Secondary Bioactive Metabolites from Foods of Plant Origin as Theravention Agents against Neurodegenerative Disorders
Abstract
:1. Introduction
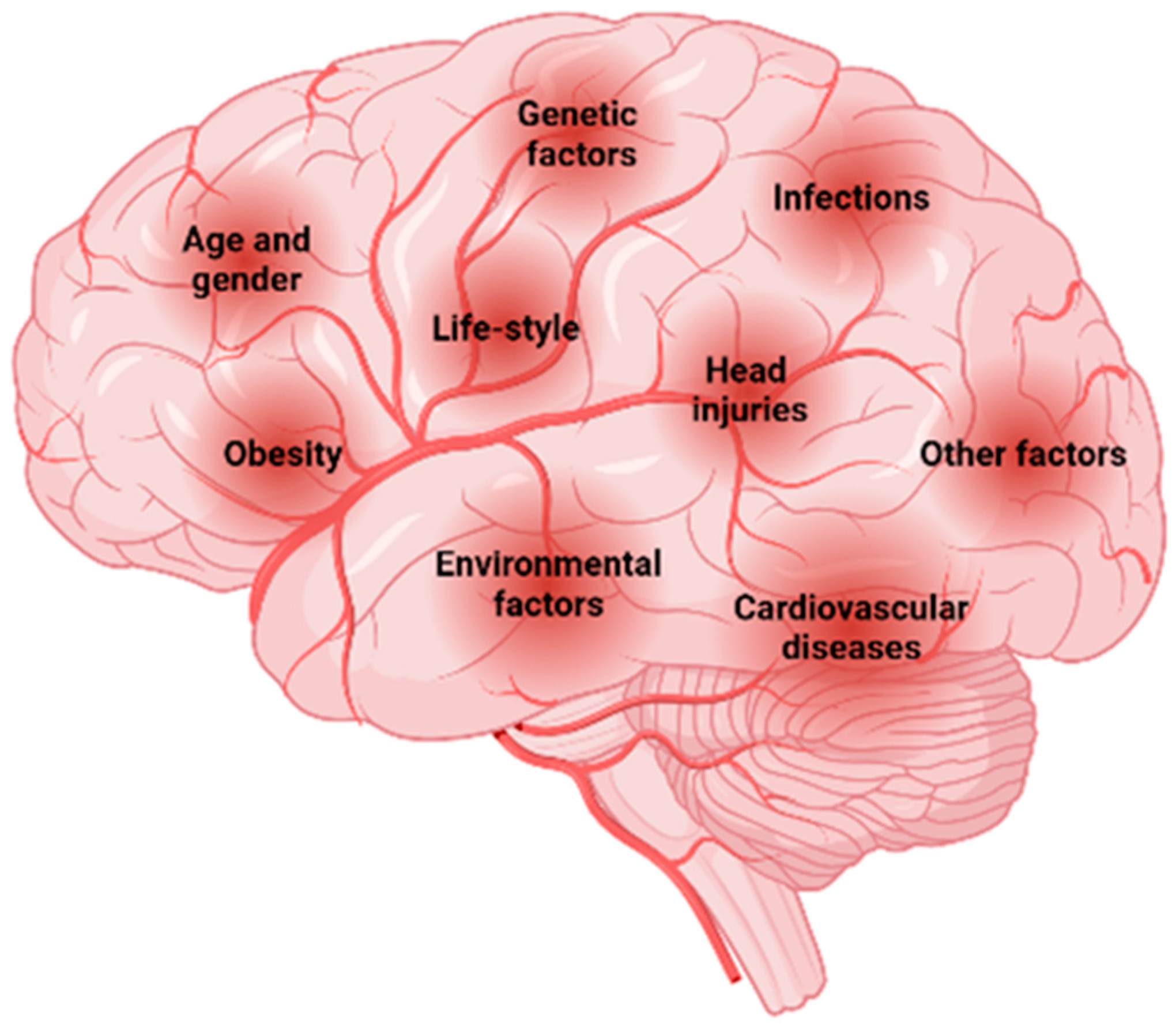
1.1. Risk Factors Associated with Neurodegenerative Disorders
1.1.1. Aging
1.1.2. Genetic Factors
1.1.3. Environmental Factors
1.1.4. Dietary Factors
1.1.5. Medical Factors
1.1.6. Other Factors
2. Current Diagnostic Strategies
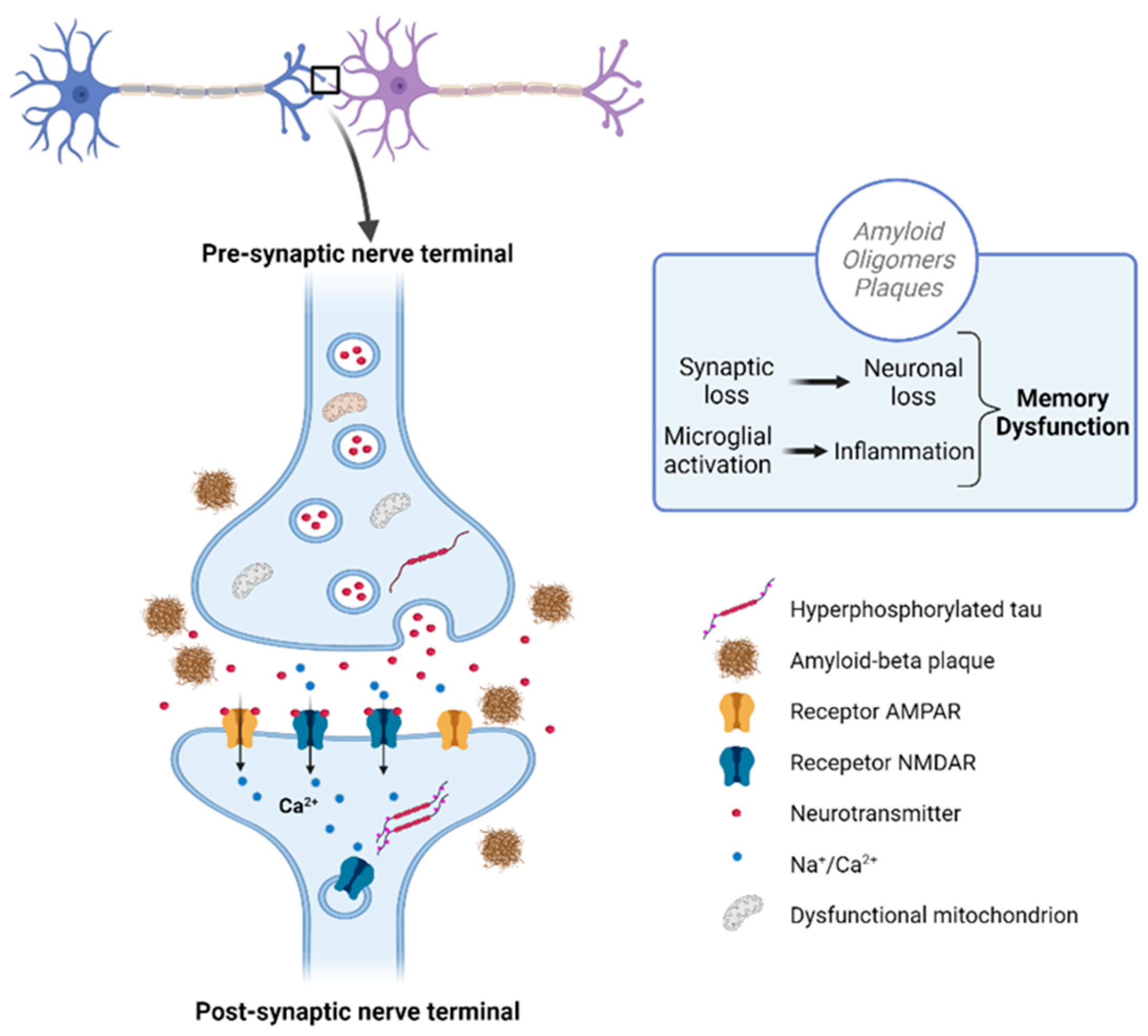
2.1. Neuropathological Techniques
2.2. Psychological and Neuropsychological Assessment
2.3. Connectivity and Electrical Activity
2.4. Neuroimaging
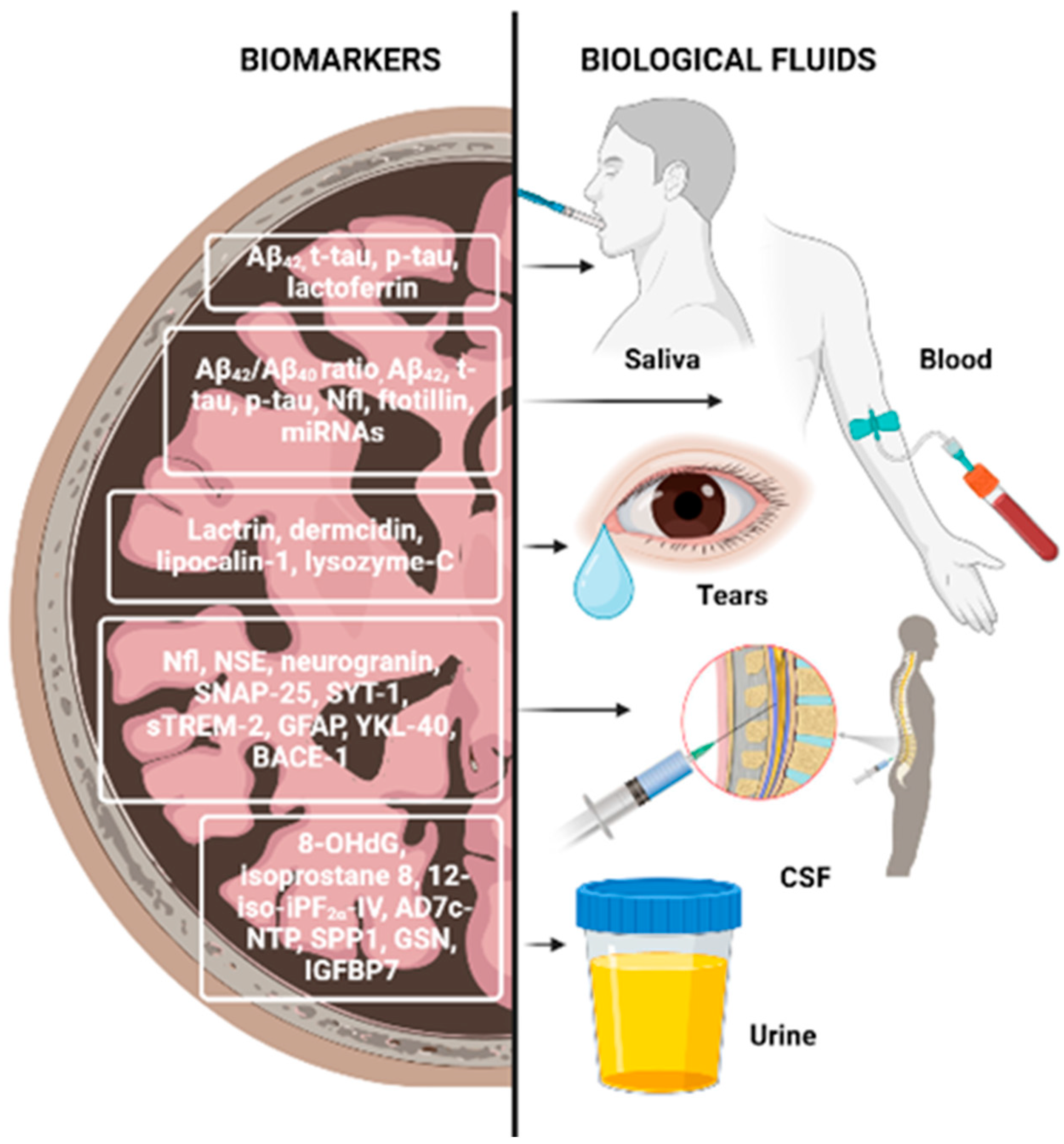
2.5. Biomarkers from Biological Fluids
3. The Potential of Secondary Bioactive Metabolites from Plant-Based Food as Theravention Agents against NDDs; Their Action Mechanisms and Bioavailability
3.1. Polyphenols
3.2. Polyunsaturated Fatty Acids
3.3. Proteins and Amino Acids
4. Plant-Based Foods and Herbs Effective against NDDs
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, J.-H. Dementia Epidemiology Fact Sheet 2022. Ann. Rehabil. Med. 2022, 46, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; Loures, C.d.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; das Graças Carvalho, M. Alzheimer’s Disease: Risk Factors and Potentially Protective Measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. North. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J.; et al. A Systematic Review and Meta-Analysis of Inflammatory Biomarkers in Parkinson’s Disease. NPJ Park. Dis. 2023, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Ingale, S.; Kasture, S. Protective Effect of Standardized Extract of Passiflora Incarnata Flower in Parkinson’s and Alzheimer’s Disease. Anc. Sci. Life 2017, 36, 200. [Google Scholar] [CrossRef] [PubMed]
- Beura, S.K.; Dhapola, R.; Panigrahi, A.R.; Yadav, P.; Kumar, R.; Reddy, D.H.; Singh, S.K. Antiplatelet Drugs: Potential Therapeutic Options for the Management of Neurodegenerative Diseases. Med. Res. Rev. 2023, 43, 1835–1877. [Google Scholar] [CrossRef] [PubMed]
- Franco, G.A.; Interdonato, L.; Cordaro, M.; Cuzzocrea, S.; Di Paola, R. Bioactive Compounds of the Mediterranean Diet as Nutritional Support to Fight Neurodegenerative Disease. Int. J. Mol. Sci. 2023, 24, 7318. [Google Scholar] [CrossRef]
- Phukan, B.C.; Roy, R.; Gahatraj, I.; Bhattacharya, P.; Borah, A. Therapeutic Considerations of Bioactive Compounds in Alzheimer’s Disease and Parkinson’s Disease: Dissecting the Molecular Pathways. Phytother. Res. 2023, 37, 5657–5699. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Morales, R.; Baglietto-Vargas, D.; Sanchez-Varo, R. Editorial: Risk Factors for Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Lellmann, A.W.; Schmidt, A.; Lehmann, A.; Amini, A.M.; Egert, S.; Schlesinger, S.; Nöthlings, U. Dietary Factors and Neurodegenerative Disorders: An Umbrella Review of Meta-Analyses of Prospective Studies. Adv. Nutr. 2020, 11, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Majnarić, L.T.; Bosnić, Z.; Guljaš, S.; Vučić, D.; Kurevija, T.; Volarić, M.; Martinović, I.; Wittlinger, T. Low Psychological Resilience in Older Individuals: An Association with Increased Inflammation, Oxidative Stress and the Presence of Chronic Medical Conditions. Int. J. Mol. Sci. 2021, 22, 8970. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.M.; Özkan, E.; Shomalizadeh, N.; Sapancı, S.; Özler, C.; Kesibi, J.; Gürsoy-Özdemir, Y. Assessing the Role of Primary Healthy Microglia and Gap Junction Blocker in Hindering Alzheimer’s Disease Neuroinflammatory Type: Early Approaches for Therapeutic Intervention. Front. Neurosci. 2023, 16, 1041461. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Nott, A.; Holtman, I.R. Genetic Insights into Immune Mechanisms of Alzheimer’s and Parkinson’s Disease. Front. Immunol. 2023, 14, 1168539. [Google Scholar] [CrossRef]
- Wang, H.; Yang, F.; Zhang, S.; Xin, R.; Sun, Y. Genetic and Environmental Factors in Alzheimer’s and Parkinson’s Diseases and Promising Therapeutic Intervention via Fecal Microbiota Transplantation. NPJ Park. Dis. 2021, 7, 70. [Google Scholar] [CrossRef]
- López González, I.; Garcia-Esparcia, P.; Llorens, F.; Ferrer, I. Genetic and Transcriptomic Profiles of Inflammation in Neurodegenerative Diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and Tauopathies. Int. J. Mol. Sci. 2016, 17, 206. [Google Scholar] [CrossRef]
- Koretsky, M.J.; Alvarado, C.; Makarious, M.B.; Vitale, D.; Levine, K.; Bandres-Ciga, S.; Dadu, A.; Scholz, S.W.; Sargent, L.; Faghri, F.; et al. Genetic Risk Factor Clustering within and across Neurodegenerative Diseases. Brain 2023, 146, 4486–4494. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Farhan, J.A.; Mroczko, J.; Winkel, I.; Perkowski, M.; Mroczko, B. Common and Trace Metals in Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2023, 24, 15721. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Bargues-Carot, A.; Riaz, Z.; Wickham, H.; Zenitsky, G.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10808. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Daneshzad, E.; Jafari, A.; Bellissimo, N.; Azadbakht, L. Association of Nut and Legume Consumption with Framingham 10 Year Risk of General Cardiovascular Disease in Older Adult Men: A Cross-Sectional Study. Clin. Nutr. ESPEN 2021, 42, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.R.; Bäckman, K.; Scarmeas, N.; Stern, Y.; Manly, J.J.; Mayeux, R.; Gu, Y. Dietary Fatty Acids and Risk of Alzheimer’s Disease and Related Dementias: Observations from the Washington Heights-Hamilton Heights-Inwood Columbia Aging Project (WHICAP). Alzheimers Dement. 2020, 16, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Kim, J.-H.; Kim, J.H.; Cho, S.J.; Nam, E.S.; Choi, H.G. The Occurrence of Alzheimer’s Disease and Parkinson’s Disease in Individuals with Osteoporosis: A Longitudinal Follow-up Study Using a National Health Screening Database in Korea. Front. Aging Neurosci. 2021, 13, 786337. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ludvigsson, J.F.; Ingre, C.; Piehl, F.; Wirdefeldt, K.; Zagai, U.; Ye, W.; Fang, F. Hospital-Treated Infections in Early- and Mid-Life and Risk of Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis: A Nationwide Nested Case-Control Study in Sweden. PLoS Med. 2022, 19, e1004092. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Lee, J.; Asthana, K.; Vakil Monfared, R.; Chen, J.; Alhassen, S.; Samad, M.; Wood, M.; Mayer, E.A.; Baldi, P. The Hidden Link between Circadian Entropy and Mental Health Disorders. Transl. Psychiatry 2022, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, F.; Restifo, D.; Laws, S.; Pawar, A.; Parikh, N.S. Impact of Tobacco Smoking on Disease-Specific Outcomes in Common Neurological Disorders: A Scoping Review. J. Clin. Neurosci. 2024, 122, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yang, Q.; B Joshi, R.; Liu, Y.; Akbar, M.; Song, B.-J.; Zhou, S.; Wang, X. Role of Alcohol Drinking in Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2316. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New Insights into Atypical Alzheimer’s Disease in the Era of Biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Lavitrano, M.; Salvatore, E.; Combi, R. Molecular and Imaging Biomarkers in Alzheimer’s Disease: A Focus on Recent Insights. J. Pers. Med. 2020, 10, 61. [Google Scholar] [CrossRef]
- Shusharina, N.; Yukhnenko, D.; Botman, S.; Sapunov, V.; Savinov, V.; Kamyshov, G.; Sayapin, D.; Voznyuk, I. Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression. Diagnostics 2023, 13, 573. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Luebke, M.; Parulekar, M.; Thomas, F.P. Fluid Biomarkers for the Diagnosis of Neurodegenerative Diseases. Biomark. Neuropsychiatry 2023, 8, 100062. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fernandes, S.L.; WeiKoh, J.E.; Ciaccio, E.J.; Fabell, M.K.M.; Tanik, U.J.; Rajinikanth, V.; Yeong, C.H. Automated Detection of Alzheimer’s Disease Using Brain MRI Images—A Study with Various Feature Extraction Techniques. J. Med. Syst. 2019, 43, 302. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.Q.; Hampel, H. Neurodegenerative Disease Biomarkers: Guideposts for Disease Prevention through Early Diagnosis and Intervention. Prog. Neurobiol. 2011, 95, 491–495. [Google Scholar] [CrossRef]
- Jiao, L.-L.; Dong, H.-L.; Liu, M.-M.; Wu, P.-L.; Cao, Y.; Zhang, Y.; Gao, F.-G.; Zhu, H.-Y. The Potential Roles of Salivary Biomarkers in Neurodegenerative Diseases. Neurobiol. Dis. 2024, 193, 106442. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef]
- Parsafar, S.; Nayeri, Z.; Aliakbari, F.; Shahi, F.; Mohammadi, M.; Morshedi, D. Multiple Neuroprotective Features of Scutellaria Pinnatifida–Derived Small Molecule. Heliyon 2020, 6, e04737. [Google Scholar] [CrossRef]
- Baghel, R.S.; Choudhary, B.; Pandey, S.; Pathak, P.K.; Patel, M.K.; Mishra, A. Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods 2023, 12, 3642. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dal-Pan, A.; Gillard, E.; Calon, F.; Pallet, V. Protective Effects of Berry Polyphenols against Age-Related Cognitive Impairment. Nutr. Aging 2015, 3, 89–106. [Google Scholar] [CrossRef]
- Sheikh, I.; Sharma, V.; Tuli, H.S.; Aggarwal, D.; Sankhyan, A.; Vyas, P.; Sharma, A.K.; Bishayee, A. Cancer Chemoprevention by Flavonoids, Dietary Polyphenols and Terpenoids. Biointerface Res. Appl. Chem. 2021, 11, 8502–8537. [Google Scholar] [CrossRef]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic Impact of Grape Leaves Polyphenols on Certain Biochemical and Neurological Markers in AlCl3-Induced Alzheimer’s Disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef] [PubMed]
- Pogačnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kwang, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F.M. Potential for Brain Accessibility and Analysis of Stability of Selected Flavonoids in Relation to Neuroprotection in Vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Yan, J.; Zhou, Q.; Wang, X. Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols. Int. J. Mol. Sci. 2022, 23, 13886. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Vahed, F.M.; Liu, Q.; Siddiqui, S.A.; Kharazmi, M.S.; Jafari, S.M. Natural Bioactive Molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases. ACS Omega 2023, 8, 3667–3683. [Google Scholar] [CrossRef]
- Yammine, S.; Delsart, C.; Vitrac, X.; Peuchot, M.M.; Ghidossi, R. Characterisation of Polyphenols and Antioxidant Potential of Red and White Pomace By-Product Extracts Using Subcritical Water Extraction. OENO One 2020, 54, 263–278. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Ojo, O.A.; Okesola, M.A.; Akinyemi, A.J.; Talabi, J.Y.; Idowu, O.T.; Fadaka, A.O.; Boligon, A.A.; Anraku de Campos, M.M. In Vitro Antioxidant Activities and Inhibitory Effects of Phenolic Extract of Senecio Biafrae (Oliv and Hiern) against Key Enzymes Linked with Type II Diabetes Mellitus and Alzheimer’s Disease. Food Sci. Nutr. 2018, 6, 1803–1810. [Google Scholar] [CrossRef]
- Abdulqadir, I.; Ahmed, S.G.; Kuliya, A.G.; Tukur, J.; Yusuf, A.A.; Musa, A.U. Hematological Parameters of Human Immunodeficiency Virus Positive Pregnant Women on Antiretroviral Therapy in Aminu Kano Teaching Hospital Kano, North Western Nigeria. J. Lab. Physicians 2018, 10, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic Ingredients and Therapeutic Potential of Stachys Cretica Subsp. Smyrnaea for the Management of Oxidative Stress, Alzheimer’s Disease, Hyperglycemia, and Melasma. Ind. Crops Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic Acid Is a Dual α/β-Secretase Modulator That Reverses Cognitive Impairment and Remediates Pathology in Alzheimer Mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective Effect of Caffeic Acid against Alzheimer’s Disease Pathogenesis via Modulating Cerebral Insulin Signaling, β-Amyloid Accumulation, and Synaptic Plasticity in Hyperinsulinemic Rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef] [PubMed]
- Giacomeli, R.; Izoton, J.C.; dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective Effects of Curcumin Lipid-Core Nanocapsules in a Model Alzheimer’s Disease Induced by β-Amyloid 1-42 Peptide in Aged Female Mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Rahul; Ara, G.; Afzal, M.; Varshney, H.; Gaur, K.; Subhan, I.; Mantasha, I.; Shahid, M. Beneficial Effects of Apigenin on the Transgenic Drosophila Model of Alzheimer’s Disease. Chem. Biol. Interact. 2022, 366, 110120. [Google Scholar] [CrossRef] [PubMed]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-Mediated Cleavage of Amyloid Beta(1–42) Peptide: Potential Relevance to Alzheimer’s Disease: Potential Relevance to Alzheimer’s Disease. Neurobiol. Ageing 2020, 94, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yan, Y.; He, X.Y.; Yang, H.; Liang, B.Y.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. Biomed. Res. Int. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Baiseitova, A.; Shah, A.B.; Kim, J.Y.; Ban, Y.J.; Kim, J.H.; Nafiah, M.A.; Park, K.H. O-Alkylated Quercetins with Selective Acetylcholinesterase and β-Secretase Inhibitions from Melicope Glabra Leaves, and Their Flavonols Profile by LC-ESI-Q-TOF/MS. J. Funct. Foods 2021, 84, 104602. [Google Scholar] [CrossRef]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, N.F.G. Neuroprotective Effect of Quercetin Nanoparticles: A Possible Prophylactic and Therapeutic Role in Alzheimer’s Disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef]
- Cascella, M.; Bimonte, S.; Muzio, M.R.; Schiavone, V.; Cuomo, A. The Efficacy of Epigallocatechin-3-Gallate (Green Tea) in the Treatment of Alzheimer’s Disease: An Overview of Pre-Clinical Studies and Translational Perspectives in Clinical Practice. Infect. Agent. Cancer 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Xicota, L.; Rodriguez-Morato, J.; Dierssen, M.; de la Torre, R. Potential Role of (-)-Epigallocatechin-3-Gallate (EGCG) in the Secondary Prevention of Alzheimer Disease. Curr. Drug Targets 2017, 18, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.N.; Barlock, B.J.; DaSilva, N.A.; Johnson, S.L.; Liu, C.; Ma, H.; Nelson, R.; Akhlaghi, F.; Seeram, N.P. Anti-Neuroinflammatory Effects of a Food-Grade Phenolic-Enriched Maple Syrup Extract in a Mouse Model of Alzheimer’s Disease. Nutr. Neurosci. 2021, 24, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Y.; Huang, X.; Yue, T. Controlled Release of Protein from Core–Shell Nanofibers Prepared by Emulsion Electrospinning Based on Green Chemical. J. Appl. Polym. Sci. 2015, 132, 41811. [Google Scholar] [CrossRef]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The Effect of Curcumin on Cognition in Alzheimer’s Disease and Healthy Aging: A Systematic Review of Pre-Clinical and Clinical Studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- Nebrisi, E. El Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef] [PubMed]
- Yarim, G.F.; Kazak, F.; Yarim, M.; Sozmen, M.; Genc, B.; Ertekin, A.; Gokceoglu, A. Apigenin Alleviates Neuroinflammation in a Mouse Model of Parkinson’s Disease. Int. J. Neurosci. 2022, 132, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, D.; Jiang, P.; Tang, X.; Lang, Q.; Yu, Q.; Zhang, S.; Che, Y.; Feng, X. Resveratrol Synergizes with Low Doses of L-DOPA to Improve MPTP-Induced Parkinson Disease in Mice. Behav. Brain Res. 2019, 367, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic Insights and Perspectives Involved in Neuroprotective Action of Quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-Secretase, Cholinesterases, Histone Deacetylase and Tyrosinase) Inhibitors from Olive (Olea Europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Fornal, E. The Impact of Flavonols on Cardiovascular Risk. Nutrients 2022, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Iglesias, N.; Nadjar, A.; Sierra, A.; Valero, J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. Int. J. Mol. Sci. 2022, 23, 3399. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, M.R.; Ceccarelli, V.; Codini, M.; Fettucciari, K.; Calvitti, M.; Cataldi, S.; Albi, E.; Vecchini, A.; Beccari, T. The Polyunsaturated Fatty Acid EPA, but Not DHA, Enhances Neurotrophic Factor Expression through Epigenetic Mechanisms and Protects against Parkinsonian Neuronal Cell Death. Int. J. Mol. Sci. 2022, 23, 16176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiong, J.-Y.; Chai, Y.-Q.; Huang, L.; Tang, Z.-Y.; Zhang, X.-F.; Liu, B.; Zhang, J.-T. Possible Antidepressant Mechanisms of Omega-3 Polyunsaturated Fatty Acids Acting on the Central Nervous System. Front. Psychiatry 2022, 13, 933704. [Google Scholar] [CrossRef]
- Yan, L.; Xie, Y.; Satyanarayanan, S.K.; Zeng, H.; Liu, Q.; Huang, M.; Ma, Y.; Wan, J.-B.; Yao, X.; Su, K.-P.; et al. Omega-3 Polyunsaturated Fatty Acids Promote Brain-to-Blood Clearance of β-Amyloid in a Mouse Model with Alzheimer’s Disease. Brain Behav. Immun. 2020, 85, 35–45. [Google Scholar] [CrossRef]
- Sarparast, M.; Dattmore, D.; Alan, J.; Lee, K.S.S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients 2020, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.; Kumari, P.; Singh, A.; Taneja, N.K.; Chopra, R. Amelioration for Oxidative Stability and Bioavailability of N-3 PUFA Enriched Microalgae Oil: An Overview. Crit. Rev. Food Sci. Nutr. 2024, 64, 2579–2600. [Google Scholar] [CrossRef]
- Saini, S.; Saxena, S.; Samtiya, M.; Puniya, M.; Dhewa, T. Potential of Underutilized Millets as Nutri-Cereal: An Overview. J. Food Sci. Technol. 2021, 58, 4465–4477. [Google Scholar] [CrossRef] [PubMed]
- Nana, G.; Mitra, S.; Watson, H.; Young, C.; Wood, H.M.; Perry, S.L.; Race, A.D.; Quirke, P.; Toogood, G.J.; Loadman, P.M.; et al. Luminal Bioavailability of Orally Administered ω-3 PUFAs in the Distal Small Intestine, and Associated Changes to the Ileal Microbiome, in Humans with a Temporary Ileostomy. J. Nutr. 2021, 151, 2142–2152. [Google Scholar] [CrossRef]
- Nagao, K. Cognition and Nutrition: The Role of Dietary Protein and Amino Acids in Cognitive Health. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Pogačnik, L.; Ota, A.; Poklar Ulrih, N. An Overview of Crucial Dietary Substances and Their Modes of Action for Prevention of Neurodegenerative Diseases. Cells 2020, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Abdi-Moghadam, Z.; Darroudi, M.; Mahmoudzadeh, M.; Mohtashami, M.; Jamal, A.M.; Shamloo, E.; Rezaei, Z. Functional Yogurt, Enriched and Probiotic: A Focus on Human Health. Clin. Nutr. ESPEN 2023, 57, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Lackie, R.E.; Maciejewski, A.; Ostapchenko, V.G.; Marques-Lopes, J.; Choy, W.-Y.; Duennwald, M.L.; Prado, V.F.; Prado, M.A.M. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front. Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Okur, M.E.; Erim, P.D.; Çağlar, E.Ş.; Özgenç, E.; Gündoğdu, E.; Köprülü, R.E.P.; Karantas, I.D.; Üstündağ Okur, N. Protein and Gene Delivery Systems for Neurodegenerative Disorders: Where Do We Stand Today? Pharmaceutics 2022, 14, 2425. [Google Scholar] [CrossRef]
- Gaudichon, C.; Calvez, J. Determinants of Amino Acid Bioavailability from Ingested Protein in Relation to Gut Health. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 55–61. [Google Scholar] [CrossRef]
- Trommelen, J.; Tomé, D.; van Loon, L.J.C. Gut Amino Acid Absorption in Humans: Concepts and Relevance for Postprandial Metabolism. Clin. Nutr. Open Sci. 2021, 36, 43–55. [Google Scholar] [CrossRef]
- Kashyap, S.; Shivakumar, N.; Varkey, A.; Duraisamy, R.; Thomas, T.; Preston, T.; Devi, S.; Kurpad, A. V Ileal Digestibility of Intrinsically Labeled Hen’s Egg and Meat Protein Determined with the Dual Stable Isotope Tracer Method in Indian Adults. Am. J. Clin. Nutr. 2018, 108, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Varkey, A.; Shivakumar, N.; Devi, S.; Reddy, B.H.R.; Thomas, T.; Preston, T.; Sreeman, S.; Kurpad, A. V True Ileal Digestibility of Legumes Determined by Dual-Isotope Tracer Method in Indian Adults. Am. J. Clin. Nutr. 2019, 110, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, S.M.; Montoya, C.A.; Scholten, P.T.; Rutherfurd, S.M.; Moughan, P.J. Cooking Conditions Affect the True Ileal Digestible Amino Acid Content and Digestible Indispensable Amino Acid Ccore (DIAAS) of Bovine Meat as Determined in Pigs. J. Nutr. 2018, 148, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.M.; Mathai, J.K.; Berg, E.P.; Stein, H.H. Pork Products Have Digestible Indispensable Amino Acid Scores (DIAAS) That Are Greater than 100 When Determined in Pigs, but Processing Does Not Always Increase DIAAS. J. Nutr. 2020, 150, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.M.; Stein, H.H. Raw and Roasted Pistachio Nuts Pistacia Vera L. Are ‘Good’ Sources of Protein Based on Their Digestible Indispensable Amino Acid Score as Determined in Pigs. J. Sci. Food Agric. 2020, 100, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Hesam Shahrajabian, W.S.Q.C. Milk Thistle, Myrrh and Mint: Herbal Plants as Natural Medicines. Nutr. Food Sci. Res. 2021, 8, 59–65. [Google Scholar]
- Gomaa, A.A.; Makboul, R.M.; El-Mokhtar, M.A.; Abdel-Rahman, E.A.; Ahmed, I.A.; Nicola, M.A. Terpenoid-Rich Elettaria Cardamomum Extract Prevents Alzheimer-like Alterations Induced in Diabetic Rats via Inhibition of GSK3β Activity, Oxidative Stress and pro-Inflammatory Cytokines. Cytokine 2019, 113, 405–416. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and Grape Extract-Loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum Annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Kumari, U.; Mitra Mazumder, P. Ameliorative Effects of Apple Cider Vinegar on Neurological Complications via Regulation of Oxidative Stress Markers. J. Food Biochem. 2020, 44, e13504. [Google Scholar] [CrossRef] [PubMed]
- Tzekaki, E.E.; Papaspyropoulos, A.; Tsolaki, M.; Lazarou, E.; Kozori, M.; Pantazaki, A.A. Restoration of BMI1 Levels after the Administration of Early Harvest Extra Virgin Olive Oil as a Therapeutic Strategy against Alzheimer’s Disease. Exp. Gerontol. 2021, 144, 111178. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Chun, Y.S.; October, N.; Yang, H.O.; Maharaj, V. Potential of South African Medicinal Plants Targeting the Reduction of Aβ42 Protein as a Treatment of Alzheimer’s Disease. J. Ethnopharmacol. 2019, 231, 363–373. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Hammam, W.E.; El-Mahdy El-Tantawi, M.; Yassin, N.A.Z.; Kirollos, F.N.; Abdelhameed, M.F.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. Apple Leaves and Their Major Secondary Metabolite Phlorizin Exhibit Distinct Neuroprotective Activities: Evidence from in Vivo and in Silico Studies. Arab. J. Chem. 2021, 14, 103188. [Google Scholar] [CrossRef]
- Massop, C.; Ndé, W.; Awounfack, C.F.; Gamo, F.Z. Extra-Virgin Avocado (Persea Americana Mill., Laucaceae) Oil Improves Cognitive Impairment in D-Galactose-Induced Alzheimer’s Disease Model on Ovariectomized Wistar Rat. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Al-Hakim, N.A.; Fidrianny, I.; Anggadiredja, K.; Mauludin, R. Effect of Banana (Musa Sp.) Peels Extract in Nanoemulsion Dosage Forms for the Improvement of Memory: In Vitro & In Vivo Studies. Pharm. Nanotechnol. 2022, 10, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, B.C.; Oyeleye, S.I.; Okeke, B.M.; Oboh, G. Anti-Cholinesterase and Antioxidant Properties of Alkaloid and Phenolic-Rich Extracts from Pawpaw (Carica Papaya) Leaf: A Comparative Study. Flavour. Fragr. J. 2021, 36, 47–54. [Google Scholar] [CrossRef]
- Ksiezak-Reding, H.; Ho, L.; Santa-Maria, I.; Diaz-Ruiz, C.; Wang, J.; Pasinetti, G.M. Ultrastructural Alterations of Alzheimer’s Disease Paired Helical Filaments by Grape Seed-Derived Polyphenols. Neurobiol. Aging 2012, 33, 1427–1439. [Google Scholar] [CrossRef]
- Schimidt, H.L.; Carrazoni, G.S.; Garcia, A.; Izquierdo, I.; Mello-Carpes, P.B.; Carpes, F.P. Strength Training or Green Tea Prevent Memory Deficits in a β-Amyloid Peptide-Mediated Alzheimer’s Disease Model. Exp. Gerontol. 2021, 143, 111186. [Google Scholar] [CrossRef]
- Yadav, Y.C.; Singh, A.; Kannaujia, S.K.; Yadav, R. Neuroprotective Effect of Citrus Limon Juice against Scopolamine Induced Amnesia in Wistar Rats: Role of Cholinergic Neurotransmission Monitoring and Beta-Actin Signaling. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100191. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, D.; Li, X.M.; Li, D.; Zhou, G.; Xu, K.P.; Kang, F.H.; Zou, Z.X.; Xu, P.S.; et al. Anti-Cholinesterase Activities of Constituents Isolated from Lycopodiastrum Casuarinoides. Fitoterapia 2019, 139, 104366. [Google Scholar] [CrossRef] [PubMed]
- Penumala, M.; Zinka, R.B.; Shaik, J.B.; Mallepalli, S.K.R.; Vadde, R.; Amooru, D.G. Phytochemical Profiling and in Vitro Screening for Anticholinesterase, Antioxidant, Antiglucosidase and Neuroprotective Effect of Three Traditional Medicinal Plants for Alzheimer’s Disease and Diabetes Mellitus Dual Therapy. BMC Complement. Altern. Med. 2018, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, D. Mulberry Fruit Extract Alleviates Cognitive Impairment by Promoting the Clearance of Amyloid-β and Inhibiting Neuroinflammation in Alzheimer’s Disease Mice. Neurochem. Res. 2020, 45, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Noya, I.D.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis Elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Shehata, M.G.; Alsulami, T.; Badr, A.N.; Elbakatoshy, M.R.; Ali, H.S.; El-Sohaimy, S.A. Characterization of Orange Peel Extract and Its Potential Protective Effect against Aluminum Chloride-Induced Alzheimer’s Disease. Pharmaceuticals 2023, 16, 12. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS Profiling of Secondary Metabolites from Opuntia Ficus-Indica Cladode, Peel and Fruit Pulp Extracts and Their Antioxidant, Neuroprotective Effect in Rats with Aluminum Chloride Induced Neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Chang, Y.; Lou, L.L.; Zhang, X.; Hou, J.Y.; Yao, G.D.; Huang, X.X.; Song, S.J.; Lin, B.; Bai, M. Solanoids F.-I: Terpenoids from Solanum Lyratum with Neuroprotective Effects against H2O2-Induced SH-SY5Y Cell Injuries. Fitoterapia 2022, 163, 105346–105354. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Fathy, F.I.; Sleem, A.A.; Morsy, F.A.; Khadar, M.S.; Mansour, M.K. Anticholinesterase Activity and Metabolite Profiling of Syagrus Romanzoffiana (Cham.) Glassman Leaves and Fruits via UPLC–QTOF–PDA–MS. Nat. Prod. Res. 2021, 35, 1671–1675. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Mahrous, E.A.; Thabet, M.M.; Elnaggar, D.M.Y.; Youssef, A.M.; Elhawary, R.; Zaitone, S.A.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Mekky, R.H. Methanolic Extracts of a Selected Egyptian Vicia Faba Cultivar Mitigate the Oxidative/Inflammatory Burden and Afford Neuroprotection in a Mouse Model of Parkinson’s Disease. Inflammopharmacology 2021, 29, 221–235. [Google Scholar] [CrossRef]
- Lei, H.; Ren, R.; Sun, Y.; Zhang, K.; Zhao, X.; Ablat, N.; Pu, X. Neuroprotective Effects of Safflower Flavonoid Extract in 6-Hydroxydopamine-Induced Model of Parkinson’s Disease May Be Related to Its Anti-Inflammatory Action. Molecules 2020, 25, 5206. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kawy, M.A.; Aboulhoda, B.E.; Michel, C.G.; Sedeek, M.S.; Kirollos, F.N.; Masoud, M.A. Ameliorating Effect of Citrus Trifoliata L. Fruits Extract on Motor Incoordination, Neurodegeneration and Oxidative Stress in Parkinson’s Disease Model. Nutr. Neurosci. 2023, 27, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Tamegart, L.; Abbaoui, A.; Makbal, R.; Zroudi, M.; Bouizgarne, B.; Bouyatas, M.M.; Gamrani, H. Crocus Sativus Restores Dopaminergic and Noradrenergic Damages Induced by Lead in Meriones Shawi: A Possible Link with Parkinson’s Disease. Acta Histochem. 2019, 121, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-W.; Guo, R.-Y. Dose-Dependent Effect of Curcuma Longa for the Treatment of Parkinson’s Disease. Exp. Ther. Med. 2017, 13, 1799–1805. [Google Scholar] [CrossRef]
- Rai, S.N.; Birla, H.; Singh, S.S.; Zahra, W.; Patil, R.R.; Jadhav, J.P.; Gedda, M.R.; Singh, S.P. Mucuna Pruriens Protects against MPTP Intoxicated Neuroinflammation in Parkinson’s Disease through NF-ΚB/PAKT Signaling Pathways. Front. Aging Neurosci. 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ye, J.; Wang, S.; He, W.; Feng, Z.; Sun, H.; Chu, S.; Zhang, Z.; Chen, N. A Small Molecule 20C from Gastrodia Elata Inhibits α-Synuclein Aggregation and Prevents Progression of Parkinson’s Disease. Cell Death Dis. 2023, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, P.; Li, J.; Liu, T.; Zhang, Y.; Wang, Q.; Zhang, J.; Lu, X.; Fan, X. Neuroprotective Effects of Ginkgo Biloba Dropping Pills in Parkinson’s Disease. J. Pharm. Anal. 2021, 11, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Nicholatos, J.W.; Francisco, A.B.; Bender, C.A.; Yeh, T.; Lugay, F.J.; Salazar, J.E.; Glorioso, C.; Libert, S. Nicotine Promotes Neuron Survival and Partially Protects from Parkinson’s Disease by Suppressing SIRT6. Acta Neuropathol. Commun. 2018, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; An, H.; Yu, F.; Yang, J.; Ding, H.; Bao, Y.; Xie, H.; Huang, D. The Neuroprotective Effects of Paeoniflorin against MPP+-Induced Damage to Dopaminergic Neurons via the Akt/Nrf2/GPX4 Pathway. J. Chem. Neuroanat. 2022, 122, 102103. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, C.; Du, H.; Chen, Y.; Huang, X.; Gong, L.; You, P.; Deng, J.; Liu, Y.; Feng, H.; et al. Novel Functional Food from an Invasive Species Polygonum Cuspidatum: Safety Evaluation, Chemical Composition, and Hepatoprotective Effects. Food Qual. Saf. 2022, 6, fyac032. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Susano, P.; Martins, A.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Exploring Marine Resources against Neurological Disorders– the Neuroprotective and Anti-Inflammatory Potential of the Brown Seaweed Bifurcaria Bifurcata. J. Appl. Phycol. 2022, 34, 2671–2688. [Google Scholar] [CrossRef]
- Li, X.-L.; Xu, X.-F.; Bu, Q.-X.; Jin, W.-R.; Sun, Q.-R.; Feng, D.-P.; Zhang, Q.-J.; Wang, L.-X. Effect of Total Flavonoids from Scutellaria Baicalensis on Dopaminergic Neurons in the Substantia Nigra. Biomed. Rep. 2016, 5, 213–216. [Google Scholar] [CrossRef]
- Kumar, C.S.; Sankar, S.R.; Prabu, K. Efficacy of Hypericum Perforatum Extract on Ultrastructural Changes in Brain and Oxidative Damage in Parkinson’s Disease. Int. J. Res. Pharm. Sci. 2020, 11, 2793–2798. [Google Scholar] [CrossRef]
- Mani, S.; Sekar, S.; Barathidasan, R.; Manivasagam, T.; Thenmozhi, A.J.; Sevanan, M.; Chidambaram, S.B.; Essa, M.M.; Guillemin, G.J.; Sakharkar, M.K. Naringenin Decreases α-Synuclein Expression and Neuroinflammation in MPTP-Induced Parkinson’s Disease Model in Mice. Neurotox. Res. 2018, 33, 656–670. [Google Scholar] [CrossRef]
- Lin, M.-W.; Lin, C.C.; Chen, Y.-H.; Yang, H.-B.; Hung, S.-Y. Celastrol Inhibits Dopaminergic Neuronal Death of Parkinson’s Disease through Activating Mitophagy. Antioxidants 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Wang, X.; Islam, R.; Akash, S.; Supti, F.A.; Mitu, M.I.; Rashid, H.O.; Aktar, M.N.; Kali, M.S.K.; Jahan, F.I.; et al. Multifunctional Role of Natural Products for the Treatment of Parkinson’s Disease: At a Glance. Front. Pharmacol. 2022, 13, 976385. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Soares, G.A.B.E.; Chopra, H.; Rahman, M.M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, N.; Thapa, K.; Kaur, G.; Sharma, A.; Puri, V.; Verma, N. Update on Therapeutic Potential of Emerging Nanoformulations of Phytocompounds in Alzheimer’s and Parkinson’s Disease. J. Drug Deliv. Sci. Technol. 2023, 79, 104074. [Google Scholar] [CrossRef]
- Paramanick, D.; Singh, V.D.; Singh, V.K. Neuroprotective Effect of Phytoconstituents via Nanotechnology for Treatment of Alzheimer Diseases. J. Control. Release 2022, 351, 638–655. [Google Scholar] [CrossRef]

| Disease | Food Source | Doses | Model | Outcomes | Ref. |
|---|---|---|---|---|---|
| AD | Apple leaves | 200 and 400 mg/kg bw of extract for 9 days | Rats | The extract and phlorizin (main metabolite) exhibited strong antioxidant and BACE1 inhibitory activities | [105] |
| AD | Apple cider vinegar | 10 µM of apple cider vinegar | high-cholesterol-fed rats | Reduced oxidative stress and memory impairment and shielded cholinergic hippocampus neurons from deterioration. Reduced tau phosphorylation and amyloid aggregation. | [102] |
| AD | Avocado oil | 1 mL/kg bw | Wistar rats | The oil induced a substantial decline in neuronal loss in the CA1 and CA3 hippocampal regions. | [106] |
| AD | Banana peels | 10 μL of plant extract (50–1000 μg/mL) | Swiss Webster mice | It was found that the extract may be used in therapies for memory impairments because of its antioxidant, AChE, and tyrosinase inhibition properties. | [107] |
| AD | Carica papaya leaves | 100 µL of extract | male Wistar rats | The extract strongly reduced AChE and BChE activities | [108] |
| AD | Elettaria cardamomum extract | 100, 200, and 400 mg/kg of extract for 8 weeks | T2DM rats | In diabetic rats, the extract prevented the accumulation of tau and amyloid. In the brains of T2D rats, the extract lowered AChE and caspase-3 activity. | [99] |
| AD | Grape leaves | 100 mg/kg/day of grape leaf extract | Wistar rats | Treatment of AD rats with extract-enhanced brain function showed positive neurobehavioral changes. The neuromodulator effect of the extract was achieved through anti-amnesic activities against AlCl3-induced cerebral damages | [44] |
| AD | Grape seed | 1 to 100 µM of extract over 24 h | TMHT mouse model | By neutralizing phospho-epitopes and upsetting fibrillary structure, the extract had substantial potential for therapeutic development for disintegrating paired helical filaments. | [109] |
| AD | Grape skin and grape seed | 40 and 80 µM of extract | Model Human Blood–Brain Barrier | The production of Aβ(1–42) fibrils was significantly inhibited by grape extracts. The extracts had a stronger inhibitory impact than pure resveratrol. | [100] |
| AD | Green tea | 400 mg/kg/mL/day for 8 weeks | Wistar rats | GT reduced antioxidant capacity and improved AChE activity. | [110] |
| AD | Lemon juice | 0.6 and 1.2 mL/kg/day for 14 days | Rats | The results of this study showed that lemon juice may enhance cognitive function in rats with scopolamine-induced amnesia. | [111] |
| AD | Lycopodiastrum casuarinoides | 10 µL of triterpenoids | Isolation of compounds and test for AChE and BuChE inhibitory activities. | The triterpenoids identified showed good inhibitory effects against AChE and BuChE. | [112] |
| AD | Medicinal plant extracts | 50 µg/mL of extract for 8 h | In vitro screening | Of 33 plant extracts, 10 were determined to be active based on their capacity to considerably lower Aβ42 production. | [104] |
| AD | Medicinal plant extracts | 10 μL of plant extract (15–150 μg/mL) | Human neuroblastoma cell lines | The plant extracts exhibited high inhibitory activity against AChE, BuChE, α– and β–Glc enzymes. | [113] |
| AD | Melicope glabra leaves | 10 µL of alkylated quercetins | O-alkylated quercetins with selective AChE and β-secretase inhibitions | Influenced significant BACE1 inhibition. | [59] |
| AD | Mulberry fruit | 100 mg/kg bw of extract for 1.5–3 weeks | APP/PS1 mice | The spatial memory and learning ability of APP/PS1 mice were significantly improved. | [114] |
| AD | Olive fruit | 100 μg/mL of extract | SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans | Reduction in oxidative stress and delay of Aβ induced paralysis due to the smaller presence of Aβ aggregates. | [115] |
| AD | Orange peel extract | 200 mg/kg bw of extract for 6 weeks | Male albino rats | The extract was found to protect against AlCl3-induced neuronal damage by decreasing the activity of AChE, Aβ42 protein level, TBARS, and No level. | [116] |
| AD | Prickly pear | 100 mg/kg bw of extract of pulp and peel | Rats | Attenuated AlCl3-induced learning and memory impairment. Significantly reduced the higher brain levels of proinflammatory cytokines | [117] |
| AD | Sweet pepper | 20 µL of extract | Antioxidant activity and inhibition of key enzymes | The strongest antioxidant, anti-BChE, and anti-BACE1 activities were found in green sweet peppers. The highest AChE inhibition levels were found in yellow sweet pepper extract. | [101] |
| AD | Solanum lyratum | 12.5, 25, 50 μM of solanoids F–I for 1 h | SH-SY5Y cells | Solanoids F–I exhibited neuroprotective effects against H2O2-induced oxidative damage of human SH-SY5Y cells. | [118] |
| AD | Syagrus romanzoffiana fruit and leaves | 50 and 100 mg/kg bw of extract | AChE activity assays | Caused a decline in AChE activity and enhanced the histopathological changes in the cerebral cortex and cerebellum of the rat model of AlCl3-induced AD | [119] |
| AD | Virgin olive oil | 50 mL of virgin olive oil daily for 12 months | Management of mild cognitive impairment patients’ clinical trial | AD-related biomarkers (p-tau, Aβ1–42, Aβ1–42/Aβ-40 ratio) returned to normal levels after administration of virgin olive oil | [103] |
| PD | Beans (Vicia faba) | 600 mg/kg | Male Swiss albino mice | Antioxidant, anti-inflammatory, and neuroprotective effects | [120] |
| PD | Carthamus tinctorius | 25, 50, and 100 mg/Kg of extract | C57BL/6 mice | Exerted neuroprotective effects on 6-OHDA-induced dyskinesia and dopaminergic neuron degeneration in PD mice; reduced the secretion of inflammatory factors via the attenuation of microglial NLRP3 inflammasome activation | [121] |
| PD | Citrus trifoliata | 50 and 100 mg/kg | Manganese animal | Reduction of the striatal myeloperoxidase activity; renewal of dopamine, GABA and AChE; amelioration of neuronal apoptosis, microgliosis, and peri-neuronal vacuolation | [122] |
| PD | Crocus Saitva | 50 mg/kg of Crocus sativus hydroethanolic extract | Meriones | Prevention of the development of PD resulting from lead (Pb)-induced nervous system damage, increasing TH levels in several brain areas including the substantia nigra compacta, locus coeruleus, dorsal striatum, and medial forebrain bundle. | [123] |
| PD | Curcuma Longa | 0.001–0.4 mg/mL | SH-SY5Y human neuroblastoma cells | Amelioration of salsolinol-induced toxicity, reduction of mitochondria-derived ROS, and downregulation of caspase-3 activity. | [124] |
| PD | Florida beans (mucuna pruriens) | 12.5–17.5 mg/kg | Swiss Albino mice | Improvement of motor response; reduction of dyskinesia. | [125] |
| PD | Gastrodia elata | 2.5–40 μM of 20C (polyphenols) | PC12 cells | Amelioration of mitochondrial dysfunction; alleviation of PD by inhibition of α-Syn aggregation and maturation; maintaining the homeostasis of mitochondrial dynamics. | [126] |
| PD | Ginkgo Biloba | 30–1500 μg/mL (leaf extract) in vitro; 50 mg/kg in vivo | Male C57BL/6 mice and SH-SY5Y human neuroblastoma cells | Protection of dopaminergic neurons against 6-OHDA and MPTP/MPP+-induced neurotoxicity | [127] |
| PD | Ginseng | 2.5–40 mg/kg | Male Wistar rats | Attenuation of damage caused by toxicants in the nigra and the striatum by increased number of TH-positive cells; improved motor function. | [127] |
| PD | Nicotiana tabacum | 200 μg/mL of extract | Mouse embryonic fibroblasts | Nicotine suppresses SIRT6 which confers resistance to neuron and cell death | [128] |
| PD | Paeonia lactiflora | 1, 5, 10, 50, and 100 μM/L | Isolated primary neurons from pregnant female C57BL/6 (in vivo) | Neuroprotective effects against dopaminergic neuron degeneration, MPP+-induced ferroptosis via de Akt/Nrf2/GPC4 signaling pathway and, cerebral ischemia reperfusion-induced neuroinflammation and oxidative stress via Akt/Nrf2 activation. | [129] |
| PD | Passiflora incarnata | 150 and 300 mg/kg (BEPIF: n-butanol extract of Passiflora incarnata flower) | Swiss albino mice and Sprague Dawley rats (in vivo) | Antioxidant activity that led to significant DPPH scavenging and H2O2 scavenging ability; reduced haloperidol-induced catalepsy and number of jaw movements induced by tacrine (an animal model of Parkinson tremors); protective effect in PD. | [7] |
| PD | Polygonum Cuspidatum | 20–80 mg/kg of extract | Kun Ming mice | Restores MPTP-induced motor behavioral deficits; improves exercise endurance and circadian activity of MPTP-exposed mice; protects against MPTP-induced loss of dopaminergic nigrostriatal system; inhibits neuronal apoptosis. | [130] |
| PD | Seaweed Bifurcaria bifurcata | 100 µg/mL | SH-SY5Y human neuroblastoma cells | Neuroprotective effects were mediated by the mitigation of ROS generation and mitochondrial dysfunctions, together with the reduction of Caspase-3 activity. | [131] |
| PD | Scutellaria baicalensis | 5 mg/kg/per day of Scutellaria baicalensis stem-leaf total flavonoid | C57BL/6J male mice | Reduced damage to the dopaminergic neurons; inhibition of oxidation; alleviation in the damage of oxygen free radicals to dopaminergic neurons. | [132] |
| PD | Scutellaria pinnatifida | 0.2, 0.3, 0.5, and 0.8 mM of neobaicalein | SH-SY5Y human neuroblastoma cells | Neobaicalein acted against oxidative stress, inflammation, and neurotoxins. | [40] |
| PD | St John’s Wort (Hypericum Perforatum) | 1 mL; 300 mg/kg | C57BL/6 male mice | Reduced oxidative stress and improvement in ultrastructural changes in brain tissue of PD model; antioxidant and anti-inflammatory properties; neuroprotection against MPTP-induced PD model | [133] |
| PD | Tomatoes, grapefruits | 25, 50, and 100 mg/kg b. wt., p.o | C57BL/6 male mice | Neuroprotection against MPTP-induced neurodegeneration in C57BL/6J mice; reduction of oxidative stress; neuroprotection by reducing neuroinflammation and improvement of motor function in MPTP-intoxicated mice | [134] |
| PD | Thunder God Vine (Tripterygium wilfordii) | 0.1–3 µM celastrol | SH-SY5Y human neuroblastoma cells | Celastrol provided neuroprotection in PD by activating mitophagy to degrade damaged mitochondria and further inhibited dopaminergic neuronal apoptosis. | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, T.M.; Sousa, P.; Campos, C.; Perestrelo, R.; Câmara, J.S. Secondary Bioactive Metabolites from Foods of Plant Origin as Theravention Agents against Neurodegenerative Disorders. Foods 2024, 13, 2289. https://doi.org/10.3390/foods13142289
Gomes TM, Sousa P, Campos C, Perestrelo R, Câmara JS. Secondary Bioactive Metabolites from Foods of Plant Origin as Theravention Agents against Neurodegenerative Disorders. Foods. 2024; 13(14):2289. https://doi.org/10.3390/foods13142289
Chicago/Turabian StyleGomes, Telma Marisa, Patrícia Sousa, Catarina Campos, Rosa Perestrelo, and José S. Câmara. 2024. "Secondary Bioactive Metabolites from Foods of Plant Origin as Theravention Agents against Neurodegenerative Disorders" Foods 13, no. 14: 2289. https://doi.org/10.3390/foods13142289





