. [Google Scholar] [CrossRef]
- Li, D.; Yi, J.; Han, G.; Qiao, L. MALDI-TOF Mass Spectrometry in Clinical Analysis and Research. ACS Meas. Sci. Au 2022, 2, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Barbano, D.; Diaz, R.; Zhang, L.; Sandrin, T.; Gerken, H.; Dempster, T. Rapid Characterization of Microalgae and Microalgae Mixtures Using Matrix-Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectrometry (MALDI-TOF MS). PLoS ONE 2015, 10, e0135337. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. OECD Environment Health and Safety Publications Series on Testing and Assessment No. 47; Organisation for Economic Co-operation and Development: Paris, France, 2004; pp. 1–170. [Google Scholar]
- Ministry of Health and Welfare of Taiwan. Health Food Control Act; Ministry of Health and Welfare: Taipei City, Taiwan, 2020; pp. 1–100.
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Su, K.Y.; Yu, C.Y.; Chen, Y.W.; Huang, Y.T.; Chen, C.T.; Wu, H.F.; Chen, Y.L. A flavonoid and principal component of Saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int. J. Med. Sci. 2014, 11, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Office of Laboratory Animal Welfare. Applied Research Ethics National Association, Guide of Institutional Animal Care and Use Committee, 2nd ed.; National of Institutes of Health Office of Laboratory Animal Walfare: Bethesda, MD, USA, 2002; pp. 5–210.
- Yeh, T.S.; Chuang, H.L.; Huang, W.C.; Chen, Y.M.; Huang, C.C.; Hsu, M.C. Astragalus membranaceus Improves Exercise Performance and Ameliorates Exercise-Induced Fatigue in Trained Mice. Molecules 2014, 19, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 364741. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Huang, W.C.; Liu, C.C.; Wang, M.F.; Ho, C.S.; Huang, W.P.; Hou, C.C.; Chuang, H.L.; Huang, C.C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef] [PubMed]
- Khapre, I.; Samantaray, S.M. Microalgal Nutraceuticals: Opportunity for Nutritional Market. Int. J. Sci. Res. 2019, 8, 1201–1206. [Google Scholar]
- Neal, W.N.; Cederberg, K.L.; Jeng, B.; Sasaki, J.E.; Motl, R.W. Is Symptomatic Fatigue Associated with Physical Activity and Sedentary Behaviors Among Persons with Multiple Sclerosis? Neurorehabil. Neural Repair 2020, 34, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Nemet, D.; Wolach, B.; Eliakim, A. Proteins and amino acid supplementation in sports: Are they truly necessary? Isr. Med. Assoc. 2005, 7, 328–332. [Google Scholar] [PubMed]
- Blomstrand, E.; Hassmén, P.; Ekblom, B.; Newsholme, E.A. Administration of branched-chain amino acids during sustained exercise--effects on performance and on plasma concentration of some amino acids. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, Z.; Luo, Y.; Yang, F.; Cao, F.; Luo, F.; Lin, Q. Dietary Polysaccharides Exert Anti-Fatigue Functions via the Gut-Muscle Axis: Advances and Perspectives. Foods 2023, 12, 3083. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Fasciolo, G.; Salbitani, G.; Venditti, P. Chlorella sorokiniana Dietary Supplementation Increases Antioxidant Capacities and Reduces ROS Release in Mitochondria of Hyperthyroid Rat Liver. Antioxidants 2020, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Ma, M.; Sui, Z. Structure and Function of Polysaccharides and Oligosaccharides in Foods. Foods 2023, 12, 3872. [Google Scholar] [CrossRef]
- Wang, P.; Wang, D.; Hu, J.; Tan, B.K.; Zhang, Y.; Lin, S. Natural Bioactive Peptides to Beat Exercise-Induced Fatigue: A Review. Food Biosci. 2021, 43, 101298. [Google Scholar] [CrossRef]
- Devlin, J.; Paton, B.; Poole, L.; Sun, W.; Ferguson, C.; Wilson, J.; Kemi, O.J. Blood lactate clearance after maximal exercise depends on active recovery intensity. J. Sports Med. Phys. Fit. 2014, 54, 271–278. [Google Scholar] [PubMed]
- Chen, Y.; Wang, J.; Jing, Z.; Ordovas, J.M.; Wang, J.; Shen, L. Anti-fatigue and anti-oxidant effects of curcumin supplementation in exhaustive swimming mice via Nrf2/Keap1 signal pathway. Curr. Res. Food Sci. 2022, 5, 1148–1157. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Chen, K. Lactate clearance as a useful biomarker for the prediction of all-cause mortality in critically ill patients: A systematic review study protocol. BMJ Open 2014, 4, e004752. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.; Stewart, M.; Thind, A. Examining the symptom of fatigue in primary care: A comparative study using electronic medical records. BMJ Health Care Inform. 2015, 22, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Dittner, A.J.; Wessely, S.C.; Brown, R.G. The assessment of fatigue: A practical guide for clinicians and researchers. J. Psychosom. Res. 2004, 56, 157–170. [Google Scholar] [CrossRef]
- Carolina, R.A.; Corsi, A.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef] [PubMed]
| Time Point | Vehicle | 1X | 2X | 5X |
|---|---|---|---|---|
| Lactate (mmol/L) | ||||
| Before swimming (A) | 3.5 ± 0.3 a | 3.5 ± 0.2 a | 3.4 ± 0.3 a | 3.5 ± 0.3 a |
| After swimming (B) | 7.1 ± 0.7 c | 6.1 ± 0.5 b | 6.1 ± 0.5 b | 5.5 ± 0.2 a |
| After a 20 min rest (C) | 5.5 ± 0.6 c | 4.4 ± 0.3 b | 4.0 ± 0.2 ab | 3.6 ± 0.3 a |
| Rate of lactate production and clearance | ||||
| Production rate = B/A | 2.03 ± 0.11 c | 1.74 ± 0.15 b | 1.80 ± 0.15 b | 1.57 ± 0.14 a |
| Clearance rate = (B − C)/B | 0.22 ± 0.14 a | 0.28 ± 0.09 ab | 0.34 ± 0.08 b | 0.35 ± 0.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-A.; Cheng, P.-H.; Hsu, Y.-J.; Cheng, S.-F.; Lin, M.-H.A.; Huang, C.-C. Effects of Chlorich®EnergyBoost on Enhancing Physical Performance and Anti-Fatigue Properties in Mice. Foods 2024, 13, 2232. https://doi.org/10.3390/foods13142232
Yang S-A, Cheng P-H, Hsu Y-J, Cheng S-F, Lin M-HA, Huang C-C. Effects of Chlorich®EnergyBoost on Enhancing Physical Performance and Anti-Fatigue Properties in Mice. Foods. 2024; 13(14):2232. https://doi.org/10.3390/foods13142232
Chicago/Turabian StyleYang, Shih-An, Po-Hsun Cheng, Yi-Ju Hsu, Shu-Feng Cheng, Meng-Hsueh Amanda Lin, and Chi-Chang Huang. 2024. "Effects of Chlorich®EnergyBoost on Enhancing Physical Performance and Anti-Fatigue Properties in Mice" Foods 13, no. 14: 2232. https://doi.org/10.3390/foods13142232






