Development and Validation of a High-Performance Liquid Chromatography Diode Array Detector Method to Measure Seven Neonicotinoids in Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Analytical Standards
2.3. Sample Preparation
2.3.1. Sample Extraction and Cleanup with the QuEChERS Method
2.3.2. Sample Extraction and Cleanup with STRATA X PRO Cartridges
2.4. HPLC (High-Performance Liquid Chromatography)’s Parameters
2.5. Method Validation
2.5.1. Experimental Designs
2.5.2. Calibration Standards
2.6. Statistical Analysis
3. Results
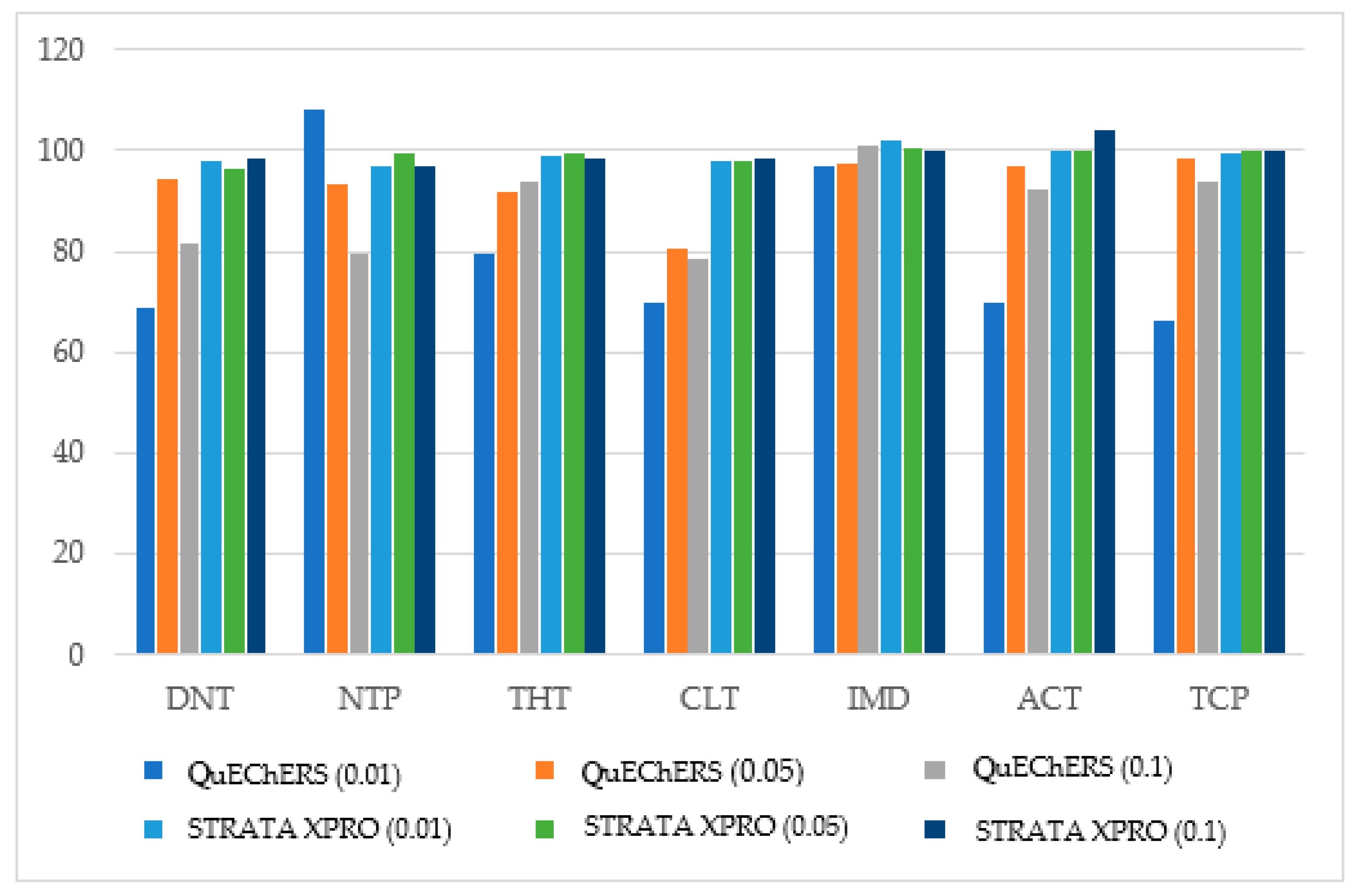
3.1. Cleanup Procedure Optimization
3.2. Method Validation
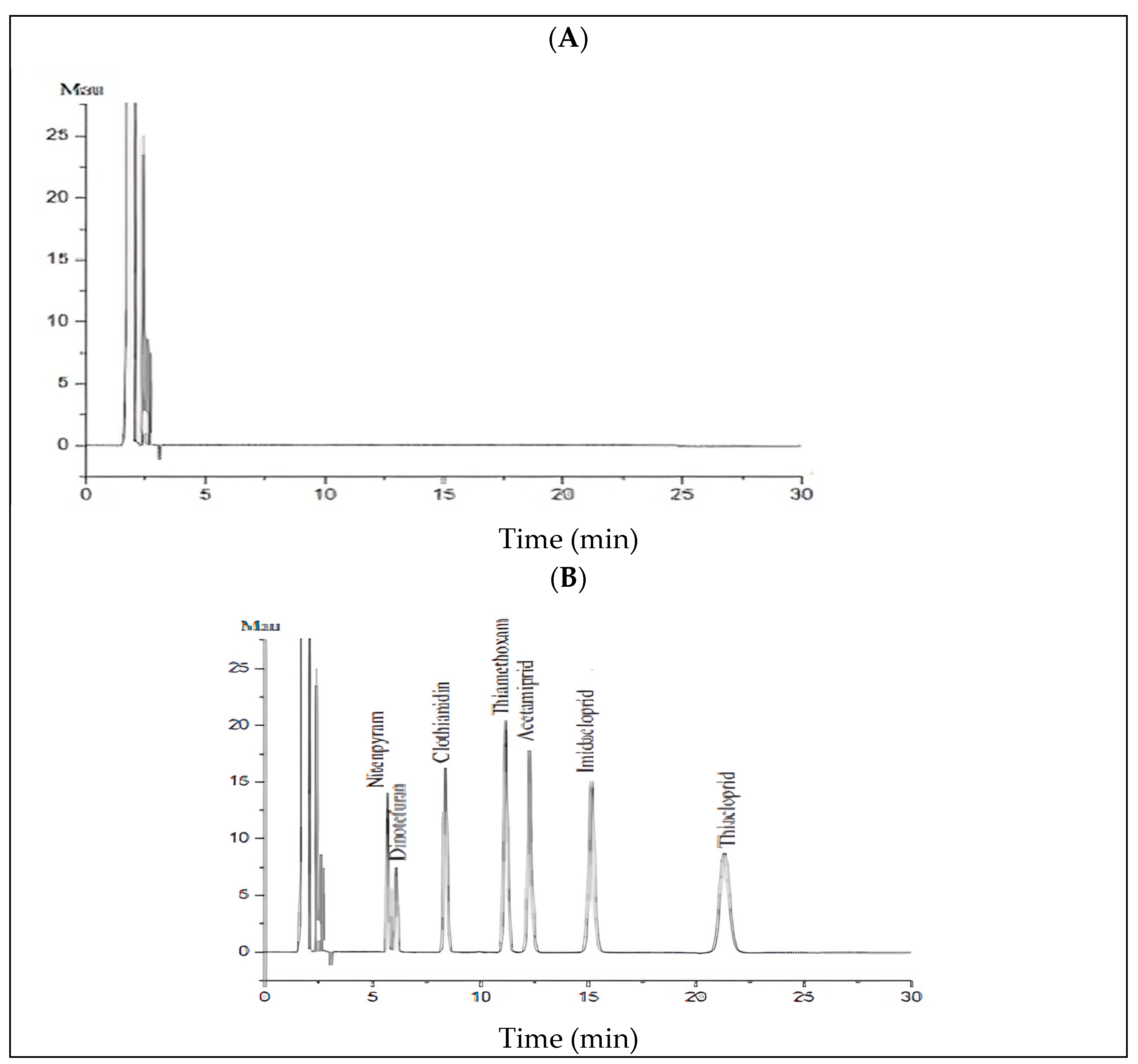
3.2.1. Selectivity
3.2.2. Linearity
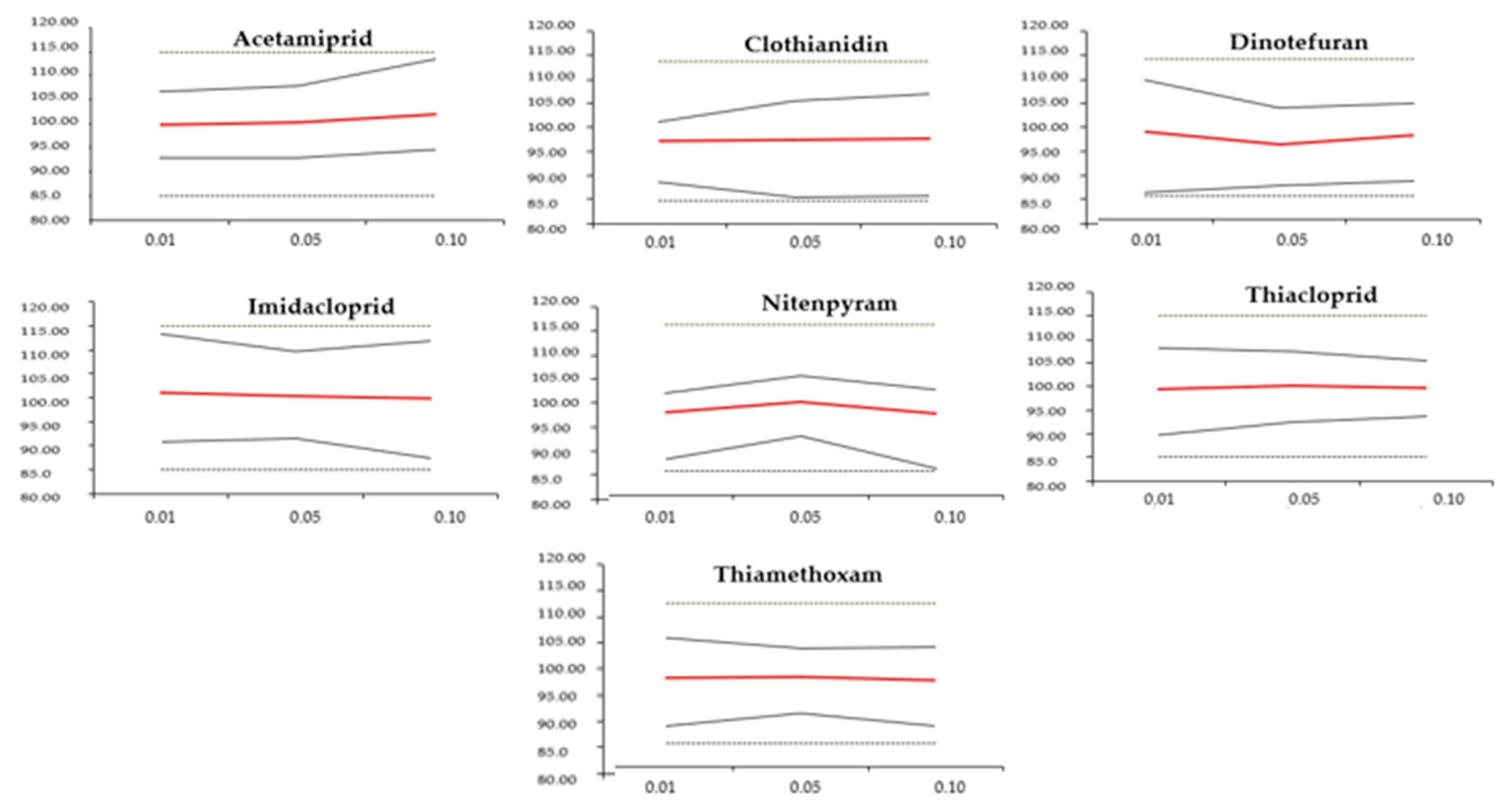
3.2.3. Trueness, Precision, and Accuracy Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Report from the Commission to the European Parliament and the Council on the Implementation of Regulation (EC) No 1185/2009 of the European Parliament and of the Council of 25 November 2009 Concerning Statistics on Pesticides. Available online: https://eur-lex.europa.eu/eli/reg/2009/1185/oj (accessed on 8 December 2021).
- Insetticide Market Straits Research. Available online: https://straitsresearch.com/report/insecticides-market#:~:text=Market%20Overview,which%20it%20is%20carried%20out (accessed on 8 December 2021).
- Zhang, C.; Wang, X.; Kaur, P.; Gan, J. A critical review on the accumulation of neonicotinoid insecticides in pollen and nectar: Influencing factors and implications for pollinator exposure. Sci. Total Environ. 2023, 899, 165670. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, W.; Wang, S.; Shi, L.; Li, X.; Ma, Z.; Zhang, Q.; Li, H. Determination of eight neonicotinoid insecticides in Chinese cabbage using a modified QuEChERS method combined with ultra performance liquid chromatography-tandem mass spectrometry. Food Chem. 2022, 387, 132935. [Google Scholar] [CrossRef] [PubMed]
- Shareefdeen, Z.; Elkamel, A. Toxic and Environmental Effects of Neonicotinoid Based Insecticides. Appl. Sci. 2024, 14, 3310. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Lv, B.; Liu, Z.; Han, J.; Li, J.; Zhao, Y.; Wu, Y. Dietary exposure to neonicotinoid insecticides and health risks in the Chinese general population through two consecutive total diet studies. Environ. Int. 2020, 135, 105399. [Google Scholar] [CrossRef] [PubMed]
- Costas-Ferreira, C.; Faro, L.R.F. Neurotoxic Effects of Neonicotinoids on Mammals: What Is There beyond the Activation of Nicotinic Acetylcholine Receptors?—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, W.; Zhang, L.; Cao, L.; Ling, J.; Liao, K.; Shen, G.; Du, W.; Chen, K.; Zhao, M.; et al. First Evidence of Neonicotinoid Insecticides in Human Bile and Associated Hepatotoxicity Risk. J. Hazard. Mater. 2023, 446, 130715. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, S. Human exposure to neonicotinoids and the associated health risks: A review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Q.-K.; Xu, Z.-R.; Xu, C.-L.; Zhao, S.-C.; Luo, Y.-S.; Sun, X.; Qi, Z.-Q.; Wang, H.-L. Thiamethoxam exposure induces endoplasmic reticulum stress and affects ovarian function and oocyte development in mice. J. Agric. Food Chem. 2021, 69, 1942–1952. [Google Scholar] [CrossRef]
- Zuščíková, L.; Bažány, D.; Greifová, H.; Knížatová, N.; Kováčik, A.; Lukáč, N.; Jambor, T. Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review. Toxics 2023, 11, 598. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Wang, Y.; Lu, Q.; Ruan, H.; Luo, J.; Yang, M. A Comprehensive Review on the Pretreatment and Detection Methods of Neonicotinoid Insecticides in Food and Environmental Samples. Food Chem. X 2022, 15, 100375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Chen, W.-J.; Wu, S.; Lei, Q.; Zhou, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Environmental occurrence, toxicity concerns, and biodegradation of neonicotinoid insecticides. Environ. Res. 2022, 218, 114953. [Google Scholar] [CrossRef] [PubMed]
- Erminia Schiano, M.; Sodano, F.; Cassiano, C.; Magli, E.; Seccia, S.; Grazia Rimoli, M.; Albrizio, S. Monitoring of seven pesticide residues by LC-MS/MS in extra virgin olive oil samples and risk assessment for consumers. Food Chem. 2024, 442, 138498. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Wang, Y.; Chang, Y.; Liu, P.; Chen, Y.; Liu, Y.; Zhu, G.; Guo, Y. Trace Immunosensing of Multiple Neonicotinoid Insecticides by a Novel Broad-Specific Antibody Obtained from a Rational Screening Strategy. Biosensors 2022, 12, 716. [Google Scholar] [CrossRef] [PubMed]
- Chatzaki, V.; Montoro, M.; El-Rashid, R.; Jensen, A.B.; Lecocq, A. A New Approach for Detecting Sublethal Effects of Neonicotinoids on Bumblebees Using Optical Sensor Technology. Insects 2023, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Guo, J.; Chen, S.; Wang, Y. Electrochemical sensing mechanisms of neonicotinoid pesticides and recent progress in utilizing functional materials for electrochemical detection platforms. Talanta 2024, 273, 125937. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Belda, M.; Garrido, I.; Campillo, N.; Viñas, P.; Hellín, P.; Flores, P.; Fenoll, J. Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid insecticides in fruits and vegetables by liquid chromatography and mass spectrometry after dispersive liquid−liquid microextraction. Food Chem. 2016, 202, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Speltini, A. Neonicotinoids: An overview of the newest sample preparation procedures of environmental, biological and food matrices. Adv. Sample Prep. 2023, 8, 100094. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, Y.; Zhao, Q.; Xiang, W.; Jiao, B.; Su, X. MIL-101(Cr) based d-SPE/UPLC-MS/MS for determination of neonicotinoid insecticides in beverages. Microchem. J. 2022, 175, 10791. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Li, H.; Peng, F.; Qiu, B.; Yang, Z. Development of QuEChERS-DLLME method for determination of neonicotinoid pesticide residues in grains by liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 331, 127190. [Google Scholar] [CrossRef]
- Kachangoon, R.; Vichapong, J.; Santaladchaiyakit, Y.; Burakham, R.; Srijaranai, S. An Eco-Friendly Hydrophobic Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction for the Determination of Neonicotinoid Insecticide Residues in Water, Soil and Egg Yolk Samples. Molecules 2020, 25, 2785. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Wang, S.; Bai, B.; Wu, N.; Ye, T.; Xu, F.; Kong, C. Zeolite H-Beta as a Dispersive Solid-Phase Extraction Sorbent for the Determination of Eight Neonicotinoid Insecticides Using Ultra-High-Performance Liquid Chromatography—Tandem Mass Spectrometry. Appl. Sci. 2022, 12, 4316. [Google Scholar] [CrossRef]
- Lu, J.; Wang, R.; Luan, J.; Li, Y.; He, X.; Chen, L.; Zhang, Y. A functionalized magnetic covalent organic framework for sensitive determination of trace neonicotinoid residues in vegetable samples. J. Chromatogr. A 2020, 1618, 460898. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Sousa, J.; Oliveira do Nascimento, H.; Hiago de Oliveira Gomes, H.; Ferreira do Nascimento, R. Pesticide residues in groundwater and surface water: Recent advances in solid-phase extraction and solid-phase microextraction sample preparation methods for multiclass analysis by gas chromatography-mass spectrometry. Microchem. J. 2021, 168, 106359. [Google Scholar] [CrossRef]
- Danek, M.; Fang, X.; Tang, J.; Plonka, J.; Barchanska, H. Simultaneous determination of pesticides and their degradation products in potatoes by MSPD-LC-MS/MS. J. Food Compos. Anal. 2021, 104, 104129. [Google Scholar] [CrossRef]
- Yang, B.; Tu, M.; Wang, S.; Ma, W.; Zhu, Y.; Ma, Z.; Li, X. Neonicotinoid insecticides in plant-derived Foodstuffs: A review of separation and determination methods based on liquid chromatography. Food Chem. 2024, 444, 138695. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M. Validation of analytical methods based on accuracy profiles. J. Chromatogr. A 2007, 1158, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Nguyen-Huu, J.J.; Boulanger, B.; Chapuzet, E.; Cohen, N.; Compagnon, P.A.; Dewé, W.; Feinberg, M.; Laurentie, M.; Mercier, N.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal: Part IV. Examples of application. J. Pharm. Biomed. Anal. 2008, 48, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Vial, J.; Jardy, A. Experimental Comparison of the Different Approaches to Estimate LOD and LOQ of an HPLC Method. Anal. Chem. 1999, 71, 2672–2677. [Google Scholar] [CrossRef]
- Dini, I.; Seccia, S.; Senatore, A.; Coppola, D.; Morelli, E. Development and Validation of an Analytical Method for Total Polyphenols Quantification in Extra Virgin Olive Oils. Food Anal. Methods 2019, 13, 457–464. [Google Scholar] [CrossRef]
- Loskutov, I.G.; Khlestkina, E.K. Wheat, Barley, and Oat Breeding for Health Benefit Components in Grain. Plants 2021, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, L.M.; Meyer, T.R. Determination of imidacloprid and triadimefon in white pine by gas chromatography/mass spectrometry. J. Agric. Food Chem. 1998, 46, 3133–3138. [Google Scholar] [CrossRef]
- Ko, A.-Y.; Rahman, M.M.; Abd El-Aty, A.M.; Jang, J.; Park, J.-H.; Cho, S.-K.; Shim, J.-H. Development of a simple extraction and oxidation procedure for the residue analysis of imidacloprid and its metabolites in lettuce using gas chromatography. Food Chem. 2014, 148, 402–409. [Google Scholar] [CrossRef]
- Wang, J.; Chow, W.; Cheung, W. Application of a tandem mass spectrometer and core–shell particle column for the determination of 151 pesticides in grains. JAFC 2011, 59, 8589–8608. [Google Scholar] [CrossRef] [PubMed]
- Gritti, F.; Leonardis, I.; Shock, D.; Stevenson, P.; Shalliker, A.; Guiochon, G. Performance of columns packed with the new shell particles, Kinetex-C18. J. Chrom. A 2010, 1217, 1589–1603. [Google Scholar] [CrossRef]
- Stoev, G.; Stoyanov, A. Comparison of the Reliability of the Identification with Diode Array Detector and Mass Spectrometry. J. Chromatogr. A 2007, 1150, 302–311. [Google Scholar] [CrossRef]
| NEOs | λmax (nm) | Rt | UV Spectrum |
|---|---|---|---|
Acetamiprid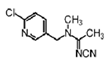 | 245 | 12.5 |  |
Imidacloprid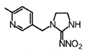 | 270 | 15.1 | 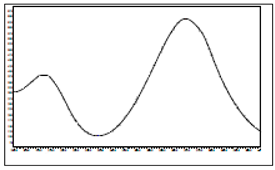 |
Nitenpyram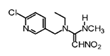 | 270 | 6.2 |  |
Thiacloprid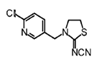 | 245 | 22.0 | 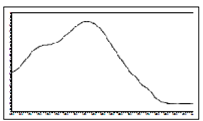 |
Clothianidin | 270 | 7.9 |  |
Thiamethoxam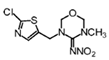 | 255 | 11.3 | 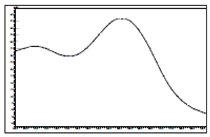 |
Dinotefuran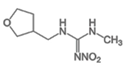 | 270 | 6.4 |  |
| NEOs | Slope | Intercept | r2 | LOQ (mg/kg) | LOD (mg/kg) | |
|---|---|---|---|---|---|---|
| DNT | Solvent Matrix | 153.24 155.12 | 0.68 0.47 | 0.9996 0.9991 | 0.01 | 0.003 |
| NTP | Solvent Matrix | 201.76 203.95 | 0.20 0.04 | 0.9997 0.9990 | 0.01 | 0.003 |
| THT | Solvent Matrix | 148.82 149.94 | 0.20 0.04 | 0.9998 0.9999 | 0.01 | 0.003 |
| CLT | Solvent Matrix | 171.43 169.82 | −0.44 −0.03 | 0.9999 0.9998 | 0.01 | 0.003 |
| IMD | Solvent Matrix | 191.57 187.05 | 0.59 0.93 | 0.9992 0.9992 | 0.01 | 0.003 |
| ACT | Solvent Matrix | 214.56 218.85 | 0.68 0.47 | 0.9995 0.9990 | 0.01 | 0.003 |
| TCP | Solvent Matrix | 176.86 175.50 | −0.44 −0.03 | 0.9997 0.9993 | 0.05 | 0.02 |
| NEOs | Intra-Day | Inter-Day | Relative Bias | β-Expectation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSD (%) | RSD (%) | (%) | Tolerance Limit (%) | |||||||||
| mg/kg | mg/kg | mg/kg | mg/kg | |||||||||
| 0.01 | 0.05 | 0.1 | 0.01 | 0.05 | 0.1 | 0.01 | 0.05 | 0.1 | 0.01 | 0.05 | 0.1 | |
| DNT | 2.1 | 2.0 | 1.3 | 1.7 | 1.9 | 1.9 | −1.0 | −3.8 | −1.7 | [11.2; −13.2] [8.1; −15.7] [7.0; −10.4] | ||
| NTP | 1.2 | 1.3 | 1.4 | 0.3 | 0.5 | 0.6 | −2.9 | −0.8 | 3.2 | [3.9; −9.7] [5.3; −6.9] [4.8; 11.2] | ||
| THT | 1.6 | 1.1 | 1.2 | 0.5 | 1.2 | 1.1 | 1.0 | 0.8 | 1.4 | [8.4; −10.4] [6.2; −7.8] [7.0; −9.8] | ||
| CLT | 0.9 | 1.4 | 1.5 | 0.6 | 1.9 | 2.2 | 2.3 | 2.0 | 1.8 | [4.2; −8.8] [8.3; −12.3] [9.7; 13.3] | ||
| IMD | 1.5 | 1.2 | 1.7 | 2.1 | 1.8 | 2.0 | 1.0 | 0.3 | 0.2 | [12.2; −10.2 ] [9.4; −8.8] [12.0; −12.4] | ||
| ACT | 0.6 | 0.9 | 1.2 | 2.2 | 1.6 | 1.9 | 0.1 | 0.2 | 2.0 | [6.7; −6.9] [7.6; −7.2] [11.5; −7.5] | ||
| TCP | 1.3 | 0.7 | 0.9 | 1.4 | 0.9 | 0.8 | 0.5 | 0.1 | 0.2 | [8.8; −9.8] [7.4; −7.6] [5.8; −6.2] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seccia, S.; Albrizio, S.; Morelli, E.; Dini, I. Development and Validation of a High-Performance Liquid Chromatography Diode Array Detector Method to Measure Seven Neonicotinoids in Wheat. Foods 2024, 13, 2235. https://doi.org/10.3390/foods13142235
Seccia S, Albrizio S, Morelli E, Dini I. Development and Validation of a High-Performance Liquid Chromatography Diode Array Detector Method to Measure Seven Neonicotinoids in Wheat. Foods. 2024; 13(14):2235. https://doi.org/10.3390/foods13142235
Chicago/Turabian StyleSeccia, Serenella, Stefania Albrizio, Elena Morelli, and Irene Dini. 2024. "Development and Validation of a High-Performance Liquid Chromatography Diode Array Detector Method to Measure Seven Neonicotinoids in Wheat" Foods 13, no. 14: 2235. https://doi.org/10.3390/foods13142235
APA StyleSeccia, S., Albrizio, S., Morelli, E., & Dini, I. (2024). Development and Validation of a High-Performance Liquid Chromatography Diode Array Detector Method to Measure Seven Neonicotinoids in Wheat. Foods, 13(14), 2235. https://doi.org/10.3390/foods13142235







