Application of Carboxymethyl Cellulose and Glycerol Monostearate as Binder Agents for Protein Powder Production from Honey Bee Brood Using Foam-Mat Drying Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
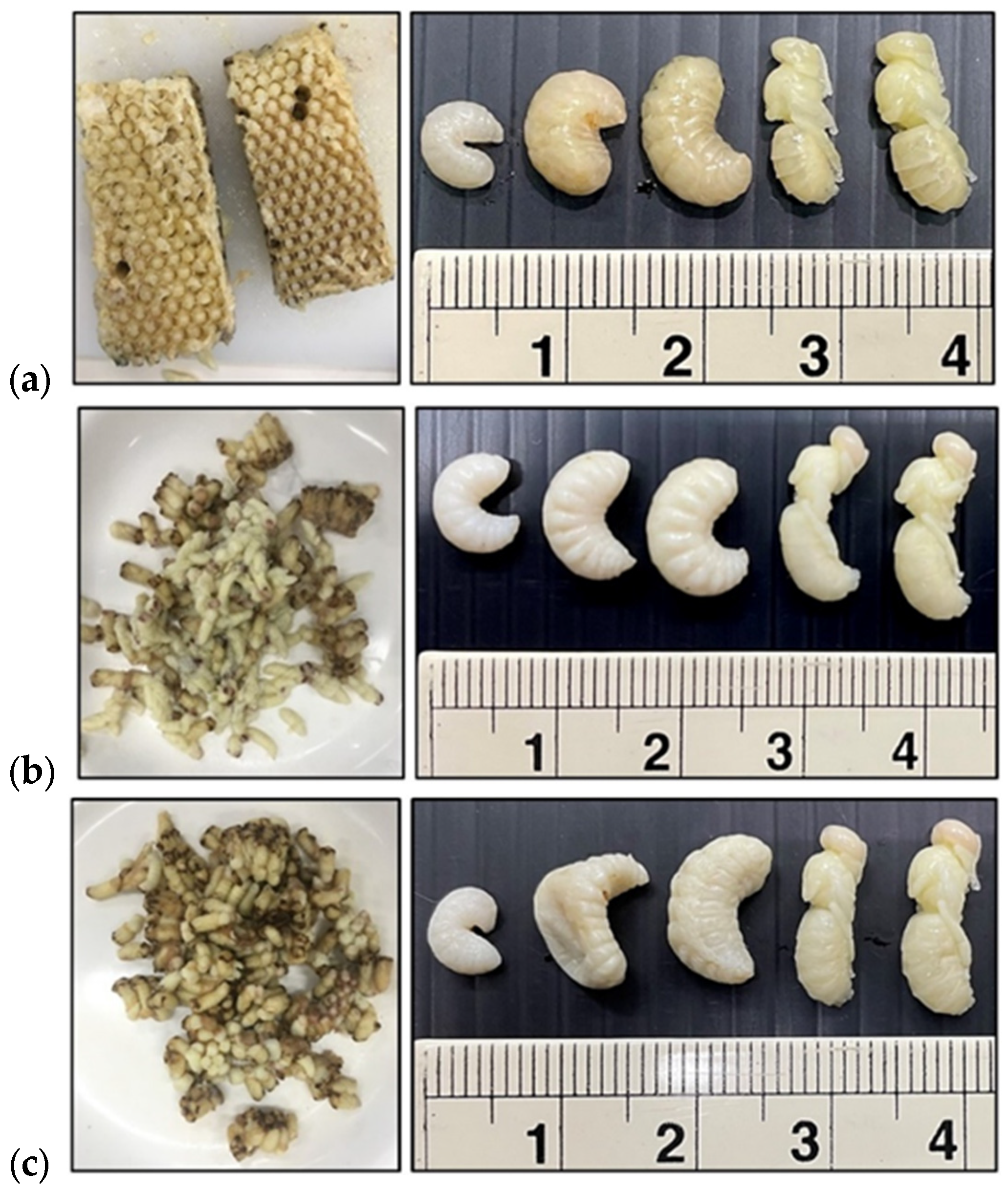
2.2. Bee Brood Preparation
2.3. Experimental Design for Bee Brood Protein Powder Formulation and Foam-Mat Drying Condition
2.4. Nutritional and Amino Acids Component of Bee Brood
2.4.1. Proximate Analysis
2.4.2. Amino Acids Determination by HPLC
2.5. Bee Brood Protein Powder Qualities
2.5.1. Powder Yield
2.5.2. Color Space Analysis
2.5.3. Water Activity (aw)
2.5.4. Solubility
2.5.5. Bulk and Tapped Densities of Powder
2.5.6. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nutritional and Amino Acid Compositions of Different Methods for Bee Brood Preparation
3.2. Influence of Bee Brood Quantity and Binder Agents on the Characteristics of Dried Foam-Mat Bee Brood Protein Powder
3.3. Optimal Formulation and Physicochemical Characterization of Dried Foam-Mat Bee Brood Protein Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finke, M.D. Nutrient Composition of Bee Brood and its Potential as Human Food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia-Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Lecocq, A.; Foley, K.; Jensen, A.B. Drone brood production in Danish apiaries and its potential for human consumption. J. Apic. Res. 2018, 57, 331–336. [Google Scholar] [CrossRef]
- Ghosh, S.; Meyer-Rochow, V.B.; Jung, C. Honey bees and their brood: A potentially valuable resource of food, worthy of greater appreciation and scientific attention. J. Ecol. Environ. 2021, 45, 31. [Google Scholar] [CrossRef]
- Jensen, A.B.; Evans, J.; Jonas-Levi, A.; Benjamin, O.; Martinez, I.; Dahle, B.; Roos, N.; Lecocq, A.; Foley, K. Standard methods for Apis mellifera brood as human food. J. Apic. Res. 2019, 58, 1–28. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Liceaga, A.M. Processing insects for use in the food and feed industry. Curr. Opin. Insect. Sci. 2021, 48, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Mishyna, M.; Martinez, J.J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Hardy, Z.; Jideani, V.A. Foam-mat drying technology: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2560–2572. [Google Scholar] [CrossRef]
- Sangamithra, A.; Sivakumar, V.; John, S.G.; Kannan, K. Foam mat drying of food materials: A review. J. Food Process. Preserv. 2015, 39, 3165–3174. [Google Scholar] [CrossRef]
- Kamali, R.; Dadashi, S.; Dehghannya, J.; Ghaffari, H. Numerical simulation and experimental investigation of foam-mat drying for producing banana powder as influenced by foam thickness. Appl. Food Res. 2022, 2, 100075. [Google Scholar] [CrossRef]
- Warepam, S.C.; Jena, S. Optimization of pomelo (Citrus grandis L. Osbeck) juice foam composition: Effect of foam composition on foam quality. J. Food Process. Preserv. 2022, 46, e16771. [Google Scholar] [CrossRef]
- Reis, F.R.; de Moraes, A.C.S.; Masson, M.L. Impact of Foam-Mat Drying on Plant-Based Foods Bioactive Compounds: A Review. Plant Foods Hum. Nutr. 2021, 76, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Association of International Official Analytic Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC International: Arlington, VA, USA, 2010. [Google Scholar]
- Somjai, C.; Siriwoharn, T.; Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Chaiyana, W.; Srinuanpan, S.; Wiriyacharee, P. Effect of drying process and long-term storage on characterization of Longan pulps and their biological aspects: Antioxidant and cholinesterase inhibition activities. LWT 2022, 154, 112692. [Google Scholar] [CrossRef]
- Thakur, C.; Verma, A.K.; Sharma, P.C.; Kaushal, M.; Vaidya, D.; Sharma, R.C.; Shivani. Effect of foaming agents on foaming properties, drying time and powder yield of rainy season Psidium guajava fruits cv. Shweta. J. Pharm. Innov. 2021, 9, 2574–2581. [Google Scholar]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Omidi, S.; Aarabi, A.; Zaki Dizaji, H.; Shahdadi, F. Microwave-assisted foam mat drying of red beet pulp: Influence of milk protein concentrate (MPC) and maltodextrin as a foaming agent, optimization and quality attribute. J. Food Meas. Charact. 2024, 18, 2505–2525. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Bußler, S.; Psarianos, M.; Rossi, G.; Schlüter, O.K. Edible insect processing pathways and implementation of emerging technologies. J. Insects Food Feed. 2021, 7, 877–900. [Google Scholar] [CrossRef]
- Nyangena, D.N.; Mutungi, C.; Imathiu, S.; Kinyuru, J.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Effects of Traditional Processing Techniques on the Nutritional and Microbiological Quality of Four Edible Insect Species Used for Food and Feed in East Africa. Foods 2020, 9, 574. [Google Scholar] [CrossRef]
- Djikeng, T.F.; Mouto Ndambwe, C.M.; Ngangoum, E.S.; Tiencheu, B.; Tambo Tene, S.; Achidi, A.U.; Womeni, H.M. Effect of different processing methods on the proximate composition, mineral content and functional properties of snail (Archachatina marginata) meat. J. Agric. Food Res. 2022, 8, 100298. [Google Scholar] [CrossRef]
- Manditsera, F.A.; Luning, P.A.; Fogliano, V.; Lakemond, C.M.M. Effect of domestic cooking methods on protein digestibility and mineral bioaccessibility of wild harvested adult edible insects. Food Res. Int. 2019, 121, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Markmanuel, D.; Godwin, J. Effects of Culinary Methods on The Proximate Composition of an Edible Insect (Rhynchophorus Phoenicis) Larvae Obtained from Bayelsa State, Nigeria. Eur. J. Agric. Food Sci. 2020, 2, 1–7. [Google Scholar] [CrossRef]
- Somjai, C.; Siriwoharn, T.; Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Wiriyacharee, P. Utilization of Maillard reaction in moist-dry-heating system to enhance physicochemical and antioxidative properties of dried whole longan fruit. Heliyon 2021, 7, e07094. [Google Scholar] [CrossRef] [PubMed]
- de Cól, C.D.; Tischer, B.; Hickmann Flôres, S.; Rech, R. Foam-mat drying of bacaba (Oenocarpus bacaba): Process characterization, physicochemical properties, and antioxidant activity. Food Bioprod. Process. 2021, 126, 23–31. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ismail-Fitry, M.R.; Rozzamri, A.; Bakar, J. Effect of foam-mat drying on kinetics and physical properties of Japanese threadfin bream (Nemipterus japonicus) powder. J. Food Process. Preserv. 2022, 46, e16376. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sharma, P.C.; Verma, A.; Thakur, C.; Saini, R.; Shivani. Improving the Powder Yield and Foaming Characteristics of Papaya Leaf Juice Treated with CMC (Carboxy-Methyl-Cellulose) and GMS (Glycerol-Mono-Stearate). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2364–2373. [Google Scholar] [CrossRef]
- Brar, A.S.; Kaur, P.; Kaur, G.; Subramanian, J.; Kumar, D.; Singh, A. Optimization of Process Parameters for Foam-Mat Drying of Peaches. Int. J. Fruit Sci. 2020, 20 (Suppl. 3), S1495–S1518. [Google Scholar] [CrossRef]
- Silva, J.M.; Crozatti, T.T.d.S.; Saqueti, B.H.F.; Chiavelli, L.U.R.; Matioli, G.; Santos, O.O. Optimization of foam mat drying using Central Composite Design to produce mixed juice powder: A process and characterization study. Food Bioprod. Process. 2024, 146, 58–68. [Google Scholar] [CrossRef]
- Bahriye, G.; Dadashi, S.; Dehghannya, J.; Ghaffari, H. Influence of processing temperature on production of red beetroot powder as a natural red colorant using foam-mat drying: Experimental and modeling study. Food Sci. Nutr. 2023, 11, 6955–6973. [Google Scholar] [CrossRef]

| Level Code | Variables and Factors Level | |||
|---|---|---|---|---|
| S_BB Content (g) | GMS Content (g) | CMC Content (g) | ||
| − | 10 | 6 | 0.5 | |
| 0 | 20 | 10 | 1.0 | |
| + | 30 | 14 | 1.5 | |
| Run | Code | S_BB | GMS | CMC |
| 1 | (1) | − | − | − |
| 2 | a | + | − | − |
| 3 | b | − | + | − |
| 4 | ab | + | + | − |
| 5 | c | − | − | + |
| 6 | ac | + | − | + |
| 7 | bc | − | + | + |
| 8 | abc | + | + | + |
| 9 | cp1 | 0 | 0 | 0 |
| 10 | cp2 | 0 | 0 | 0 |
| 11 | cp3 | 0 | 0 | 0 |
| Parameters | Methods for Bee Brood Preparation | ||
|---|---|---|---|
| Control (Raw Bee Brood) | Boiled Bee Brood (B_BB) | Steamed Bee Brood (S_BB) | |
| Moisture (%) ns | 4.61 ± 0.80 | 3.31 ± 0.28 | 4.57 ± 0.74 |
| Protein (g/100 g db) | 40.40 ± 0.30 b | 39.18 ± 0.07 c | 44.71 ± 0.07 a |
| Carbohydrate (g/100 g db) | 27.89 ± 0.08 b | 32.81 ± 0.17 a | 26.92 ± 0.48 b |
| Lipid (g/100 g db) | 29.83 ± 0.33 a | 25.32 ± 0.08 c | 27.31 ± 0.10 b |
| Ash (g/100 g db) | 4.27 ± 0.01 a | 4.07 ± 0.08 a | 3.35 ± 0.04 b |
| Fiber (g/100 g db) ns | 2.43 ± 0.04 | 2.05 ± 0.10 | 2.50 ± 0.34 |
| Amino Acids (mg/g db) | Methods for Bee Brood Preparation | ||
|---|---|---|---|
| Control (Raw Bee Brood) | Boiled Bee Brood (B_BB) | Steamed Bee Brood (S_BB) | |
| Aspartic acid | ND | 0.58 ± 0.01 a | 0.44 ± 0.01 b |
| Threonine | ND | 1.27 ± 0.01 b | 1.52 ± 0.01 a |
| Serine | 1.96 ± 0.04 a | 0.35 ± 0.01 b | 0.28 ± 0.01 b |
| Glutamic acid | 5.96 ± 0.04 c | 6.67 ± 0.06 a | 6.21 ± 0.04 b |
| Proline | 7.23 ± 0.15 a | 5.82 ± 0.13 b | 5.30 ± 0.13 b |
| Glycine | 23.87 ± 0.01 c | 45.29 ± 0.10 a | 34.97 ± 0.11 b |
| Alanine | 16.19 ± 0.16 a | 9.34 ± 0.11 b | 4.45 ± 0.05 c |
| Cysteine | 1.67 ± 0.01 c | 2.13 ± 0.02 b | 2.44 ± 0.02 a |
| Valine | 2.48 ± 0.03 a | 0.12 ± 0.01 b | 0.10 ± 0.01 b |
| Methionine | 0.85 ± 0.01 a | 0.76 ± 0.01 b | 0.57 ± 0.01 c |
| Isoleucine | 1.22 ± 0.04 a | 0.95 ± 0.02 b | 0.65 ± 0.01 c |
| Leucine | 1.55 ± 0.02 a | 1.43 ± 0.02 b | 1.20 ± 0.01 c |
| Tyrosine | 1.05 ± 0.01 c | 1.20 ± 0.01 b | 1.37 ± 0.01 a |
| Phenylalanine | 1.37 ± 0.01 a | 0.98 ± 0.02 b | 0.71 ± 0.01 c |
| Histidine | 1.94 ± 0.03 c | 4.79 ± 0.03 a | 4.43 ± 0.04 b |
| Lysine | 0.31 ± 0.01 b | 0.51 ± 0.02 a | 0.54 ± 0.01 a |
| Arginine | ND | 0.58 ± 0.01 a | 0.44 ± 0.01 b |
| TAA | 67.65 ± 0.57 b | 82.19 ± 0.59 a | 65.18 ± 0.49 c |
| Run | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Powder Yield (%) | Color Spaces | aw | Protein Content (g/100 g) | TAA (mg/g) | Solubility (%) | |||
| L* | C* | h | ||||||
| 1 | 5.82 ± 0.01 | 69.23 ± 0.23 | 12.01 ± 0.87 | 65.74 ± 0.98 | 0.400 ± 0.009 | 10.77 ± 0.04 | 13.66 ± 0.11 | 15.14 ± 0.95 |
| 2 | 8.73 ± 0.01 | 64.79 ± 0.24 | 13.93 ± 0.96 | 66.43 ± 0.09 | 0.261 ± 0.011 | 20.35 ± 0.02 | 22.13 ± 0.12 | 23.69 ± 0.12 |
| 3 | 10.70 ± 0.02 | 71.33 ± 0.63 | 10.01 ± 0.04 | 59.47 ± 0.88 | 0.274 ± 0.011 | 5.31 ± 0.02 | 12.27 ± 0.07 | 8.12 ± 0.58 |
| 4 | 10.35 ± 0.01 | 69.46 ± 0.32 | 11.51 ± 0.27 | 63.41 ± 0.18 | 0.441 ± 0.017 | 12.88 ± 0.01 | 17.77 ± 0.08 | 10.22 ± 0.33 |
| 5 | 6.65 ± 0.02 | 75.68 ± 0.11 | 7.71 ± 0.02 | 58.92 ± 0.35 | 0.266 ± 0.019 | 10.16 ± 0.01 | 9.99 ± 0.10 | 27.72 ± 0.12 |
| 6 | 10.98 ± 0.01 | 70.10 ± 0.80 | 11.43 ± 0.44 | 63.95 ± 0.25 | 0.234 ± 0.021 | 18.08 ± 0.02 | 20.14 ± 0.08 | 17.25 ± 0.27 |
| 7 | 9.74 ± 0.02 | 77.66 ± 0.08 | 8.84 ± 0.11 | 58.03 ± 0.21 | 0.290 ± 0.010 | 5.06 ± 0.03 | 6.87 ± 0.0.6 | 16.71 ± 0.41 |
| 8 | 11.30 ± 0.02 | 75.07 ± 0.14 | 8.11 ± 0.92 | 60.77 ± 0.03 | 0.365 ± 0.014 | 11.39 ± 0.02 | 13.58 ± 0.03 | 18.74 ± 0.86 |
| 9 | 9.41 ± 0.01 | 70.79 ± 0.01 | 11.32 ± 0.01 | 63.31 ± 0.23 | 0.229 ± 0.013 | 13.32 ± 0.01 | 15.64 ± 0.12 | 18.63 ± 0.76 |
| 10 | 9.01 ± 0.01 | 69.18 ± 0.39 | 11.70 ± 0.85 | 64.39 ± 0.90 | 0.238 ± 0.020 | 13.40 ± 0.01 | 14.56 ± 0.11 | 19.41 ± 0.43 |
| 11 | 9.68 ± 0.01 | 73.88 ± 0.04 | 11.41 ± 0.02 | 65.23 ± 0.02 | 0.269 ± 0.018 | 12.79 ± 0.02 | 13.29 ± 0.04 | 17.86 ± 0.91 |
| Responses | Equations | Adjusted R2 | C.V. (%) |
|---|---|---|---|
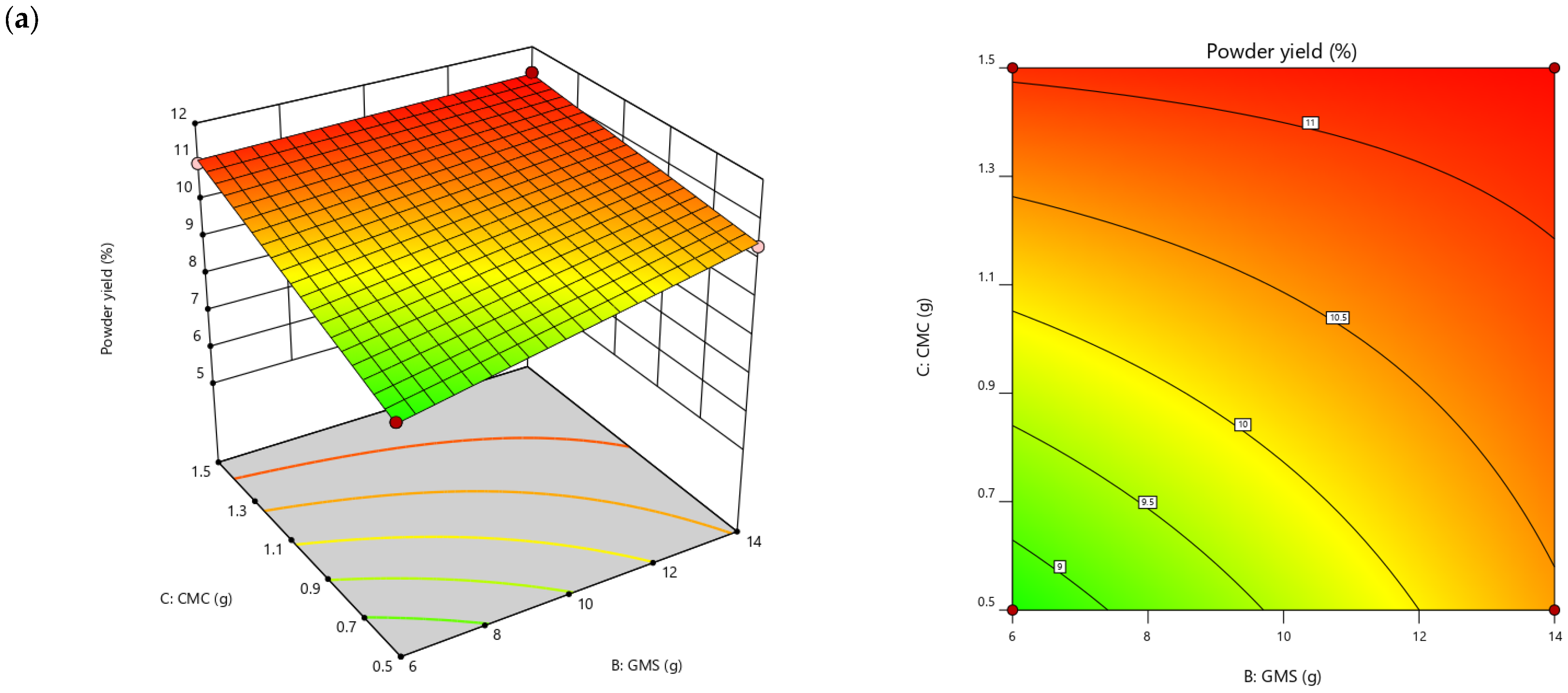
| Powder yield (%) | =−0.70 + 0.21A + 0.88B + 1.03C − 0.02AB + 0.08AC − 0.19BC | 0.9767 | 2.83 |
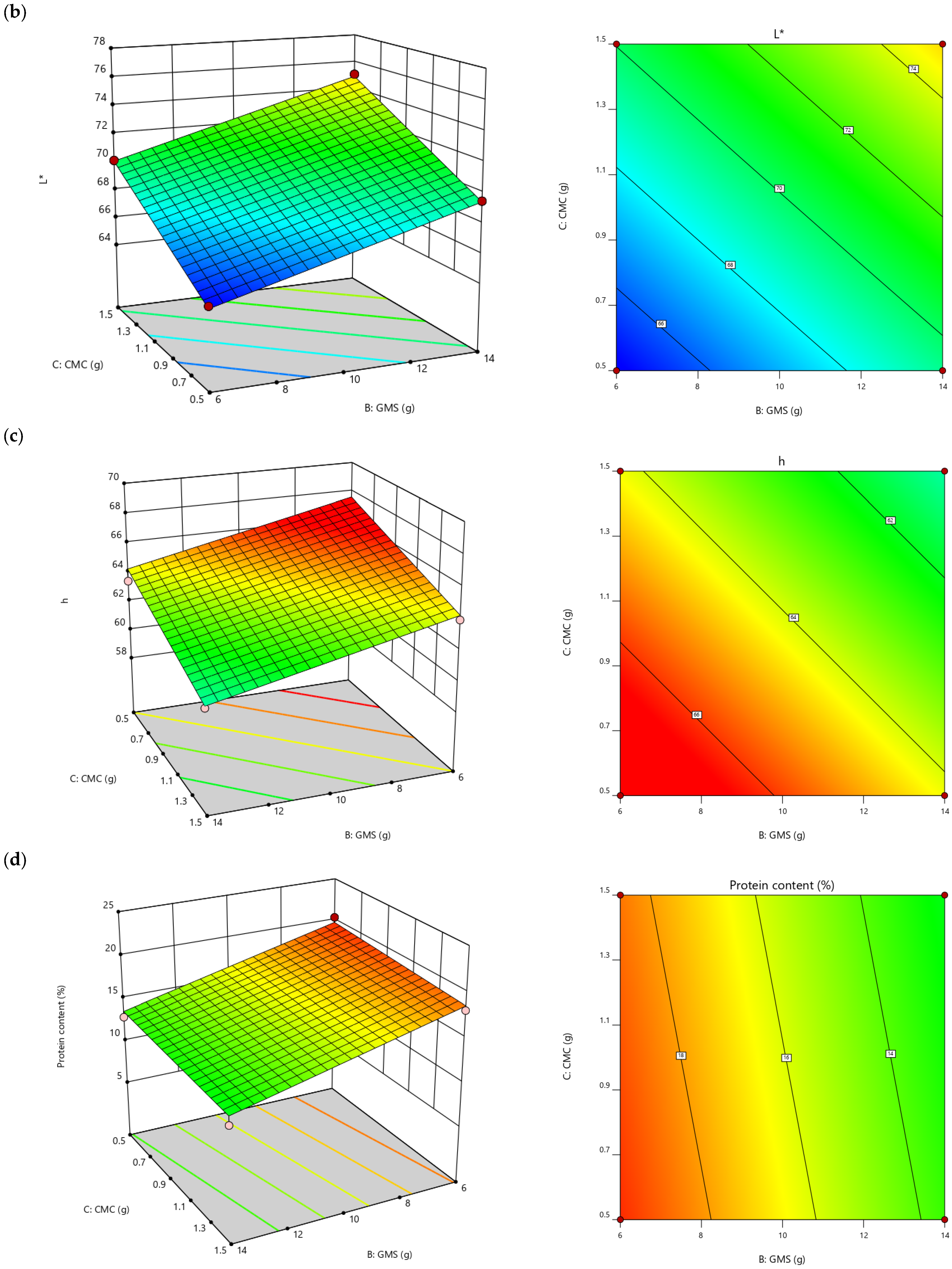
| L* | =67.62 − 0.31A + 0.07B + 6.75C + 0.02AB − 0.05AC + 0.01BC | 0.7841 | 2.40 |
| h | =67.11 + 0.15A − 0.42B − 3.35C | 0.6444 | 2.77 |
| Protein content (g/100 g) | =13.17 + 0.39A − 0.77B − 1.16C | 0.9491 | 8.51 |
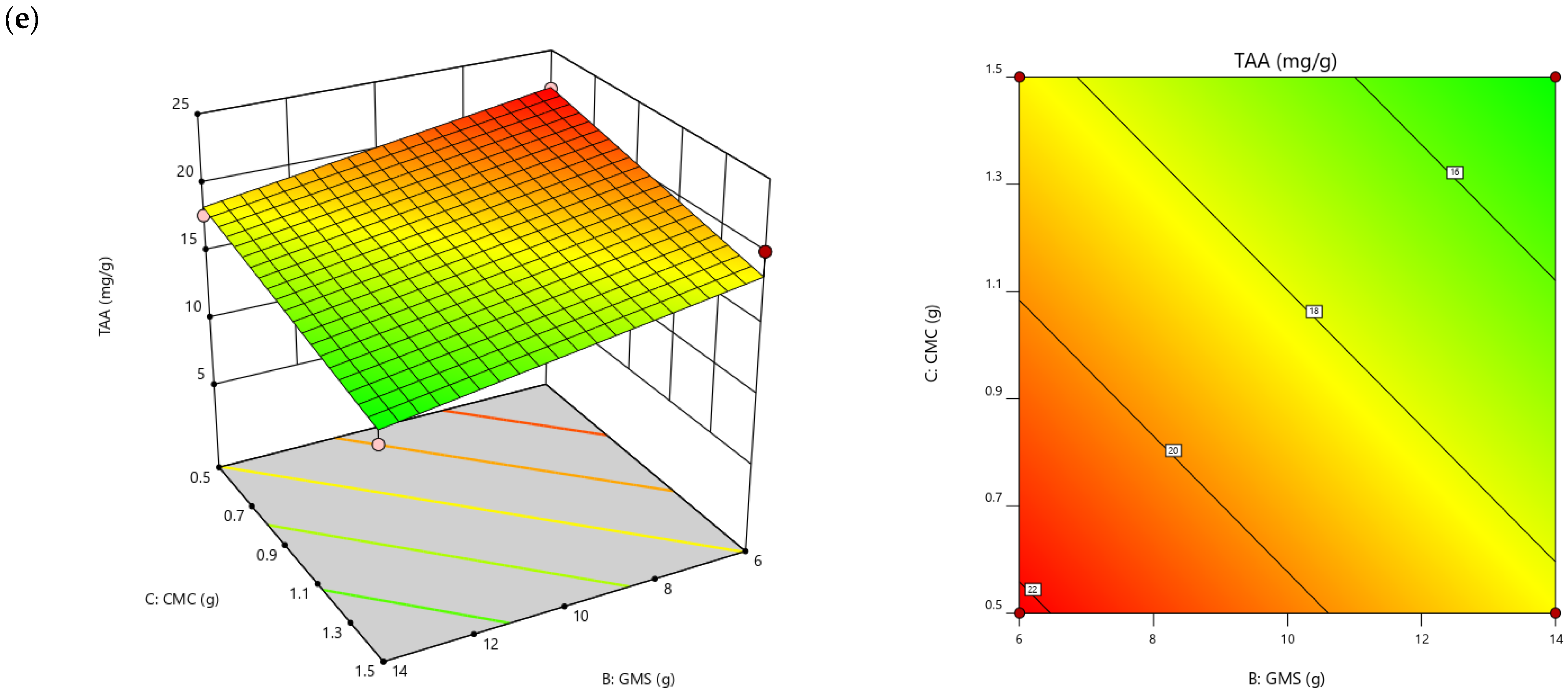
| TAA (mg/g) | =15.46 + 0.39A − 0.48B − 3.81C | 0.9173 | 8.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaipoot, S.; Phongphisutthinant, R.; Wiriyacharee, P.; Kanthakat, G.; Wongwatcharayothin, W.; Somjai, C.; Danmek, K.; Chuttong, B. Application of Carboxymethyl Cellulose and Glycerol Monostearate as Binder Agents for Protein Powder Production from Honey Bee Brood Using Foam-Mat Drying Technique. Foods 2024, 13, 2265. https://doi.org/10.3390/foods13142265
Chaipoot S, Phongphisutthinant R, Wiriyacharee P, Kanthakat G, Wongwatcharayothin W, Somjai C, Danmek K, Chuttong B. Application of Carboxymethyl Cellulose and Glycerol Monostearate as Binder Agents for Protein Powder Production from Honey Bee Brood Using Foam-Mat Drying Technique. Foods. 2024; 13(14):2265. https://doi.org/10.3390/foods13142265
Chicago/Turabian StyleChaipoot, Supakit, Rewat Phongphisutthinant, Pairote Wiriyacharee, Gochakorn Kanthakat, Worachai Wongwatcharayothin, Chalermkwan Somjai, Khanchai Danmek, and Bajaree Chuttong. 2024. "Application of Carboxymethyl Cellulose and Glycerol Monostearate as Binder Agents for Protein Powder Production from Honey Bee Brood Using Foam-Mat Drying Technique" Foods 13, no. 14: 2265. https://doi.org/10.3390/foods13142265
APA StyleChaipoot, S., Phongphisutthinant, R., Wiriyacharee, P., Kanthakat, G., Wongwatcharayothin, W., Somjai, C., Danmek, K., & Chuttong, B. (2024). Application of Carboxymethyl Cellulose and Glycerol Monostearate as Binder Agents for Protein Powder Production from Honey Bee Brood Using Foam-Mat Drying Technique. Foods, 13(14), 2265. https://doi.org/10.3390/foods13142265











