Microbial Decontamination of Cuminum cyminum Seeds Using “Intensification of Vaporization by Decompression to the Vacuum”: Effect on Color Parameters and Essential Oil Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Microbial Inoculation
2.3. IVDV Microbial Inactivation
2.4. Bacterial Enumeration
2.5. Extraction of Oils and Their Analyses
2.5.1. Extraction of Essential Oils
2.5.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.6. Color Determination
2.7. Experimental Design
2.8. Statistical Analysis
3. Results and Discussion
3.1. Response Surface Design
3.2. Experimental Modeling
3.3. Effect of Steam Pressure and Processing Time on Microbiological Load
3.4. Effect of Steam Pressure and Processing Time on Color
3.5. Optimization of the Treatment
3.6. GC-MS Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Shi, H.; Ho, C. Effects of rosemary extracts and major constituents on lipid oxidation and soybean lipoxygenase activity. J. Am. Oil Chem. Soc. 1992, 69, 999–1002. [Google Scholar] [CrossRef]
- Trigui, M.; Ben Hsouna, A.; Tounsi, S.; Jaoua, S. Chemical composition and evaluation of antioxidant and antimicrobial activities of Tunisian Thymelaea hirsuta with special reference to its mode of action. Ind. Crops Prod. 2013, 41, 150–157. [Google Scholar] [CrossRef]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arab. J. Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical Composition of Cumin Seeds, and Biorefining Study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Saikia, D.; Parasar, D.P.; Loying, S.; Telenga, K. Phytochemical Analysis and Antibacterial Activity of Methanolic and Aqueous Extracts of Cumin (Cuminum cyminum) Seeds. 2021. Available online: www.botanyjournals.com (accessed on 18 August 2023).
- Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, D. Bioactive Properties of Extracts from Plectranthus barbatus (Coleus forskohlii) Roots Received Using Various Extraction Methods. Molecules 2022, 27, 8986. [Google Scholar] [CrossRef] [PubMed]
- Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28, 5864. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Han, L.R.; Zhang, X.; Feng, J.T. Antifungal Activity and Action Mode of Cuminic Acid from the Seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. Niveum (FON) Causing Fusarium Wilt on Watermelon. Molecules 2017, 22, 2053. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Trigui, M.; Ben Mansour, R.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.-W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Bo, W.; Zhou, Y.; Dang, S.; Wei, J.; Li, X.; Liu, M. Multiple effects of ellagic acid on human colorectal carcinoma cells identified by gene expression profile analysis. Int. J. Oncol. 2017, 50, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Shaker, M.A. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int. J. Nanomed. 2017, 12, 7405–7417. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Tabatabaei, M.J.; Jafari, R.M.; Shemirani, F.; Tavakoli, S.; Mofasseri, M.; Tofighi, Z. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2018, 34, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Deshannavar, U.B.; Ramasamy, M.; Emani, S.; Khalilpoor, N.; Issakhov, A. Study of Extraction Kinetics of Total Polyphenols from Curry Leaves. Int. J. Chem. Eng. 2021, 2021, 9988684. [Google Scholar] [CrossRef]
- Augustynowicz, D.; Lemieszek, M.K.; Strawa, J.W.; Wiater, A.; Tomczyk, M. Anticancer potential of acetone extracts from selected Potentilla species against human colorectal cancer cells. Front. Pharmacol. 2022, 13, 1027315. [Google Scholar] [CrossRef] [PubMed]
- Elviss, N.; Little, C.; Hucklesby, L.; Sagoo, S.; Surman-Lee, S.; De Pinna, E.; Threlfall, E. Microbiological study of fresh herbs from retail premises uncovers an international outbreak of salmonellosis. Int. J. Food Microbiol. 2009, 134, 83–88. [Google Scholar] [CrossRef] [PubMed]
- El Darra, N.; Gambacorta, L.; Solfrizzo, M. Multimycotoxins occurrence in spices and herbs commercialized in Lebanon. Food Control 2018, 95, 63–70. [Google Scholar] [CrossRef]
- Karam, L.; Salloum, T.; El Hage, R.; Hassan, H.; Hassan, H.F. How can packaging, source and food safety management system affect the microbiological quality of spices and dried herbs? The case of a developing country. Int. J. Food Microbiol. 2021, 353, 109295. [Google Scholar] [CrossRef]
- Mathot, A.G.; Postollec, F.; Leguerinel, I. Bacterial spores in spices and dried herbs: The risks for processed food. Compr. Rev. Food Sci. Food Saf. 2020, 20, 840–862. [Google Scholar] [CrossRef]
- Eissa, F.; Zidan, N.E.A.; Sebaei, A.S. Contamination of herbs and spices: A 23-year EU RASFF notifications analysis. J. Food Saf. 2024, 44, e13131. [Google Scholar] [CrossRef]
- RASFF. 2022. Available online: https://food.ec.europa.eu/safety/rasff-food-and-feed-safety-alerts_en (accessed on 5 June 2024).
- Debs, E. Assessment of the Microbiological Quality and Safety of Common Spices and Herbs Sold in Lebanon. J. Food Nutr. Disord. 2013, 2. [Google Scholar] [CrossRef]
- Makhlouf, J.; Carvajal-Campos, A.; Querin, A.; Tadrist, S.; Puel, O.; Lorber, S.; Oswald, I.P.; Hamze, M.; Bailly, J.-D.; Bailly, S. Morphologic, molecular and metabolic characterization of Aspergillus section Flavi in spices marketed in Lebanon. Sci. Rep. 2019, 9, 5263. [Google Scholar] [CrossRef]
- Hassan, H.F.; Koaik, L.; El Khoury, A.; Atoui, A.; El Obeid, T.; Karam, L. Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon. Toxins 2022, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Kartal, M.; Özek, T.; Aslan, S.; Hüsnü, K. Composition of essential oil of Cuminum cyminum L. according to harvesting times. Turk. J. Pharm. Sci. 2007, 4, 25–29. [Google Scholar]
- Johri, R. Cuminum cyminum and Carum carvi: An update. Pharmacogn. Rev. 2011, 5, 63–72. [Google Scholar] [CrossRef]
- Rebey, I.B.; Zakhama, N.; Karoui, I.J.; Marzouk, B. Polyphenol Composition and Antioxidant Activity of Cumin (Cuminum cyminum L.) Seed Extract Under Drought. J. Food Sci. 2012, 77, C734–C739. [Google Scholar] [CrossRef]
- Ravi, R.; Prakash, M.; Bhat, K.K. Characterization of aroma active compounds of cumin (Cuminum cyminum L.) by GC-MS, E-Nose, and sensory techniques. Int. J. Food Prop. 2013, 16, 1048–1058. [Google Scholar] [CrossRef]
- Alinian, S.; Razmjoo, J.; Zeinali, H. Flavonoids, anthocynins, phenolics and essential oil produced in cumin (Cuminum cyminum L.) accessions under different irrigation regimes. Ind. Crops Prod. 2016, 81, 49–55. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Sumrra, S.H. Cumin. In Medicinal Plants of South Asia: Novel Sources for Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–178. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Brebu, M.; Skalicka-Woźniak, K.; Luca, S.V. Phytochemical characterization and evaluation of the antioxidant and anti-enzymatic activity of five common spices: Focus on their essential oils and spent material extractives. Plants 2021, 10, 2692. [Google Scholar] [CrossRef]
- Alqethami, A.; Aldhebiani, A.Y. Medicinal plants used in Jeddah, Saudi Arabia: Phytochemical screening. Saudi J. Biol. Sci. 2020, 28, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.E.; Moberg, K.; Amin, K.N.; Wright, M.; Newkirk, J.J.; Ponder, M.A.; Acuff, G.R.; Dickson, J.S. Processes to Preserve Spice and Herb Quality and Sensory Integrity During Pathogen Inactivation. J. Food Sci. 2017, 82, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Sarkar, P.K. Microbiological quality of some retail spices in India. Food Res. Int. 2003, 36, 469–474. [Google Scholar] [CrossRef]
- Tateo, F.; Bononi, M. Determination of ethylene chlorohydrin as marker of spices fumigation with ethylene oxide. J. Food Compos. Anal. 2006, 19, 83–87. [Google Scholar] [CrossRef]
- ASTA. 2024. Available online: https://www.astaspice.org/advocacy-regulatory-issues/public-policy-2/processing-treatments/ (accessed on 5 June 2024).
- Schottroff, F.; Lasarus, T.; Stupak, M.; Hajslova, J.; Fauster, T.; Jäger, H. Decontamination of herbs and spices by gamma irradiation and low-energy electron beam treatments and influence on product characteristics upon storage. J. Radiat. Res. Appl. Sci. 2021, 14, 380–395. [Google Scholar] [CrossRef]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World Market Development and Consumer Acceptance of Irradiation Technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Raul, F.; Gossé, F.; Delincé, H.; Hartwig, A.; Marchioni, E.; Miesch, M.; Werner, D.; Burnouf, D. Food-borne radiolytic compounds (2-alkylcyclobutanones) may promote experimental colon carcinogenesis. Nutr. Cancer 2002, 44, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.H.; Deotale, S.; Rawson, A.; Patras, A. Thermal Processing of Herbs and Spices. In Herbs, Spices and Medicinal Plants; Wiley: Hoboken, NJ, USA, 2020; pp. 1–21. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D.; Ulca, P. Efficacy of gaseous ozone against Salmonella and microbial population on dried oregano. Int. J. Food Microbiol. 2013, 165, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, I.-H.; Min, S.C. Microbial decontamination of vegetables and spices using cold plasma treatments. Korean J. Food Sci. Technol. 2013, 45, 735–741. [Google Scholar] [CrossRef]
- Bang, I.H.; Kim, Y.E.; Lee, S.Y.; Min, S.C. Microbial decontamination of black peppercorns by simultaneous treatment with cold plasma and ultraviolet C. Innov. Food Sci. Emerg. Technol. 2020, 63, 102392. [Google Scholar] [CrossRef]
- Darvish, H.; Ramezan, Y.; Khani, M.R.; Kamkari, A. Effect of low-pressure cold plasma processing on decontamination and quality attributes of Saffron (Crocus sativus L.). Food Sci. Nutr. 2022, 10, 2082–2090. [Google Scholar] [CrossRef]
- Staack, N.; Ahrné, L.; Borch, E.; Knorr, D. Effect of infrared heating on quality and microbial decontamination in paprika powder. J. Food Eng. 2008, 86, 17–24. [Google Scholar] [CrossRef]
- Ferriccioni, N.; Mateucci, R.; Zangrando, A.; Santana, S.; Campos, C.A. Effect of decontamination treatment on the quality of dehydrated thyme, coriander, and mustard. Food Sci. Technol. Int. 2019, 25, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, B.F. An innovative microwave process for microbial decontamination of spices and herbs. Afr. J. Microbiol. Res. 2013, 7, 636–645. [Google Scholar]
- El Darra, N.; Xie, F.; Kamble, P.; Khan, Z.; Watson, I. Decontamination of Escherichia coli on dried onion flakes and black pepper using Infra-red, ultraviolet and ozone hurdle technologies. Heliyon 2021, 7, e07259. [Google Scholar] [CrossRef]
- Mrad, R.; Assy, P.; Maroun, R.G.; Louka, N. Multiple optimization of polyphenols content, texture and color of roasted chickpea pre-treated by IVDV using response surface methodology. LWT-Food Sci. Technol. 2015, 62, 532–540. [Google Scholar] [CrossRef]
- Abi-Khattar, A.-M.; Rajha, H.N.; Abdel-Massih, R.M.; Habchi, R.; Maroun, R.G.; Debs, E.; Louka, N. “Intensification of Vaporization by Decompression to the Vacuum” (IVDV), a novel technology applied as a pretreatment to improve polyphenols extraction from olive leaves. Food Chem. 2020, 342, 128236. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66912.html (accessed on 13 July 2024).
- Mrad, R.; Rouphael, M.; Maroun, R.G.; Louka, N. Effect of expansion by “Intensification of Vaporization by Decompression to the Vacuum” (IVDV) on polyphenol content, expansion ratio, texture and color changes of Australian chickpea. LWT-Food Sci. Technol. 2014, 59, 874–882. [Google Scholar] [CrossRef]
- Labaky, P.; Dahdouh, L.; Grosmaire, L.; Rajha, H.N.; Ricci, J.; Delpech, C.; El Khoury, A.; Debs, E.; Wisniewski, C.; Louka, N.; et al. Improving dried mango physicochemical properties using conventional hot-air drying coupled with a novel technology “intensification of vaporization by decompression to the vacuum”. Innov. Food Sci. Emerg. Technol. 2024, 95, 103739. [Google Scholar] [CrossRef]
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-chloro-3-indolyl beta-D-Glucuronide. ISO: Geneva, Switzerland, 2001. Available online: https://www.iso.org/standard/29824.html (accessed on 13 July 2024).
- Mokrzycki Wojciech, M.T. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. Available online: https://www.researchgate.net/publication/236023905_Color_difference_Delta_E_-_A_survey (accessed on 23 May 2024).
- Farah, D.M.H.; Zaibunnisa, A.H. Optimization of cocoa beans roasting process using Response Surface Methodology based on concentration of pyrazine and acrylamide. Int. Food Res. J. 2012, 19, 1355–1359. [Google Scholar]
- Joudi-Sarighayeh, F.; Abbaspour-Gilandeh, Y.; Kaveh, M.; Szymanek, M.; Kulig, R. Response Surface Methodology Approach for Predicting Convective/Infrared Drying, Quality, Bioactive and Vitamin C Characteristics of Pumpkin Slices. Foods 2023, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Setyani, W.; Murwanti, R.; Sulaiman, T.N.S.; Hertiani, T. Application of Response Surface Methodology (RSM) for the Optimization of Ultrasound-Assisted Extraction (UAE) of Moringa oleifera: Extraction Yield, Content of Bioactive Compounds, and Biological Effects In Vitro. Plants 2023, 12, 2455. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, J.J.; Wu, J.; Acuff, J.C.; Caver, C.B.; Mallikarjunan, K.; Wiersema, B.D.; Williams, R.C.; Ponder, M.A. Inactivation of Salmonella enterica and Surrogate Enterococcus faecium on Whole Black Peppercorns and Cumin Seeds Using Vacuum Steam Pasteurization. Front. Sustain. Food Syst. 2018, 2, 48. [Google Scholar] [CrossRef]
- Rihawy, M.; Bakraji, E.; Odeh, A. PIXE and GC–MS investigation for the determination of the chemical composition of Syrian Cuminum cyminum L. Appl. Radiat. Isot. 2014, 86, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Rezzoug, S.A.; Baghdadi, M.W.; Louka, N.; Boutekedjiret, C.; Allaf, K. Study of a new extraction process: Controlled instantaneous decompression. Application to the extraction of essential oil from rosemary leaves. Flavour Fragr. J. 1998, 13, 251–258. [Google Scholar] [CrossRef]
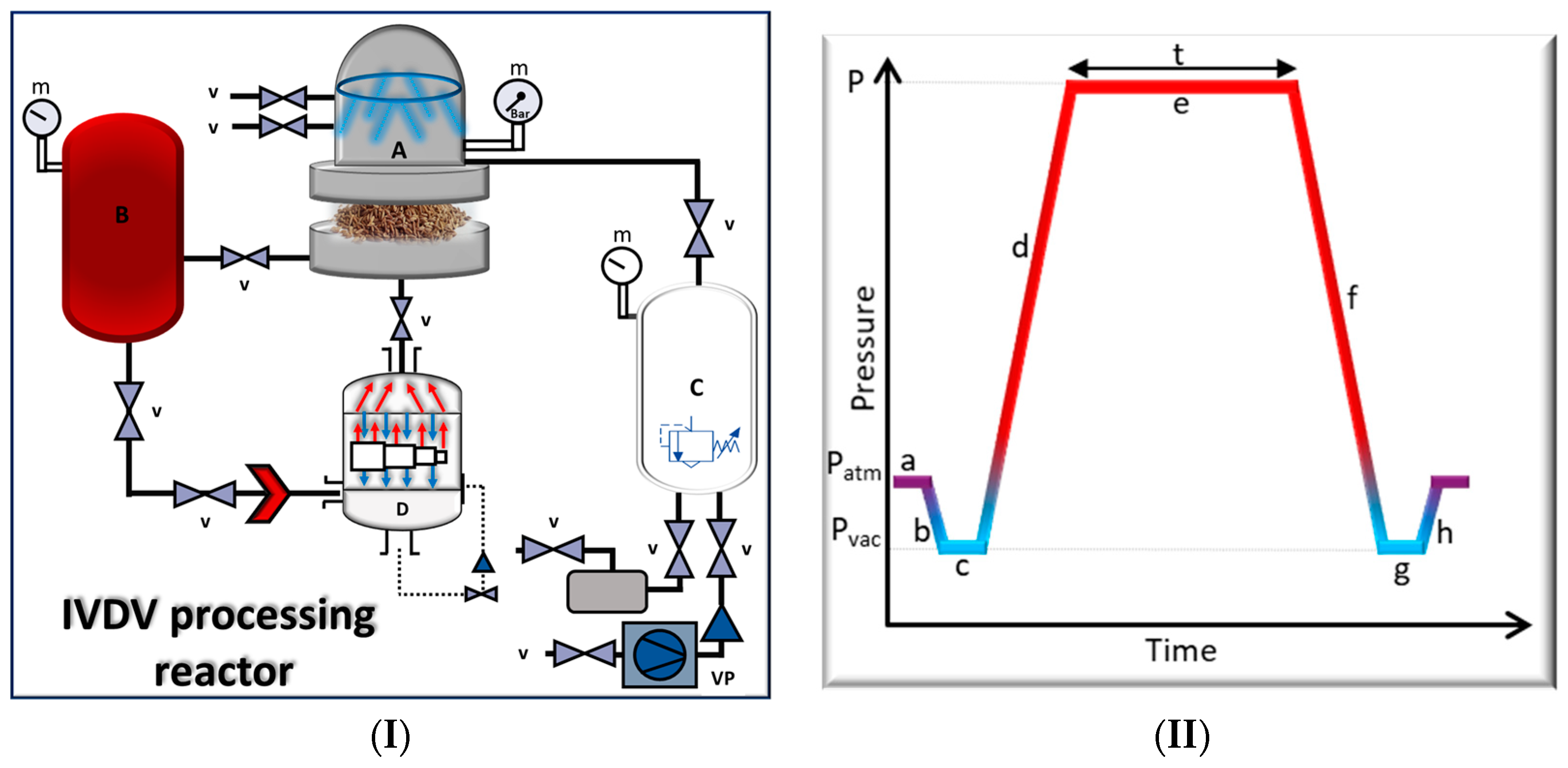
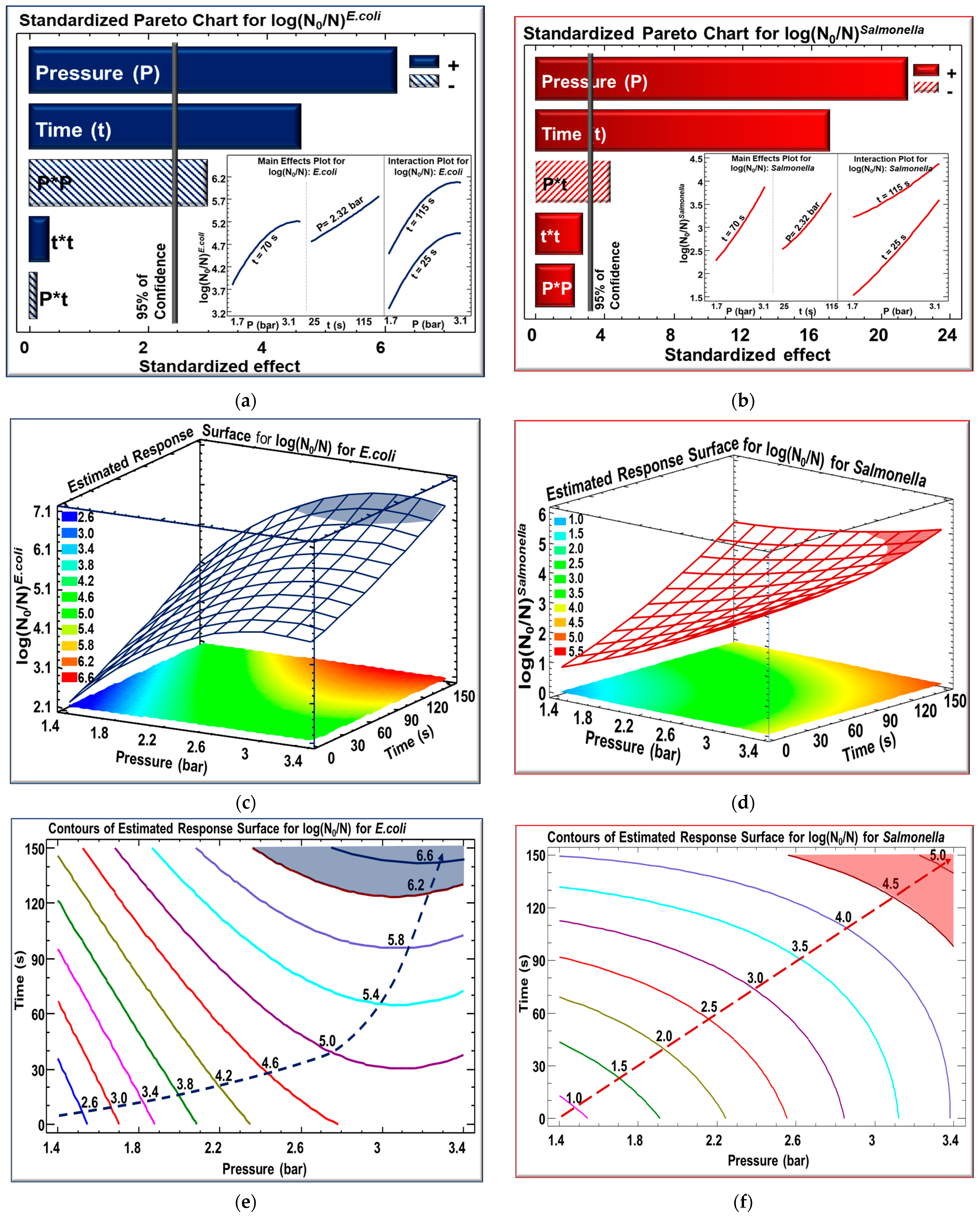


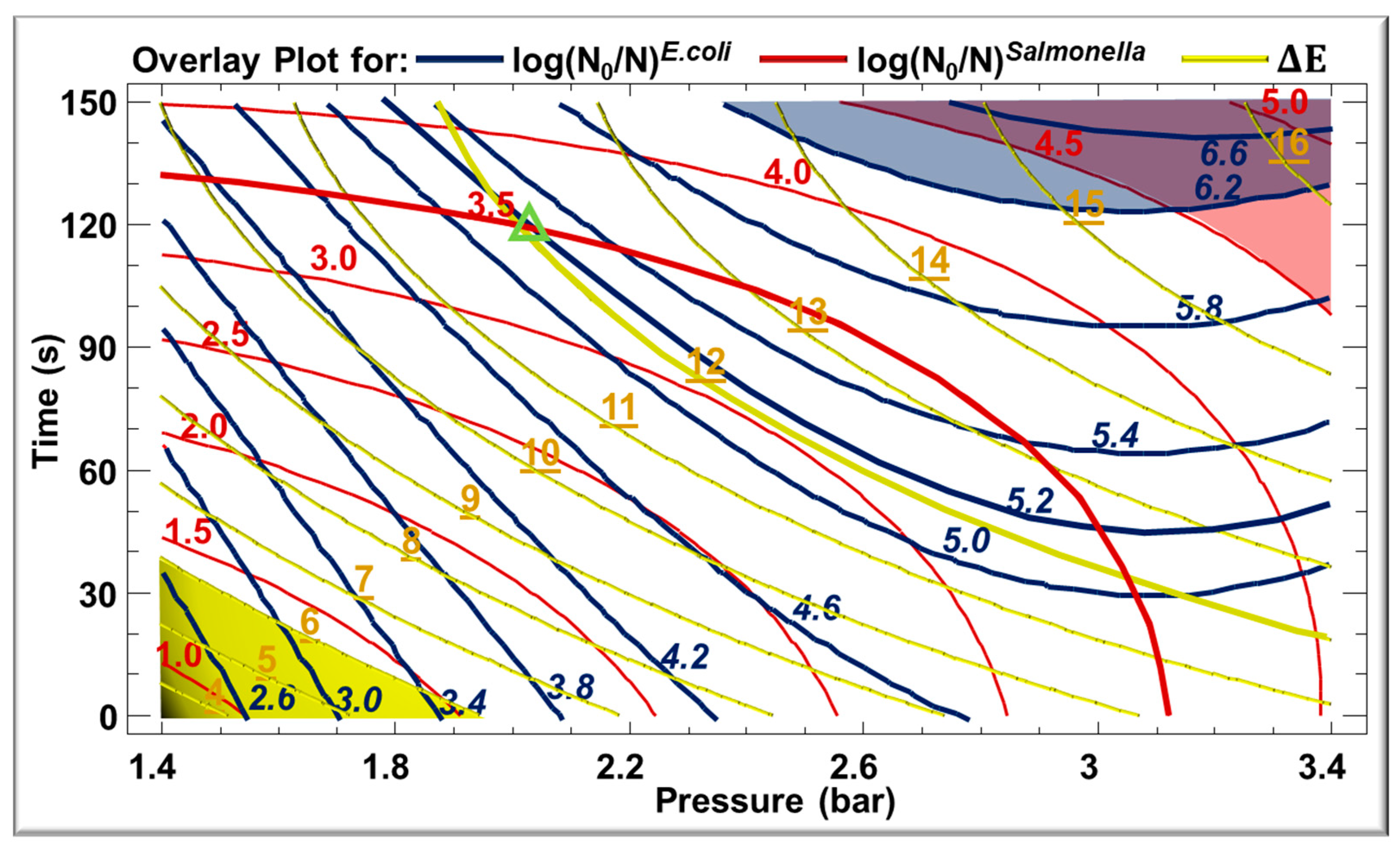
| Variables | Symbol | Coded Variable Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Steam pressure (bar) | P(T:°C) | 1.48 (111) | 1.7 (115) | 2.32 (125) | 3.13 (135) | 3.5 (139) |
| Processing time (s) | t | 2.5 | 25 | 70 | 115 | 133.45 |
| IVDV Parameters | Microbial Load | Colorimetric Response Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Run | P (bar) | t (s) | Log Reduction E. coli | Log Reduction Salmonella Typhimurium | L* | a* | b* | ΔE |
| Untreated | - | - | 92.00 | 6.77 | 43.01 | |||
| 1 | 1.7 | 25 | 0.49 | 0.10 | 88.01 | 7.98 | 48.56 | 6.94 |
| 2 | 3.13 | 25 | 1.92 | 2.18 | 83.08 | 9.67 | 50.38 | 11.93 |
| 3 | 1.7 | 115 | 1.13 | 1.30 | 85.95 | 8.91 | 49.88 | 9.40 |
| 4 | 3.13 | 115 | 2.61 | 2.60 | 80.23 | 10.54 | 50.14 | 14.27 |
| 5 | 1.48 | 70 | 1.27 | 1.25 | 85.89 | 8.32 | 48.67 | 8.47 |
| 6 | 3.5 | 70 | 2.57 | 2.50 | 80.16 | 10.68 | 51.54 | 15.11 |
| 7 | 2.32 | 2.5 | 1.00 | 0.96 | 87.10 | 7.81 | 48.18 | 7.20 |
| 8 | 2.32 | 133.45 | 3.40 | 3.42 | 80.69 | 10.13 | 51.93 | 14.79 |
| 9 | 2.32 | 70 | 2.05 | 1.70 | 84.19 | 9.45 | 51.71 | 11.99 |
| 10 | 2.32 | 70 | 1.48 | 1.48 | 86.07 | 8.43 | 48.62 | 8.33 |
| 11 | 2.32 | 70 | 1.79 | 1.70 | 83.85 | 9.23 | 50.47 | 11.32 |
| 12 | 2.32 | 70 | 2.06 | 2.25 | 82.50 | 8.84 | 48.43 | 11.13 |
| logN0/NE. Coli | =−3.6 + 5.34 × P + 0.012 × t − 0.86 × P2 |
| logN0/NSalmonella | =−0.58 + 0.75 × P + 0.023 × t − 0.0069 × P × t |
| ΔE | =−5.1 + 7.1 × P + 0.08 × t |
| Parameters | Optimum Conditions | ||
|---|---|---|---|
| logN0/NE. coli | logN0/NSalmonella | ΔE | |
| Pressure (bar) | 3.3 | 3.5 | 1.48 |
| Time (s) | 133.5 | 133.45 | 2.5 |
| logN0/NSalmonella | - | 5.1 | - |
| logN0/NE. coli | 6.35 | - | - |
| ΔE | - | - | 4.1 |
| R-squared (R2) | 91.3 | 89.6 | 95.2 |
| Area | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | RT (min) | Untreated | 1 | 2 | 3 | 4 | 5 | 6 |
| (+)-4-carene | 5.2 | 10.1 | 13.8 | 17.8 | 17.3 | 16.3 | 13.3 | 25.8 |
| P-cymene | 5.4 | 37.4 | 33.7 | 42.4 | 41.7 | 36 | 29.2 | 62.8 |
| D-limonene | 5.5 | - | 2.6 | 3.9 | 3.89 | 2.97 | 3.27 | - |
| y-terpinene | 6.37 | 41.5 | 73.2 | 114 | 106 | 83.1 | 68.5 | 138 |
| Cuminaldehyde | 13 | 787 | 573 | 656 | 652 | 753 | 882 | 1260 |
| Phellandral | 14 | 17.4 | 21.3 | 24.3 | 9.4 | 7.07 | 7.57 | 38.8 |
| Cumenol | 15 | 45.7 | 25.5 | 32.7 | 18.5 | 26.5 | 33.7 | 78.9 |
| Total | 939 | 744 | 881 | 849 | 925 | 1030 | 1600 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tannir, H.; Debs, E.; Mansour, G.; Neugart, S.; El Hage, R.; Khalil, M.I.; El Darra, N.; Louka, N. Microbial Decontamination of Cuminum cyminum Seeds Using “Intensification of Vaporization by Decompression to the Vacuum”: Effect on Color Parameters and Essential Oil Profile. Foods 2024, 13, 2264. https://doi.org/10.3390/foods13142264
Tannir H, Debs E, Mansour G, Neugart S, El Hage R, Khalil MI, El Darra N, Louka N. Microbial Decontamination of Cuminum cyminum Seeds Using “Intensification of Vaporization by Decompression to the Vacuum”: Effect on Color Parameters and Essential Oil Profile. Foods. 2024; 13(14):2264. https://doi.org/10.3390/foods13142264
Chicago/Turabian StyleTannir, Hana, Espérance Debs, Georges Mansour, Susanne Neugart, Rima El Hage, Mahmoud I. Khalil, Nada El Darra, and Nicolas Louka. 2024. "Microbial Decontamination of Cuminum cyminum Seeds Using “Intensification of Vaporization by Decompression to the Vacuum”: Effect on Color Parameters and Essential Oil Profile" Foods 13, no. 14: 2264. https://doi.org/10.3390/foods13142264








