Assessment of Multifunctional Activity of a Postbiotic Preparation Derived from Lacticaseibacillus paracasei Postbiotic-P6
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Reagents
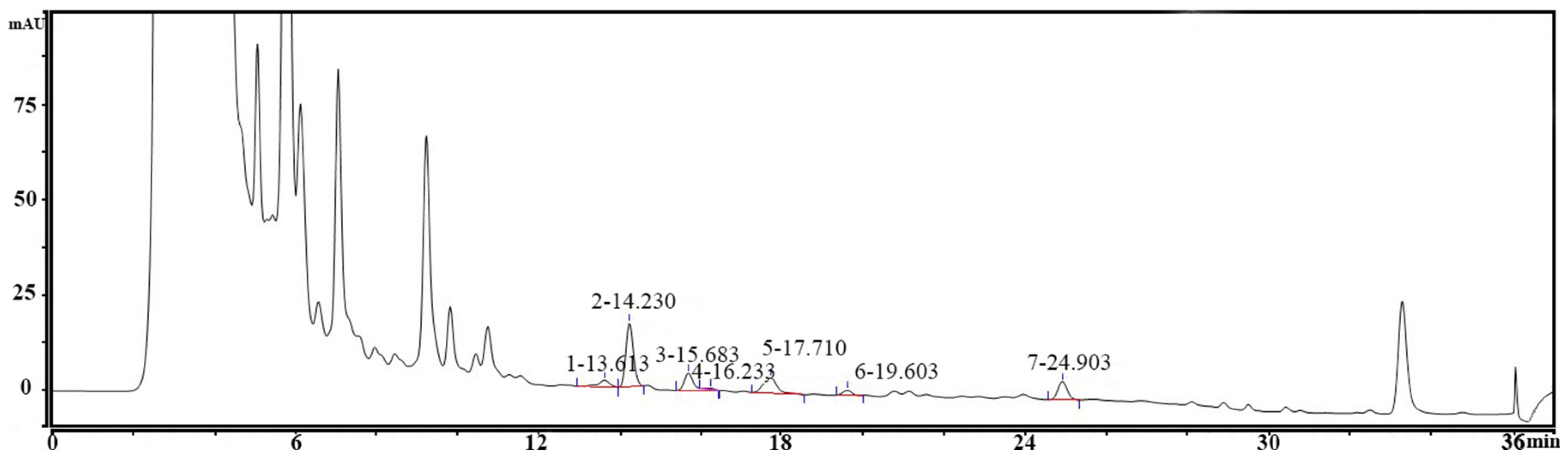
2.2. Preparation of PostbioP-6 and Determination of Biogenic Amine Production
2.3. Antihemolytic Activity Assay
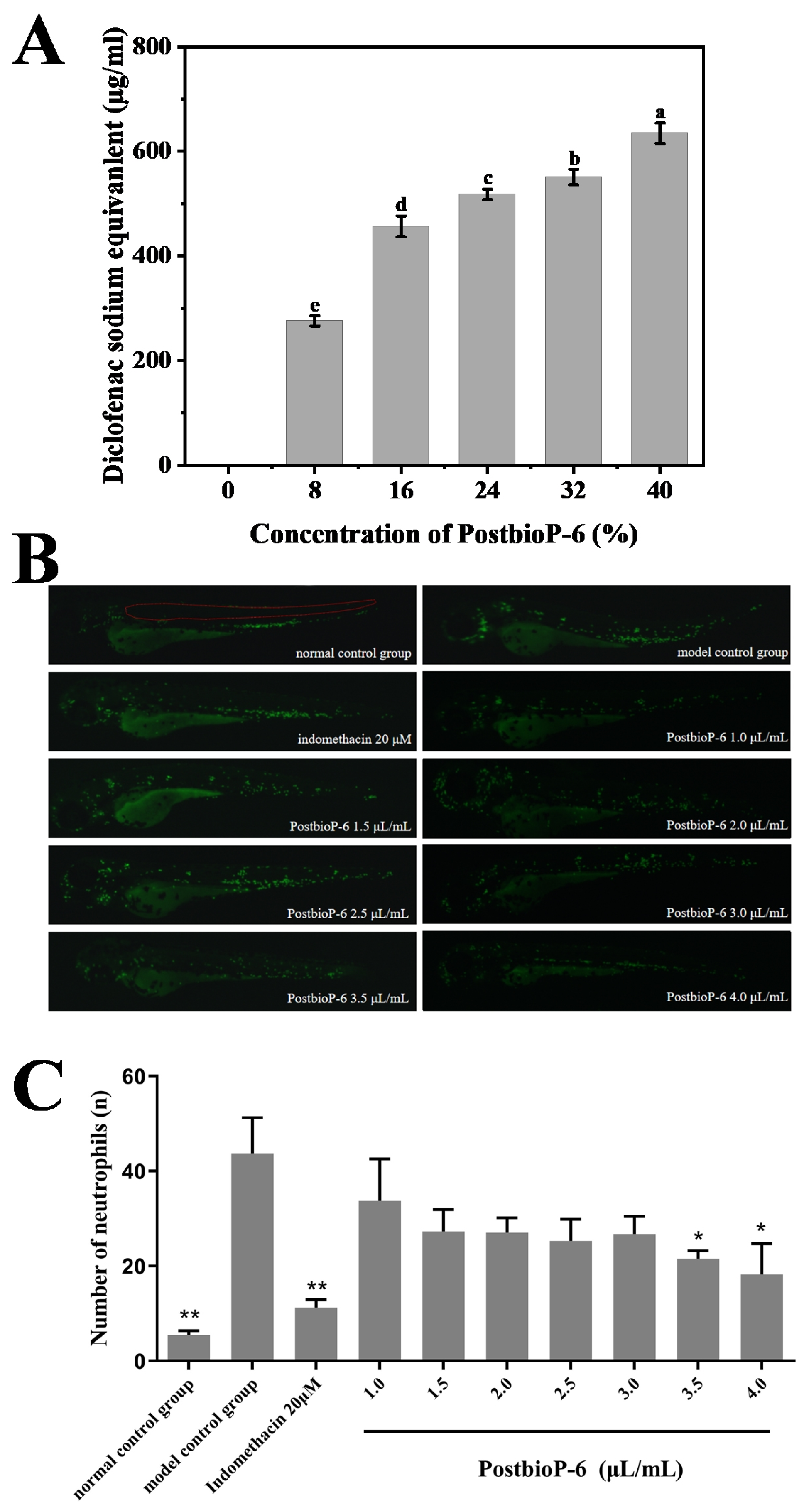
2.4. In Vitro and In Vivo Anti-Inflammatory Activity Tests
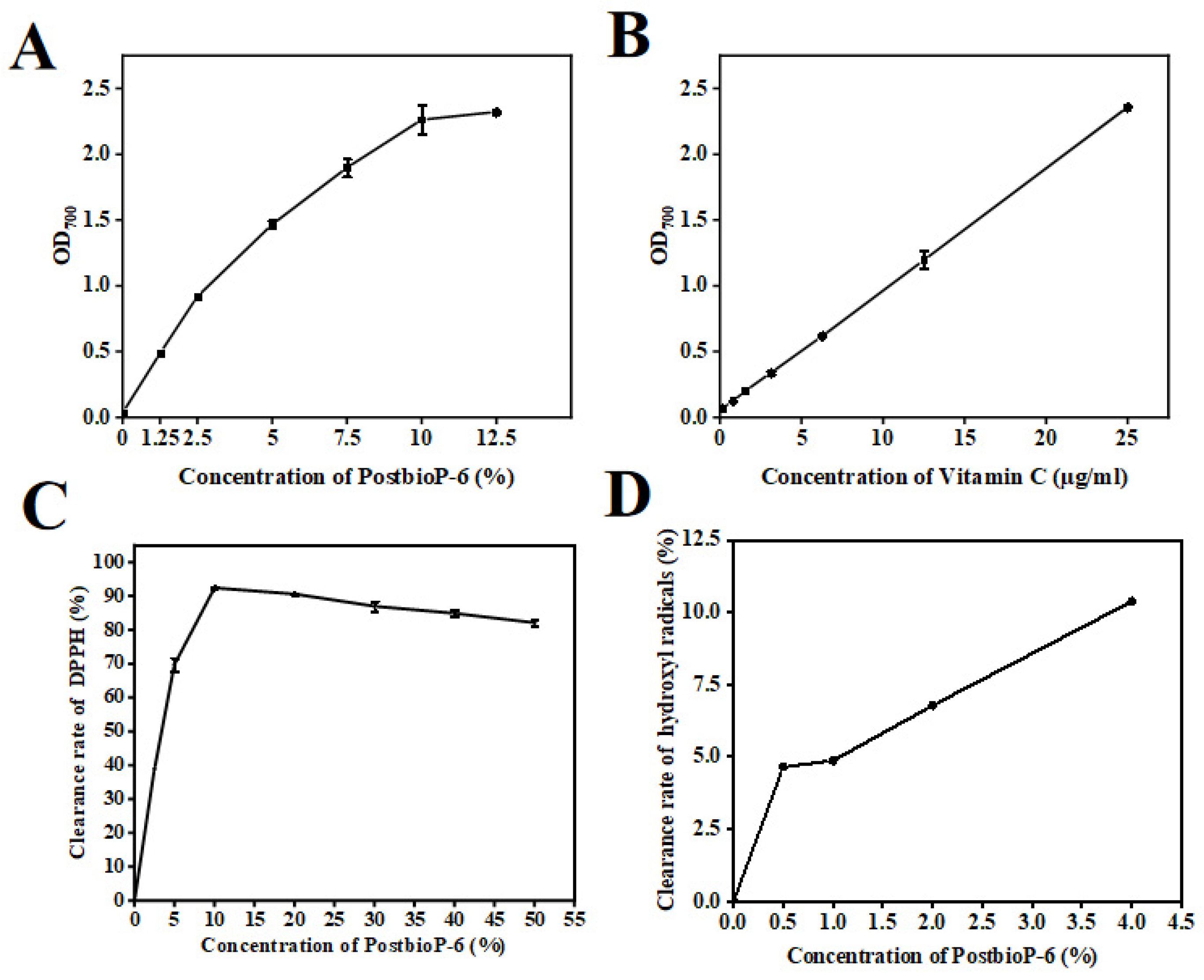
2.5. In Vitro Antioxidant Activity Assessment
2.6. Determination of Reducing Power
2.7. Measurement of DPPH Free Radical Scavenging Activity
2.8. Measurement of Hydroxyl Radical Scavenging Activity
2.9. Antibacterial Activity
2.10. The Effects of PostbioP-6 on the Peroxide Values and Malondialdehyde Content in Cookies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Determination of Biogenic Amine Production
3.2. Antihemolytic Activity of PostbioP-6
3.3. Anti-Inflammatory Activity of PostbioP-6
3.4. In Vitro Antioxidant Activity of PostbioP-6
3.5. Reducing Power
3.6. DPPH Free Radical Scavenging Activity
3.7. Hydroxyl Radical Scavenging Activity
3.8. Antimicrobial Activity
3.9. The Effects of PostbioP-6 on the Peroxide Value and Malondialdehyde Content in Cookies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aponte, M.; Murru, N.; Shoukat, M. Therapeutic, prophylactic, and functional use of probiotics: A current perspective. Front. Microbiol. 2020, 11, 562048. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bei, T.; Niu, Z.; Guo, X.; Wang, M.; Lu, H.; Gu, X.; Tian, H. Adhesion and colonization of the probiotic Lactobacillus rhamnosus labeled by dsred2 in mouse gut. Curr. Microbiol. 2019, 76, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Lenfestey, M.W.; Neu, J. Probiotics in newborns and children. Pediatr. Clin. N. Am. 2017, 64, 1271–1289. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of bifidobacteria on infant health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 2017, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of probiotics’ safety in human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Gianni, M.L.; Rescigno, M. Can postbiotics represent a new strategy for NEC? In Probiotics and Child Gastrointestinal Health: Advances in Microbiology, Infectious Diseases and Public Health; Springer: Cham, Switzerland, 2019; Volume 10, pp. 37–45. [Google Scholar] [CrossRef]
- Imperial, I.C.V.J.; Ibana, J.A. Addressing the antibiotic resistance problem with probiotics: Reducing the risk of its double-edged sword effect. Front. Microbiol. 2016, 7, 232849. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramón, D.; et al. Heat-Treated Bifidobacterium longum CECT-7347: A Whole-Cell Postbiotic with Antioxidant, Anti-Inflammatory, and Gut-Barrier Protection Properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, J.; Chen, Z.; Wang, S.; Ruan, C.; Zhou, W.; Miao, M.; Shi, H. Inhibitory effect of a microecological preparation on azoxymethane/dextran sodium sulfate-induced inflammatory colorectal cancer in mice. Front. Oncol. 2020, 10, 562189. [Google Scholar] [CrossRef] [PubMed]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef] [PubMed]
- Myeong, J.Y.; Jung, H.Y.; Chae, H.S.; Cho, H.H.; Kim, D.K.; Jang, Y.J.; Park, J.I. Protective effects of the postbiotic plantarum MD35 on bone loss in an ovariectomized mice model. Probiotics Antimicrob. Proteins 2023, 16, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzym. Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [PubMed]
- Homayouni Rad, A.; Aghebati Maleki, L.; Samadi Kafil, H.; Abbasi, A. Postbiotics: A novel strategy in food allergy treatment. Crit. Rev. Food Sci. Nutr. 2021, 61, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Banakar, M.; Pourhajibagher, M.; Etemad-Moghadam, S.; Mehran, M.; Yazdi, M.H.; Haghgoo, R.; Alaeddini, M.; Frankenberger, R. Antimicrobial effects of postbiotic mediators derived from Lactobacillus rhamnosus GG and Lactobacillus reuteri on Streptococcus mutans. Front. Biosci. 2023, 28, 88. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet. Res. 2019, 15, 315. [Google Scholar] [CrossRef]
- Kwon, M.; Lee, J.; Park, S.; Kwon, O.-H.; Seo, J.; Roh, S. Exopolysaccharide Isolated from Lactobacillus plantarum L-14 Has Anti-Inflammatory Effects via the Toll-Like Receptor 4 Pathway in LPS-Induced RAW 264.7 Cells. Int. J. Mol. Sci. 2020, 21, 9283. [Google Scholar] [CrossRef] [PubMed]
- Wuri, G.; Liu, F.; Sun, Z.; Fang, B.; Zhao, W.; Hung, W.L.; Liu, W.H.; Zhang, X.; Wang, R.; Wu, F.; et al. Lactobacillus paracasei ET-22 and derived postbiotics reduce halitosis and modulate oral microbiome dysregulation—A randomized, double-blind placebo-controlled clinical trial. Food Funct. 2023, 14, 7335–7346. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, L.; Hasannezhad, P.; Abbasi, A.; Khani, N. Antibacterial, antiviral, antioxidant, and anticancer activities of postbiotics: A review of mechanisms and therapeutic perspectives. Biointerface Res. Appl. Chem. 2021, 12, 2629–2645. [Google Scholar] [CrossRef]
- Lv, L.; Ruan, G.; Ping, Y.; Cheng, Y.; Tian, Y.; Xiao, Z.; Zhao, X.; Chen, D.; Wei, Y. Clinical study on sequential treatment of severe diarrhea irritable bowel syndrome with precision probiotic strains transplantation capsules, fecal microbiota transplantation capsules and live combined bacillus subtilis and enterococcus faecium capsules. Front. Cell. Infect. Microbiol. 2022, 12, 1025889. [Google Scholar] [CrossRef] [PubMed]
- Elkolli, M.; Elkolli, H.; Laouer, H. First Report on the Antimicrobial, Antioxidant, Antihemolytic and Antiinflammatory Activities of Extracts of Two Apiaceous Species from Eastern Algeria. Curr. Bioact. Compd. 2023, 19, 54–61. [Google Scholar] [CrossRef]
- Spickett, C.M.; Verrastro, I.; Pitt, A.R. Protein oxidation and protein redox interactions in metabolic and inflammatory diseases. Free Radic. Biol. Med. 2017, 108, S12. [Google Scholar] [CrossRef]
- Yue, C.S.; Selvi, C.; Tang, A.N.; Chee, K.N.; Ng, H.Y. Determination of biogenic amines in Malaysian traditional wine by High-Performance Liquid Chromatography (HPLC). Anal. Lett. 2021, 54, 1968–1994. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- He, B.L.; Hu, T.G.; Wu, H. Phenotypic screening of novel probiotics with potential anti-neuroinflammation activity based on cell and zebrafish models. Food Biosci. 2023, 55, 102949. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, X.; Li, F.; Zhang, Y.; Huang, Z.; Wang, Y.; Li, K.; Bao, Y.; Iqbal, M.; Fakhar-e-Alam Kulyar, M.; et al. Probiotic properties of Bacillus proteolyticus isolated from Tibetan yaks, China. Front. Microbiol. 2021, 12, 649207. [Google Scholar] [CrossRef] [PubMed]
- DÜz, M.; DoĞan, Y.N.; DoĞan, İ. Antioxidant activitiy of Lactobacillus plantarum, Lactobacillus sake and Lactobacillus curvatus strains isolated from fermented Turkish Sucuk. An. Acad. Bras. Ciênc. 2020, 92, e20200105. [Google Scholar] [CrossRef] [PubMed]
- Setyo Putri, A.Y.; Purwanta, M.; Indiastuti, D.; Kawilarang, A.P. Antibacterial Activity Test of Jatropha multifida L. sap against Staphylococcus aureus and Methicillin Resistant Staphylococcus aureus (MRSA) in vitro. Indian J. Forensic Med. Toxicol. 2021, 15, 2015–2020. [Google Scholar] [CrossRef]
- Cirlini, M.; Caligiani, A.; Palla, G.; De Ascentiis, A.; Tortini, P. Stability studies of ozonized sunflower oil and enriched cosmetics with a dedicated peroxide value determination. Ozone Sci. Eng. 2012, 34, 293–299. [Google Scholar] [CrossRef]
- Castrejon, S.E.; Ysstumirsk, A.K. Cyclodextrin enhanced fluorimetric determination of malonaldehyde by the thiobarbituric acid method. Talanta 1997, 44, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Lee, J.H.; Park, Y.K.; Lee, J.-H.; Mah, J.-H. The Occurrence of Biogenic Amines and Determination of Biogenic Amine-Producing Lactic Acid Bacteria in Kkakdugi and Chonggak Kimchi. Foods 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xing, Y.; Gao, J.; Jin, R.; Lin, R.; Weng, W.; Xie, Y.; Aweya, J.J. Lacticaseibacillus paracasei fermentation broth identified peptide, Y2Fr, and its antibacterial activity on Vibrio parahaemolyticus. Microb. Pathog. 2023, 182, 106260. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.; Foley, M.; Dluzewski, A.R.; Murray, L.J.; Anders, R.F.; Tilley, L. The Plasmodium falciparum protein RESA interacts with the erythrocyte cytoskeleton and modifies erythrocyte thermal stability. Mol. Biochem. Parasitol. 1994, 66, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Khan, M.R.; Saeed, N. Assessment of phytochemicals, antioxidant, anti-lipid peroxidation and anti-hemolytic activity of extract and various fractions of Maytenus royleanus leaves. BMC Complement. Altern. Med. 2013, 13, 143. [Google Scholar] [CrossRef]
- Ahmed, T.; Archie, S.R.; Faruk, A.; Chowdhury, F.A.; Al Shoyaib, A.; Ahsan, C.R. Evaluation of the anti-inflammatory activities of diclofenac sodium, prednisolone and atorvastatin in combination with ascorbic acid. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, H.; Rauf, A.; Ben Hadda, T. Inhibition of thermal induced protein denaturation of extract/fractions of withania somnifera and isolated withanolides. Nat. Prod. Res. 2015, 29, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.A.; Punitha, S.M.J.; Rema, M. Anti-inflammatory activity of flower extract of Cassia auriculata—An in-vitro study. Int. Res. J. Pharm. Appl. Sci. 2014, 4, 57–60. [Google Scholar]
- Chaiya, P.; Senarat, S.; Phaechamud, T.; Narakornwit, W. In vitro anti-inflammatory activity using thermally inhibiting protein denaturation of egg albumin and antimicrobial activities of some organic solvents. Mater. Today Proc. 2022, 65, 2290–2295. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Samek, O.; Marova, I. Evaluation of 3-hydroxybutyrate as an enzyme-protective agent against heating and oxidative damage and its potential role in stress response of poly (3-hydroxybutyrate) accumulating cells. Appl. Microbiol. Biotechnol. 2016, 100, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dai, M.Z.; Liu, F.S.; Cao, B.B.; Guo, J.; Shen, J.Q.; Li, C.Q. Probiotics modulate intestinal motility and inflammation in zebrafish models. Zebrafish 2020, 17, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, C.; Sun, Y.; Zhang, T.; Feng, C.; Zhang, W.; Huang, T.; Yao, G.; Zhang, H.; He, Q. Both viable Bifidobacterium longum subsp. infantis B8762 and heat-killed cells alleviate the intestinal inflammation of DSS-induced IBD rats. Microbiol. Spectr. 2024, 12, e0350923. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Khalili, M.; Ebrahimzadeh, M.A. A review on antioxidants and some of their common evaluation methods. J. Maz. Univ. Med. Sci. 2015, 24, 188–208. [Google Scholar]
- Méndez-Rodríguez, D.; Molina-Pérez, E.; Spengler-Salabarri, I.; Escalona-Arranz, J.C.; Cos, P. Composición química y actividad antioxidante de Coccoloba cowellii Britton. Rev. Cuba. Quím. 2019, 31, 185–198. [Google Scholar]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A. Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs. Antioxidants 2020, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Guo, H.N.; Abbas, Z.; Zhang, J.; Wang, J.; Cheng, Q.; Peng, S.; Yang, T.; Bai, T.; Zhou, Y.; et al. Optimizing postbiotic production through solid-state fermentation with Bacillus amyloliquefaciens J and Lactiplantibacillus plantarum SN4 enhances antibacterial, antioxidant, and anti-inflammatory activities. Front. Microbiol. 2023, 14, 1229952. [Google Scholar] [CrossRef] [PubMed]
- Faraki, A.; Rahmani, F. The antioxidant activity of Lactic acid bacteria and probiotics: A review. J. Food Saf. Hyg. 2020, 6, 168–182. [Google Scholar] [CrossRef]
- Del valle, L.G. Oxidative stress in aging: Theoretical outcomes and clinical evidences in humans. Biomed. Aging Pathol. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Hooi Ling, F.; Teck Chwen, L.; May Foong, O.; Anjas Asmara, S. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Azami, S.; Arefian, E.; Kashef, N. Postbiotics of Lactobacillus casei target virulence and biofilm formation of Pseudomonas aeruginosa by modulating quorum sensing. Arch. Microbiol. 2022, 204, 157. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sevin, S.; Karaca, B.; Haliscelik, O.; Kibar, H.; OmerOglou, E.; Kiran, F. Postbiotics secreted by Lactobacillus sakei EIR/CM-1 isolated from cow milk microbiota, display antibacterial and antibiofilm activity against ruminant mastitis-causing pathogens. Ital. J. Anim. Sci. 2021, 20, 1302–1316. [Google Scholar] [CrossRef]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical application of probiotics in skin: Adhesion, antimicrobial and antibiofilm in vitro assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef] [PubMed]


| Strain Name | Form | Antibacterial Activity |
|---|---|---|
| Staphylococcus aureus | G+ | +++ |
| Listeria monocytogenes | G+ | ++ |
| Bacillus cereus | G+ | − |
| L. paracasei | G+ | − |
| L. rhamnosus | G+ | − |
| L. plantarum | G+ | − |
| Yersinia enterocolitica enteritis | G− | +++ |
| Salmonella typhimurium | G− | ++ |
| Escherichia coli | G− | +++ |
| Pseudomonas fluorescens | G− | ++ |
| Pseudomonas putida | G− | ++ |
| Pseudomonas aeruginosa | G− | − |
| Pseudomonas fragi | G− | ++ |
| Pseudomonas lundensis | G− | + |
| Enterobacter sakazakii | G− | ++ |
| Aspergillus flavus | fungi | + |
| Penicillium citri | fungi | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Ren, X.; Song, Y.; Zhang, J.; Zhuang, H.; Peng, C.; Zhao, J.; Shen, J.; Yang, J.; Zang, J.; et al. Assessment of Multifunctional Activity of a Postbiotic Preparation Derived from Lacticaseibacillus paracasei Postbiotic-P6. Foods 2024, 13, 2326. https://doi.org/10.3390/foods13152326
Dong H, Ren X, Song Y, Zhang J, Zhuang H, Peng C, Zhao J, Shen J, Yang J, Zang J, et al. Assessment of Multifunctional Activity of a Postbiotic Preparation Derived from Lacticaseibacillus paracasei Postbiotic-P6. Foods. 2024; 13(15):2326. https://doi.org/10.3390/foods13152326
Chicago/Turabian StyleDong, Hui, Xianpu Ren, Yaxin Song, Jingwen Zhang, Haonan Zhuang, Chuantao Peng, Jinshan Zhao, Jinling Shen, Jielin Yang, Jinhong Zang, and et al. 2024. "Assessment of Multifunctional Activity of a Postbiotic Preparation Derived from Lacticaseibacillus paracasei Postbiotic-P6" Foods 13, no. 15: 2326. https://doi.org/10.3390/foods13152326
APA StyleDong, H., Ren, X., Song, Y., Zhang, J., Zhuang, H., Peng, C., Zhao, J., Shen, J., Yang, J., Zang, J., Li, D., Gupta, T. B., Guo, D., & Li, Z. (2024). Assessment of Multifunctional Activity of a Postbiotic Preparation Derived from Lacticaseibacillus paracasei Postbiotic-P6. Foods, 13(15), 2326. https://doi.org/10.3390/foods13152326






