Preparation and Performance Evaluation of Polysaccharide–Iron Complex of Eucommia ulmoides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polysaccharide Preparation from E. ulmoides
2.3. Preparation of Polysaccharide–Iron Complex
2.4. Determination of Iron Content
2.5. SEM Observation
2.6. Average Particle Size and Zeta Potential Analysis
2.7. Thermal Stability Analysis
2.8. FTIR Analysis
2.9. XRD Analysis
2.10. Molecular Weight Analysis
2.11. Antioxidant Activity In Vitro of Polysaccharide–Iron Complex
2.11.1. DPPH Radical-Scavenging Activity
2.11.2. OH Radical-Scavenging Activity
2.12. Data Processing and Analysis
3. Results and Discussion
3.1. The Iron Content of Polysaccharide–Iron Complex
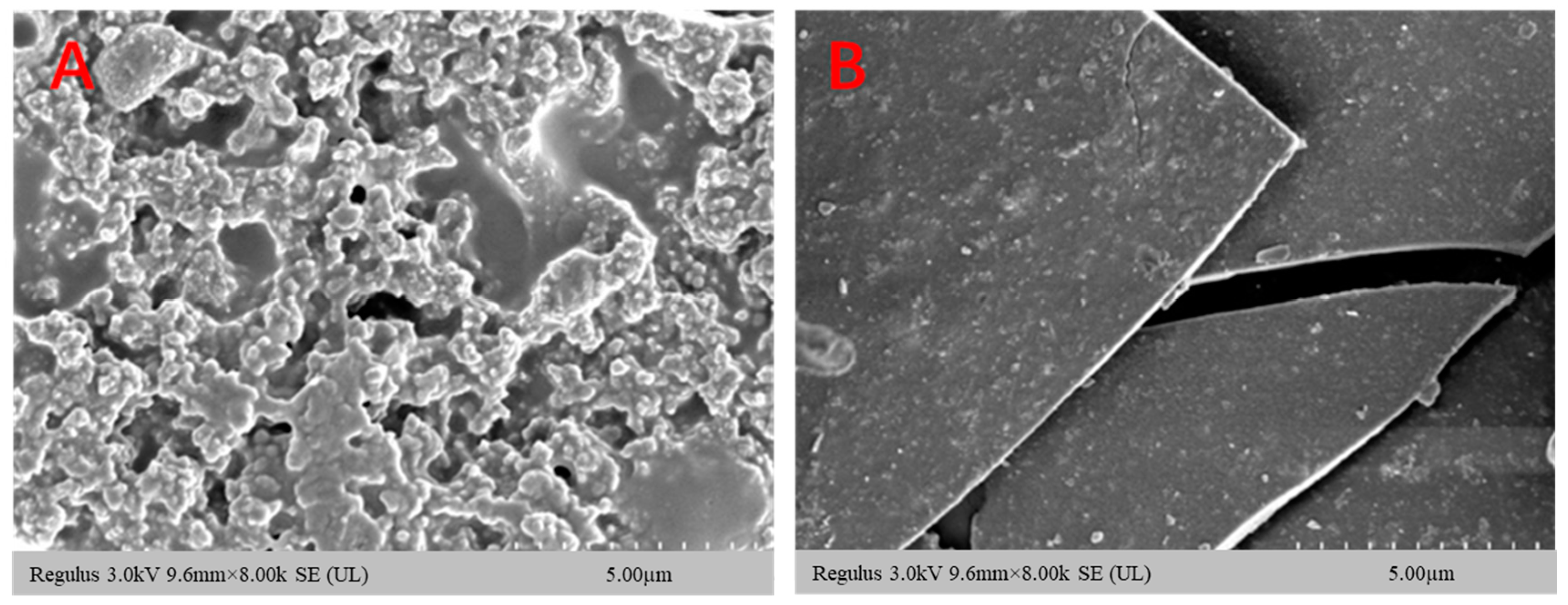
3.2. Analysis of Polysaccharide–Iron Complex Microstructure
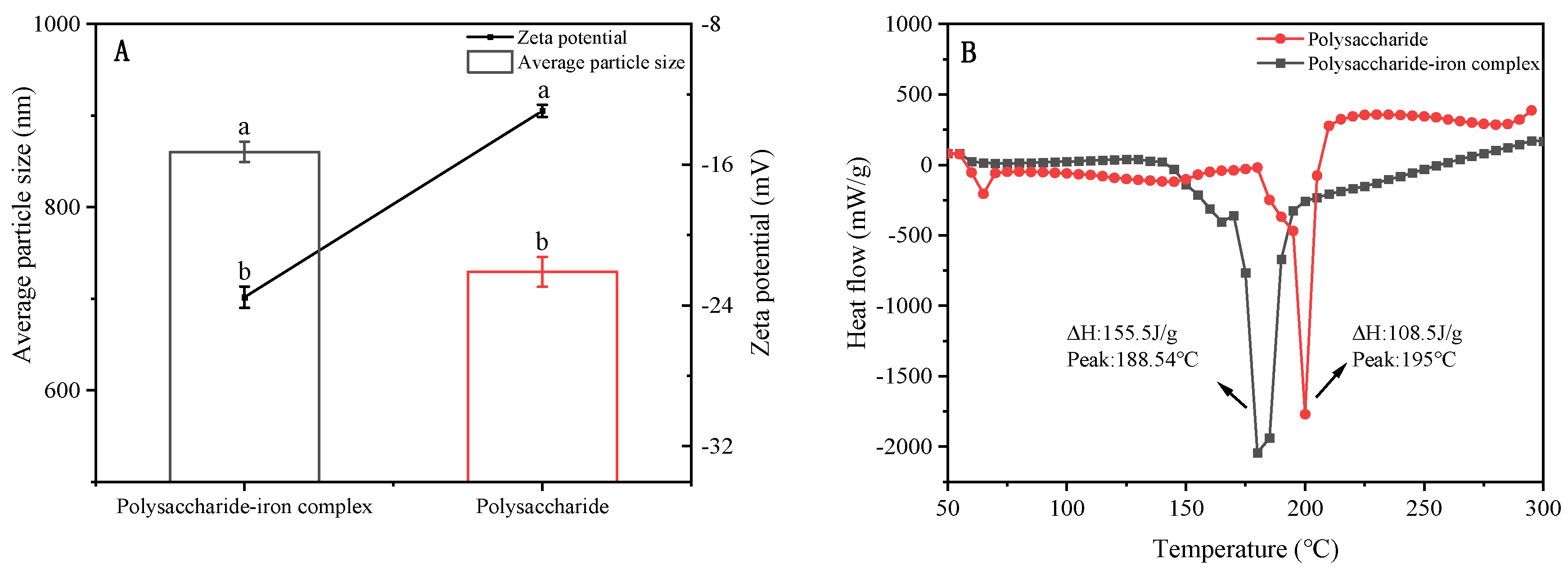
3.3. Average Particle Size and Zeta Potential of Polysaccharide–Iron Complex
3.4. Thermal Stability of Polysaccharide–Iron Complex
3.5. Infrared Spectrum Analysis of Polysaccharide–Iron Complex
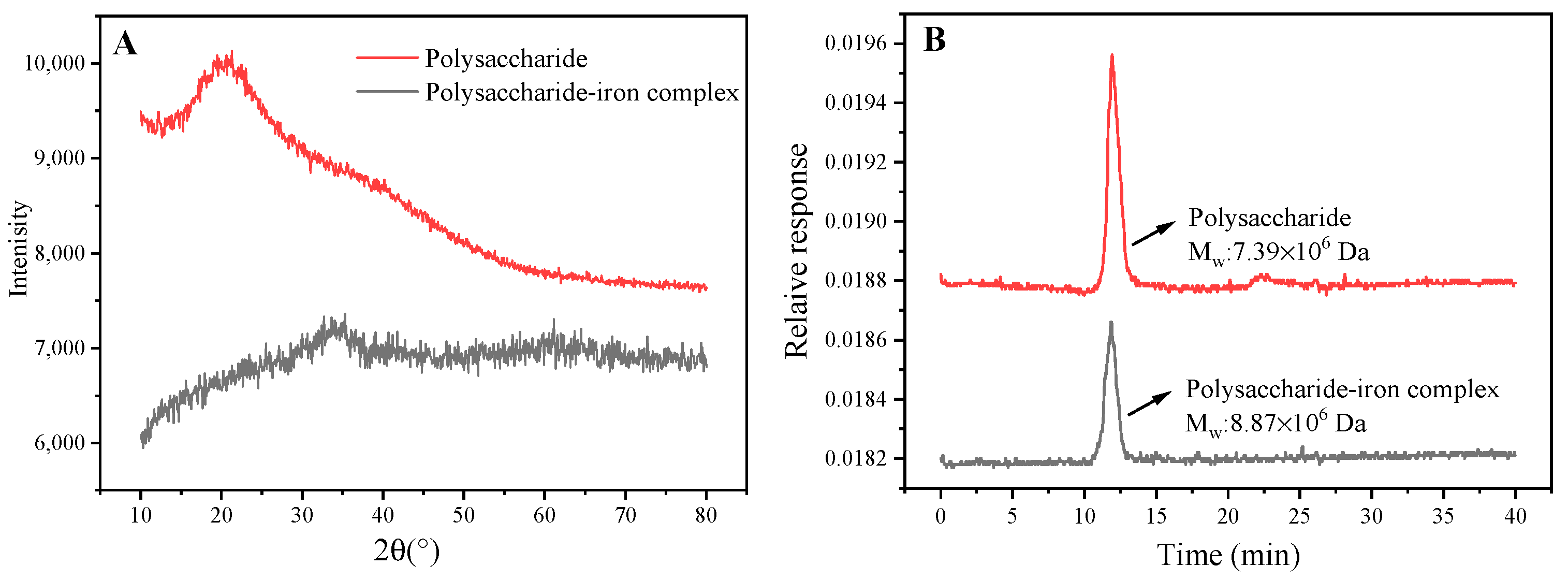
3.6. XRD Analysis of Polysaccharide–Iron Complex
3.7. Molecular Weight of Polysaccharide–Iron Complex
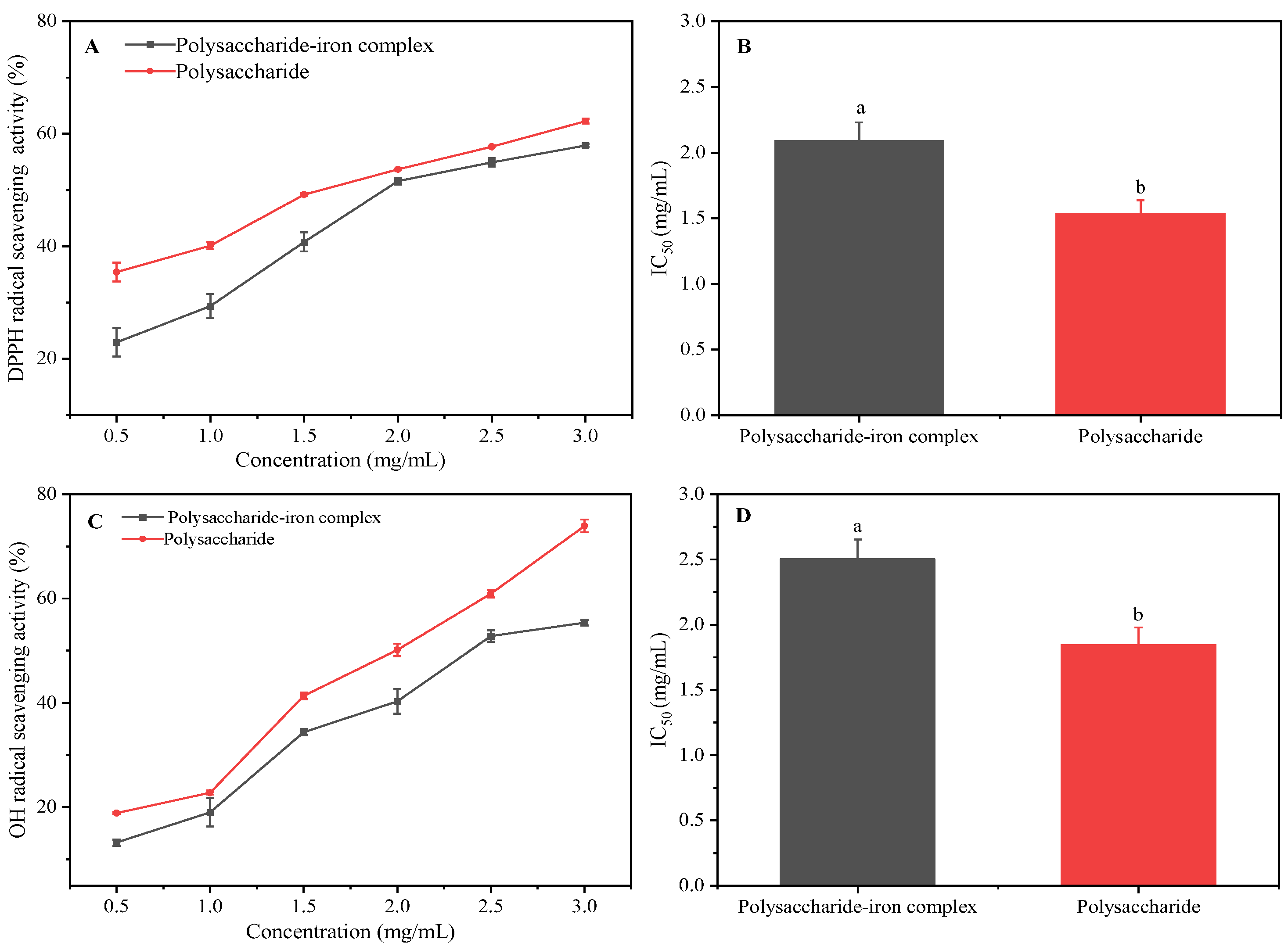
3.8. Antioxidant Activity Analysis of Polysaccharide–Iron Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Zhang, S.; Li, M.; Zhang, R.; Zhang, H.; Zheng, Y.; Zhang, D.; Wu, L. Structural characterization and biological activities of polysaccharide iron complex synthesized by plant polysaccharides: A review. Front. Nutr. 2022, 9, 1013067. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, G.L. Synthesis and antioxidant activities of garlic polysaccharide-Fe (III) complex. Int. J. Biol. Macromol. 2020, 145, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Feng, J.; Liang, Y.; Sun, H.; Yang, X.; Su, Z.; Luo, L.; Wang, J.; Zhang, W. Lycium barbarum polysaccharide-iron (III) chelate as peroxidase mimics for total antioxidant capacity assay of fruit and vegetable food. Foods 2021, 10, 2800. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: A review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Chen, S.; Guan, X.; Yong, T.; Gao, X.; Xiao, C.; Xie, Y.; Chen, D.; Hu, H.; Wu, Q. Structural characterization and hepatoprotective activity of an acidic polysaccharide from Ganoderma lucidum. Food Chem. X 2022, 13, 100204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Well. 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar] [CrossRef]
- Ganie, S.A.; Naik, R.A.; Ali, A.; Mir, T.A.; Mazumdar, N. Preparation, characterization, release and antianemic studies of guar gum functionalized iron complexes. Int. J. Biol. Macromol. 2021, 183, 1495–1504. [Google Scholar] [CrossRef]
- Yuan, S.; Dong, P.Y.; Ma, H.H.; Liang, S.L.; Li, L.; Zhang, X.F. Antioxidant and biological activities of the Lotus root polysaccharide-iron (III) complex. Molecules 2022, 27, 7106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Sun, M.; Duan, Y.; Wang, L.; Yu, N.; Peng, D.; Chen, W.; Wang, Y. Preparation, characterization, antioxidant and antianemia activities of Poria cocos polysaccharide iron (III) complex. Heliyon 2023, 9, e12819. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Ezzat, H.; Ramadan, F.; Lonappan, V.; Yasin, F.; Mohammad, N.; Thomas, M.; Alali, F. Effect of iron polysaccharide on anemia management in peritoneal dialysis and low clearance alinics. Nephrol. Dial. Transpl. 2019, 34 (Suppl. S1), gfz103.SP370. [Google Scholar] [CrossRef]
- Cherno, N.; Ozolina, S.; Nikitina, O. Preparation and characterization of iron complexes based on polysaccharides from Agaricus bisporus. East.-Eur. J. Enterp. Technol. 2014, 5, 52–57. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, D.; He, Y.; Zhang, H.; Ma, X.; Chen, S. Preparation, characterization, stability and bioactivity of fermented Tremella polysaccharide-Fe3+ complex. LWT 2024, 193, 115775. [Google Scholar] [CrossRef]
- Feng, H.; Fan, J.; Song, Z.; Du, X.; Chen, Y.; Wang, J.; Song, G. Characterization and immunoenhancement activities of Eucommia ulmoides polysaccharides. Carbohyd. Polym. 2016, 136, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Peng, M.; Tang, Q.; Liang, H.; Liang, Y.; Fang, J.; Wang, X. Comprehensive assessment of the safety of Eucommia ulmoides leaf extract for consumption as a traditional Chinese health food. J. Renew. Mater. 2023, 11, 3091–3114. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Ni, Y.; Yao, L.; Jiang, H.; Ren, X.; Fu, Y.; Zhao, C. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohyd. Polym. 2019, 12, 730557. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, R.; Wu, L.; Zhang, D.; Zheng, Y. Structural characteristics and antioxidant activity of polysaccharide-iron complex from Glehniae Radix. Int. J. Food Prop. 2020, 23, 894–907. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Wang, Y.; Xing, L. Synthesis and characterization of a new Inonotus obliquus polysaccharide-iron (III) complex. Int. J. Biol. Macromol. 2015, 75, 210–217. [Google Scholar] [CrossRef]
- Chen, P.; You, Q.; Li, X.; Chang, Q.; Zhang, Y.; Zheng, B.; Hu, X.; Zeng, H. Polysaccharide fractions from Fortunella margarita affect proliferation of Bifidobacterium adolescentis ATCC 15703 and undergo structural changes following fermentation. Int. J. Biol. Macromol. 2019, 123, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; He, F.; Fu, L.; Zhang, Y. Polysaccharide from rubescens: Extraction, optimization, characterization and antioxidant activities. RSC Adv. 2021, 11, 18974–18983. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, Y.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohyd. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.; Li, C.; Chen, J. Antioxidant and digestion properties of polysaccharides from Rosa roxburghii Tratt fruit and polysacchride-iron (III) complex. J. Food Process. Pres. 2021, 45, e15617. [Google Scholar] [CrossRef]
- Jiang, C.; Xiong, Q.; Gan, D.; Jiao, Y.; Liu, J.; Ma, L.; Zeng, X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohyd. Polym. 2013, 91, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zheng, H.Z.; Jeong, W.S.; Chung, S.K.; Qu, Z.Y.; Zou, X.; Liu, C.; Xiang, Q.; Feng, F. Synthesis, characterization, and antioxidant activity in vitro of selenium-Euryale ferox Salisb. polysaccharide. Appl. Biol. Chem. 2021, 64, 59. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science; Academic Press: New York, NY, USA, 1988. [Google Scholar]
- Jia, N.; Qiao, H.; Zhu, W.; Zhu, M.; Meng, Q.; Lu, Q.; Zu, Y. Antioxidant, immunomodulatory, oxidative stress inhibitory and iron supplementation effect of Astragalus membranaceus polysaccharide-iron (III) complex on iron-deficiency anemia mouse model. Int. J. Biol. Macromol. 2019, 132, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, N.; Wang, Q.; Zhou, J.; Liu, J.; Zhang, M.; He, C.; Chen, H. Effect of Fe (III), Zn (II), and Cr (III) complexation on the physicochemical properties and bioactivities of corn silk polysaccharide. Int. J. Biol. Macromol. 2021, 189, 847–856. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, X.M.; Han, Z.P.; Yin, L.; Zhao, M.X.; Yu, S.C. Physicochemical properties and inhibition effect on iron deficiency anemia of a novel polysaccharide-iron complex (LPPC). Bioorg. Med. Chem. Lett. 2012, 22, 489–492. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, C.; Zhao, H.; Jing, J.; Gong, N.; Lu, W. In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide–iron (III) complex. Int. J. Biol. Macromol. 2013, 60, 341–346. [Google Scholar] [CrossRef]
- Xian, H.; Wang, P.; Jing, H.; Chen, G.Q.; Cheng, D.F.; Ji, F.; Song, S.; Zhang, L. Comparative study of components and anti-oxidative effects between sulfated polysaccharide and its iron complex. Int. J. Biol. Macromol. 2018, 118, 1303–1309. [Google Scholar] [CrossRef]
- Evans, P.; Rogers, K.; Dicken, A.; Godber, S.; Prokopiou, D. X-ray diffraction tomography employing an annular beam. Opt. Express 2014, 22, 11930–11944. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, H.; Wu, Y.; Zhang, J.; Chen, Y. Preparation, characterization and antioxidant activity of a novel polysaccharide-iron (III) from Flammulina velutipes scraps. Arab. J. Chem. 2022, 15, 104190. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Fu, X. Fructus mori L. polysaccharide-iron chelates formed by self-embedding with iron (III) as the core exhibit good antioxidant activity. Food Funct. 2019, 10, 3150–3160. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wang, R.; Du, Q.; Wang, L. Structural characterization, physicochemical property, and antioxidant activity of polysaccharide components from Eucommia ulmoides leaves. Chem. Biol. Technol. Agric. 2023, 10, 122. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, J.; Li, W. Co-cultured Lepista sordida and Pholiota nameko polysaccharide-iron (III) chelates exhibit good antioxidant activity. RSC Adv. 2020, 10, 27259–27265. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, Y.; Wang, R.; Zong, W.; Zhang, L.; Wang, L. Preparation and Performance Evaluation of Polysaccharide–Iron Complex of Eucommia ulmoides. Foods 2024, 13, 2302. https://doi.org/10.3390/foods13142302
Liu M, Wang Y, Wang R, Zong W, Zhang L, Wang L. Preparation and Performance Evaluation of Polysaccharide–Iron Complex of Eucommia ulmoides. Foods. 2024; 13(14):2302. https://doi.org/10.3390/foods13142302
Chicago/Turabian StyleLiu, Mengpei, Yan Wang, Rong Wang, Wei Zong, Lihua Zhang, and Lu Wang. 2024. "Preparation and Performance Evaluation of Polysaccharide–Iron Complex of Eucommia ulmoides" Foods 13, no. 14: 2302. https://doi.org/10.3390/foods13142302




