The Influence of pH on the Emulsification Properties of Heated Whey Protein–Pectin Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Sample Preparation
2.2.1. Formation of Heated WPI-Pectin Complex
2.2.2. Emulsion Preparation
2.3. Particle Size and ξ-Potential Measurement
2.4. Rheological Properties’ Measurement
2.5. Creaming Index Measurement
2.6. Statistical Analysis
3. Results and Discussion
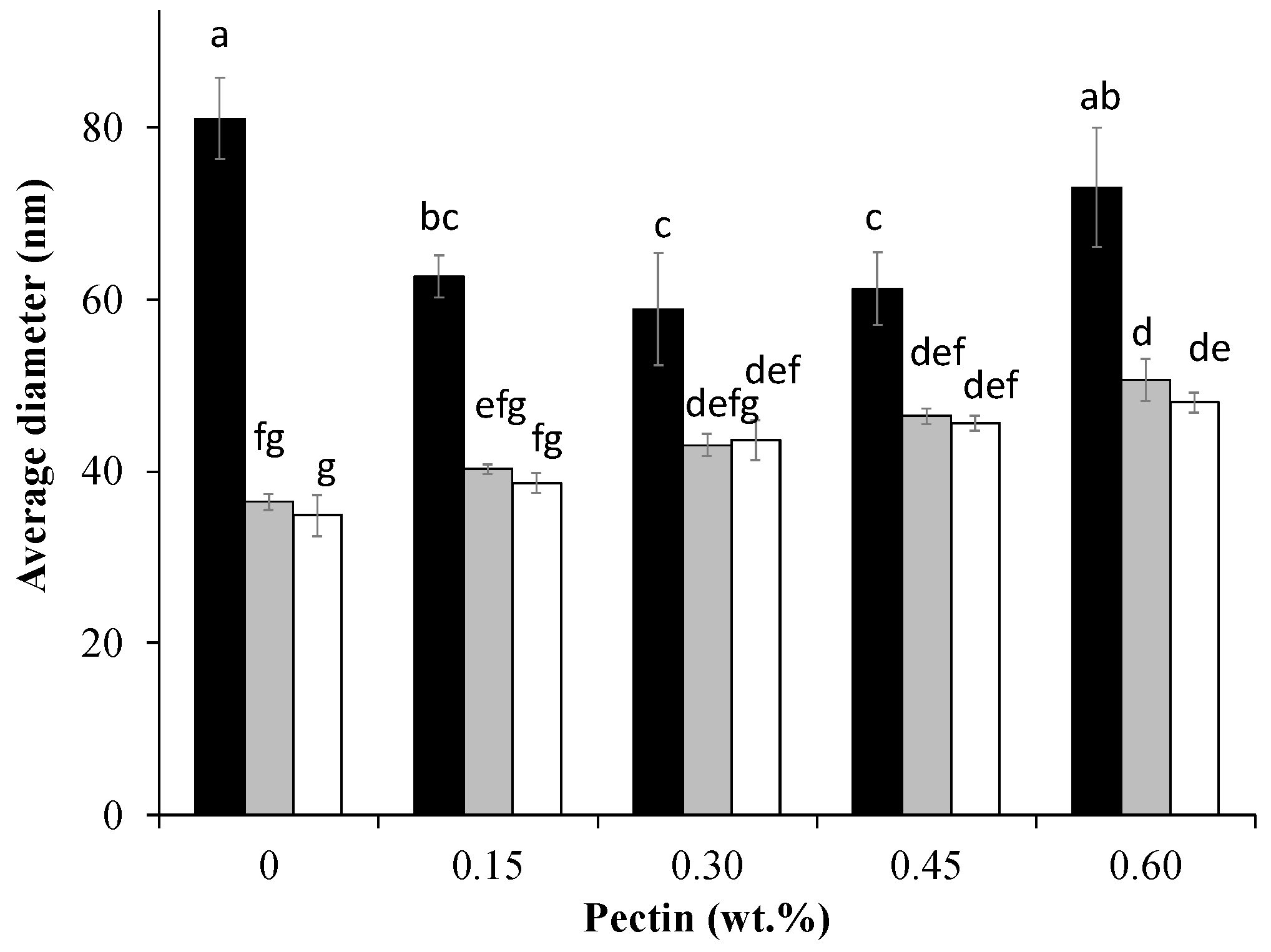
3.1. Particle Sizes and Surface Charge of Cpxs
3.2. Characterization of o/w Emulsions
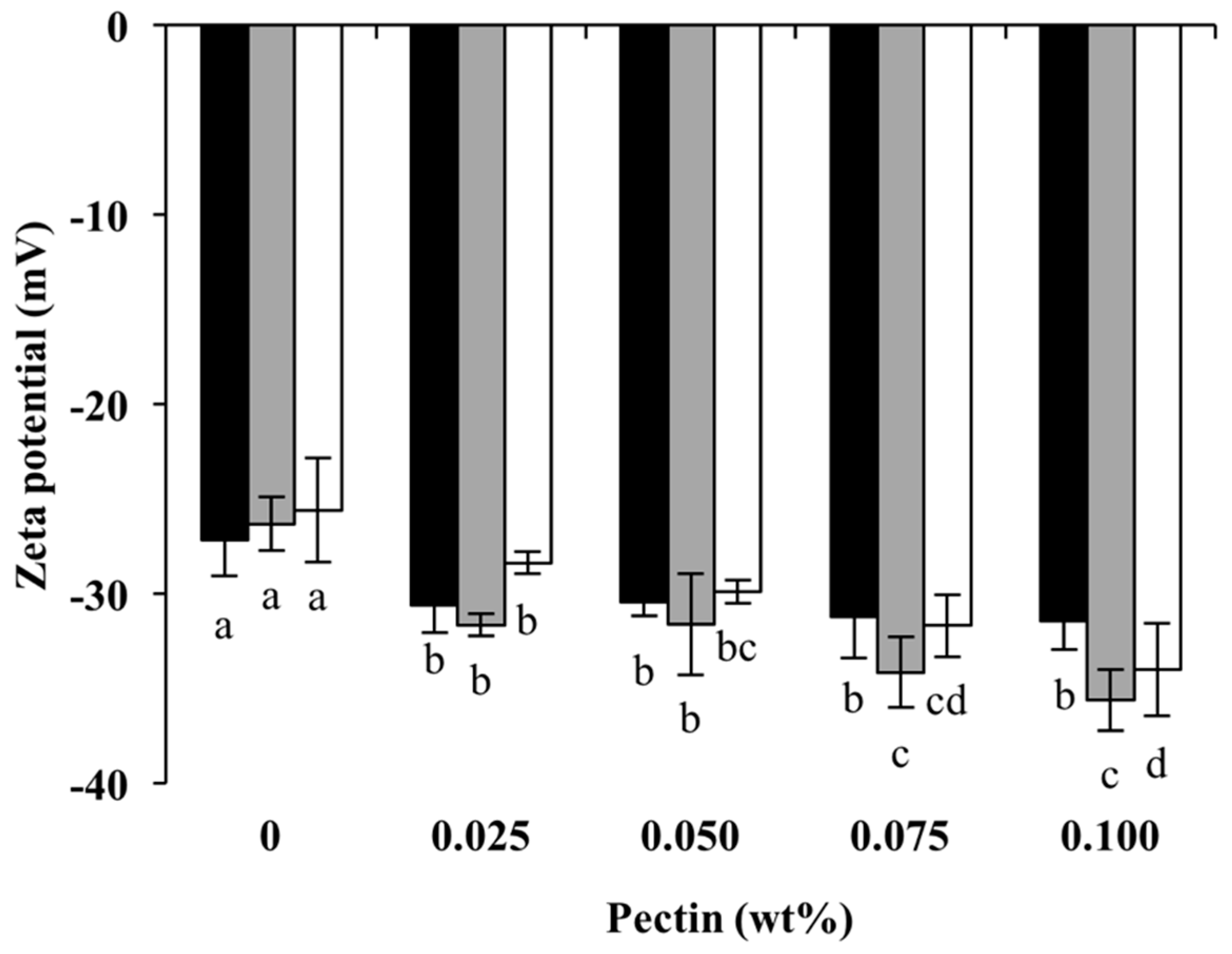
3.2.1. Surface Charge
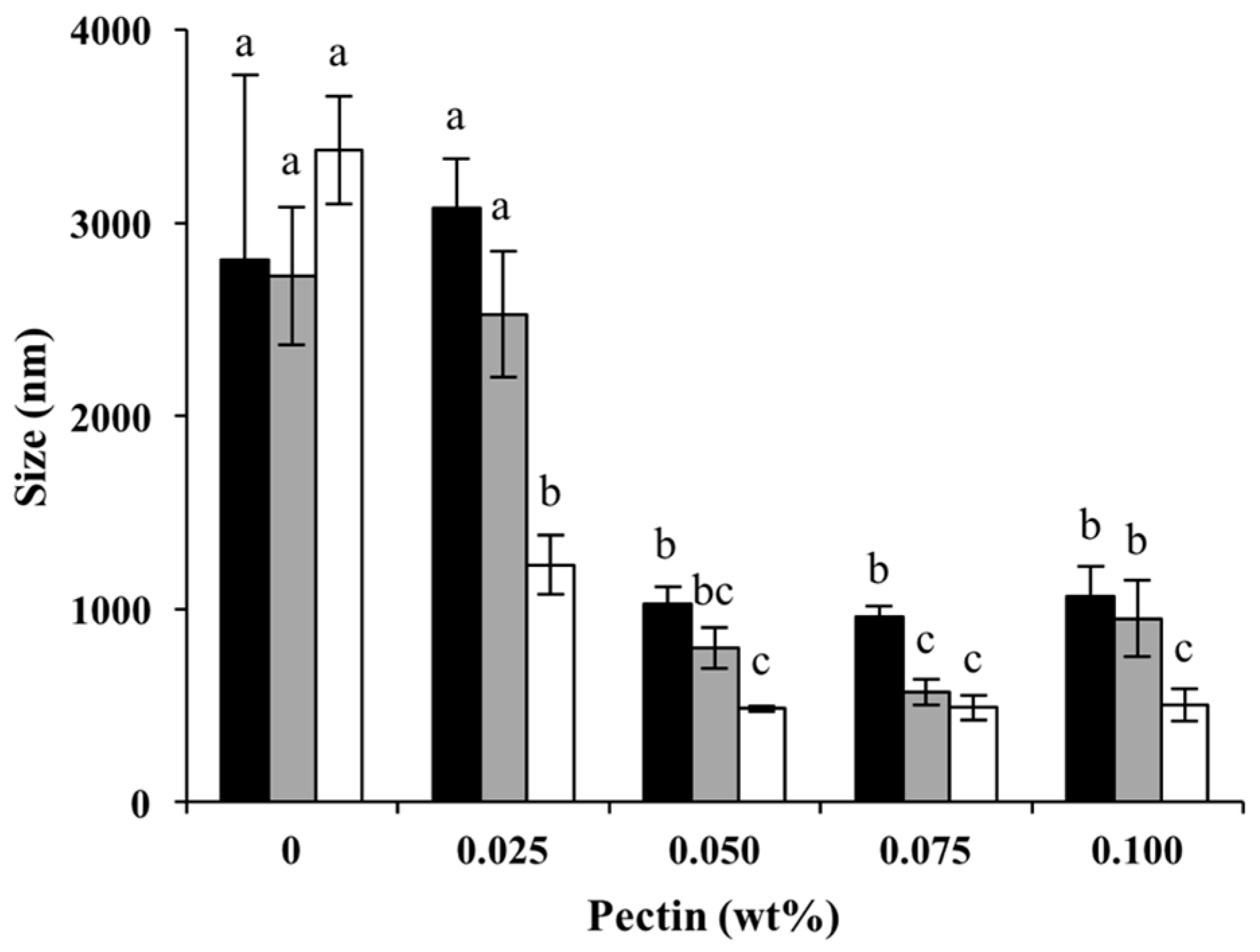
3.2.2. Droplet Size
3.2.3. Rheological Properties of Emulsions
3.2.4. Emulsion Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foegeding, E.A.; Davis, J.P.; Doucet, D.; McGuffey, M.K. Advances in modifying and understanding whey protein functionality. Trends Food Sci. Technol. 2002, 13, 151–159. [Google Scholar] [CrossRef]
- Beijerinck, M.W. Ueber Emulsionsbildung bei der Vermischung wässeriger Lösungen gewisser gelatinierender Kolloide. Z. Chem. Ind. Kolloide 1910, 7, 16–20. [Google Scholar] [CrossRef]
- Mishra, S.; Mann, B.; Joshi, V.K. Functional improvement of whey protein concentrate on interaction with pectin. Food Hydrocoll. 2001, 15, 9–15. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Lin, M.; Vardhanabhuti, B. Raman spectroscopic characterization of structural changes in heated whey protein isolate upon soluble complex formation with pectin at near neutral pH. J. Agric. Food Chem. 2012, 60, 12029–12035. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.; McClements, D. Influence of pH and pectin type on properties and stability of sodium-caseinate stabilized oil-in-water emulsions. Food Hydrocoll. 2006, 20, 607–618. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Vardhanabhuti, B. Foaming Properties of Whey Protein Isolate and lambda-Carrageenan Mixed Systems. J. Food Sci. 2015, 80, N1893–N1902. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Weinbreck, F.; de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and Technofunctional Properties of Protein-Polysaccharide Complexes: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Wagoner, T.; Vardhanabhuti, B.; Foegeding, E.A. Designing Whey Protein-Polysaccharide Particles for Colloidal Stability. Annu. Rev. Food Sci. Technol. 2016, 7, 93–116. [Google Scholar] [CrossRef]
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Tuinier, R. Polysaccharide protein interactions. Food Hydrocoll. 2001, 15, 555–563. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Schmitt, C.; Sanchez, C. Protein–polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 2007, 12, 166–178. [Google Scholar] [CrossRef]
- Huan, Y.; Zhang, S.; Vardhanabhuti, B. Influence of the molecular weight of carboxymethylcellulose on properties and stability of whey protein-stabilized oil-in-water emulsions. J. Dairy Sci. 2016, 99, 3305–3315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hsieh, F.-H.; Vardhanabhuti, B. Acid-induced gelation properties of heated whey protein–pectin soluble complex (Part I): Effect of initial pH. Food Hydrocoll. 2014, 36, 76–84. [Google Scholar] [CrossRef]
- Girard, M.; Turgeon, S.L.; Gauthier, S.F. Interbiopolymer complexing between β-lactoglobulin and low- and high-methylated pectin measured by potentiometric titration and ultrafiltration. Food Hydrocoll. 2002, 16, 585–591. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Demetriades, K.; Coupland, J.N.; McClements, D.J. Physicochemical Properties of Whey Protein-Stabilized Emulsions as affected by Heating and Ionic Strength. J. Food Sci. 1997, 62, 462–467. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Dickinson, E. Stability and rheological implications of electrostatic milk protein-polysaccharide interactions. Trends Food Sci. Technol. 1998, 9, 347–354. [Google Scholar] [CrossRef]
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Influence of pH and ι-Carrageenan Concentration on Physicochemical Properties and Stability of β-Lactoglobulin-Stabilized Oil-in-Water Emulsions. J. Agric. Food Chem. 2004, 52, 3626–3632. [Google Scholar] [CrossRef]
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Influence of pH and carrageenan type on properties of β-lactoglobulin stabilized oil-in-water emulsions. Food Hydrocoll. 2005, 19, 83–91. [Google Scholar] [CrossRef]
- Neirynck, N.; Van der Meeren, P.; Lukaszewicz-Lausecker, M.; Cocquyt, J.; Verbeken, D.; Dewettinck, K. Influence of pH and biopolymer ratio on whey protein–pectin interactions in aqueous solutions and in O/W emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 99–107. [Google Scholar] [CrossRef]
- Salminen, H.; Weiss, J. Electrostatic adsorption and stability of whey protein–pectin complexes on emulsion interfaces. Food Hydrocoll. 2014, 35, 410–419. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Roefs, S.P.; De Kruif, K.G. A model for the denaturation and aggregation of beta-lactoglobulin. Eur. J. Biochem. 1994, 226, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of Whey Proteins during Thermal Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Ryan, K.N.; Zhong, Q.; Foegeding, E.A. Use of whey protein soluble aggregates for thermal stability-a hypothesis paper. J. Food Sci. 2013, 78, R1105–R1115. [Google Scholar] [CrossRef]
- Xiong, Y.L.L. Influence of Ph and Ionic Environment on Thermal Aggregation of Whey Proteins. J. Agric. Food Chem. 1992, 40, 380–384. [Google Scholar] [CrossRef]
- Hoffmann, M.A.M.; vanMil, P.J.J.M. Heat-induced aggregation of beta-lactoglobulin: Role of the free thiol group and disulfide bonds. J. Agric. Food Chem. 1997, 45, 2942–2948. [Google Scholar] [CrossRef]
- Wagoner, T.B.; Foegeding, E.A. Whey protein–pectin soluble complexes for beverage applications. Food Hydrocoll. 2017, 63, 130–138. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Formation of biopolymer particles by thermal treatment of β-lactoglobulin–pectin complexes. Food Hydrocoll. 2009, 23, 1312–1321. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Stability of Biopolymer Particles Formed by Heat Treatment of β-lactoglobulin/Beet Pectin Electrostatic Complexes. Food Biophys. 2008, 3, 191–197. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Foegeding, E.A. Effects of dextran sulfate, NaCl, and initial protein concentration on thermal stability of β-lactoglobulin and α-lactalbumin at neutral pH. Food Hydrocoll. 2008, 22, 752–762. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Yucel, U.; Coupland, J.N.; Foegeding, E.A. Interaction between β-lactoglobulin and Dextran Sulfate at Near Neutral pH and their Effect on Thermal Stability. Food Hydrocoll. 2009, 23, 1511–1520. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Lutz, R.; Garti, N. Formation and characterization of amphiphilic conjugates of whey protein isolate (WPI)/xanthan to improve surface activity. Food Hydrocoll. 2007, 21, 379–391. [Google Scholar] [CrossRef]
- Salminen, H.; Weiss, J. Effect of Pectin Type on Association and pH Stability of Whey Protein–Pectin Complexes. Food Biophys. 2013, 9, 29–38. [Google Scholar] [CrossRef]
- Schmitt, C.; Bovay, C.; Rouvet, M.; Shojaei-Rami, S.; Kolodziejczyk, E. Whey protein soluble aggregates from heating with NaCl: Physicochemical, interfacial, and foaming properties. Langmuir 2007, 23, 4155–4166. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E. Emulsifying properties of whey protein–dextran conjugates at low pH and different salt concentrations. Colloids Surf. B Biointerfaces 2003, 31, 125–132. [Google Scholar] [CrossRef]
- Schubert, H.E.R. Product and Formulation Engineering of Emulsions. Chem. Eng. Res. Des. 2004, 82, 1137–1143. [Google Scholar] [CrossRef]
- Dickinson, E. Protein-stabilized emulsions. J. Food Eng. 1994, 22, 59–74. [Google Scholar] [CrossRef]
- Jourdain, L.; Leser, M.E.; Schmitt, C.; Dickinson, E. Stability of emulsions containing sodium caseinate and anionic polysaccharides. In Gums and Stabilisers for the Food Industry—14; Williams, P.A., Phillips, G.O., Eds.; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 272–279. [Google Scholar]
- Quemada, D.; Berli, C. Energy of interaction in colloids and its implications in rheological modeling. Adv. Colloid Interface Sci. 2002, 98, 51–85. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.M.; Berjano, M.; Guerrero, A.; Muñoz, J.; Gallegos, C. Flow behaviour and stability of light mayonnaise containing a mixture of egg yolk and sucrose stearate as emulsifiers. Food Hydrocoll. 1995, 9, 111–121. [Google Scholar] [CrossRef]
- Evans, M.; Ratcliffe, I.; Williams, P.A. Emulsion stabilisation using polysaccharide–protein complexes. Curr. Opin. Colloid Interface Sci. 2013, 18, 272–282. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S.; Richards, M.P. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocoll. 2007, 21, 555–564. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. 2—Hydrocolloids and emulsion stability. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 23–49. [Google Scholar]
- Jourdain, L.; Leser, M.E.; Schmitt, C.; Michel, M.; Dickinson, E. Stability of emulsions containing sodium caseinate and dextran sulfate: Relationship to complexation in solution. Food Hydrocoll. 2008, 22, 647–659. [Google Scholar] [CrossRef]





| Heating pH | Pectin wt% | K (Pa·sn) * | n * | R2 |
|---|---|---|---|---|
| 6.0 | 0 | 0.127 ± 0.03 a | 0.288 ± 0.06 a | 0.85 ± 0.06 |
| 0.025 | 0.133 ± 0.01 a | 0.378 ± 0.09 a | 0.93 ± 0.01 | |
| 0.050 | 0.213 ± 0.03 b | 0.262 ± 0.04 a | 0.86 ± 0.04 | |
| 0.075 | 0.156 ± 0.02 ab | 0.334 ± 0.01 a | 0.96 ± 0.01 | |
| 0.100 | 0.146 ± 0.03 ab | 0.316 ± 0.03 a | 0.91 ± 0.03 | |
| 6.5 | 0 | 0.076 ± 0.01 a | 0.478 ± 0.03 b | 0.98 ± 0.02 |
| 0.025 | 0.091 ± 0.03 ab | 0.367 ± 0.02 ab | 0.96 ± 0.02 | |
| 0.050 | 0.076 ± 0.02 a | 0.477 ± 0.06 a | 0.98 ± 0.01 | |
| 0.075 | 0.160 ± 0.02 b | 0.312 ± 0.06 a | 0.89 ± 0.03 | |
| 0.100 | 0.113 ± 0.04 ab | 0.391 ± 0.06 b | 0.98 ± 0.01 | |
| 7.0 | 0 | 0.118 ± 0.01 d | 0.405 ± 0.02 a | 0.98 ± 0.03 |
| 0.025 | 0.053 ± 0.00 b | 0.533 ± 0.01 b | 0.99 ± 0.01 | |
| 0.050 | 0.027 ± 0.01 a | 0.633 ± 0.04 b | 0.98 ± 0.00 | |
| 0.075 | 0.029 ± 0.00 a | 0.639 ± 0.05 b | 0.99 ± 0.00 | |
| 0.100 | 0.082 ± 0.01 c | 0.422 ± 0.02 a | 0.97 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Vardhanabhuti, B. The Influence of pH on the Emulsification Properties of Heated Whey Protein–Pectin Complexes. Foods 2024, 13, 2295. https://doi.org/10.3390/foods13142295
Wang Y, Vardhanabhuti B. The Influence of pH on the Emulsification Properties of Heated Whey Protein–Pectin Complexes. Foods. 2024; 13(14):2295. https://doi.org/10.3390/foods13142295
Chicago/Turabian StyleWang, Yeyang, and Bongkosh Vardhanabhuti. 2024. "The Influence of pH on the Emulsification Properties of Heated Whey Protein–Pectin Complexes" Foods 13, no. 14: 2295. https://doi.org/10.3390/foods13142295




