Characterization of Meat Metabolites and Lipids in Shanghai Local Pig Breeds Revealed by LC–MS-Based Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Measurement of Meat Quality Traits
2.3. Metabolite and Lipid Extraction from Tissues
2.4. LC–MS of Metabolites
2.5. LC–MS of Lipids
2.6. Data Analysis
3. Results
3.1. Meat Quality Traits
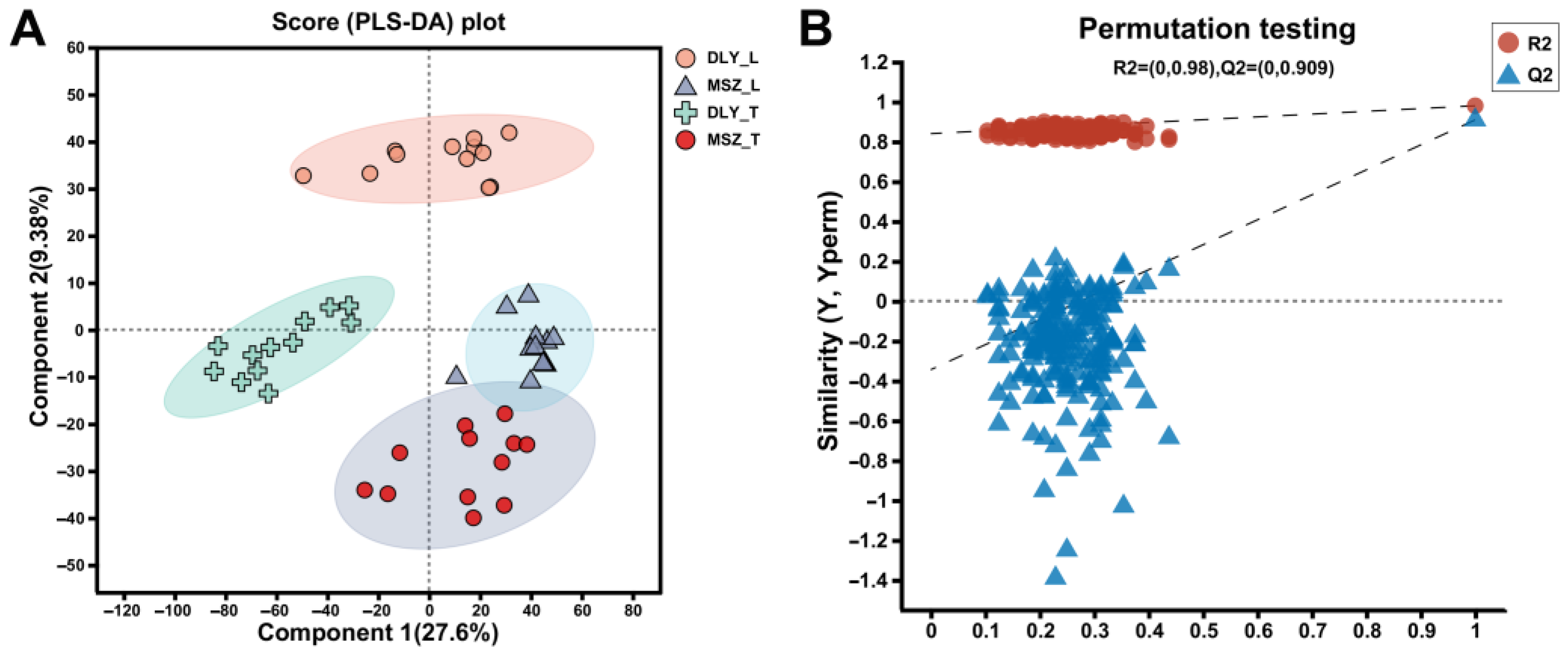
3.2. Muscle Untargeted Metabolomic Analysis
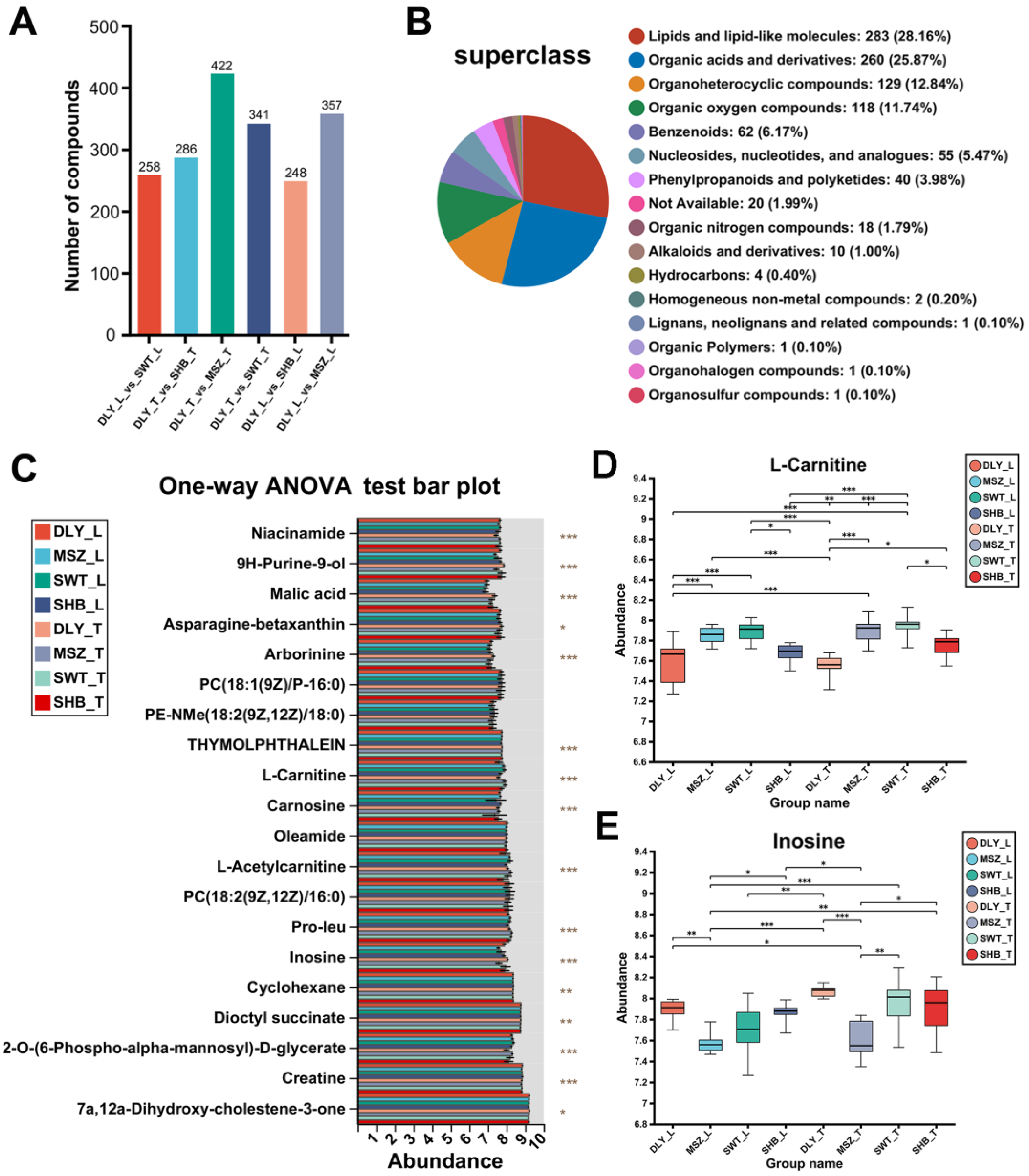
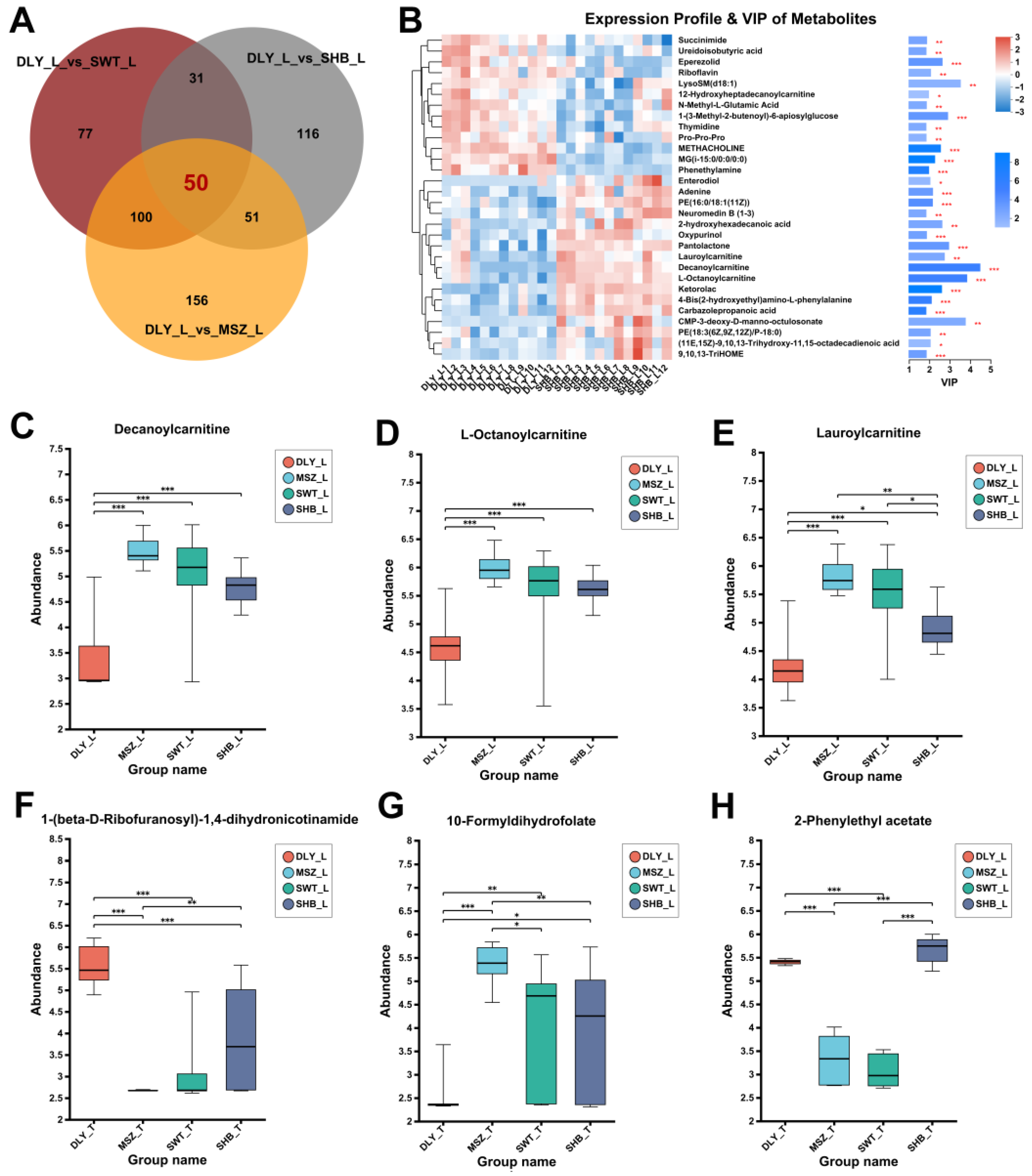
3.3. DEMs Identified between Breeds
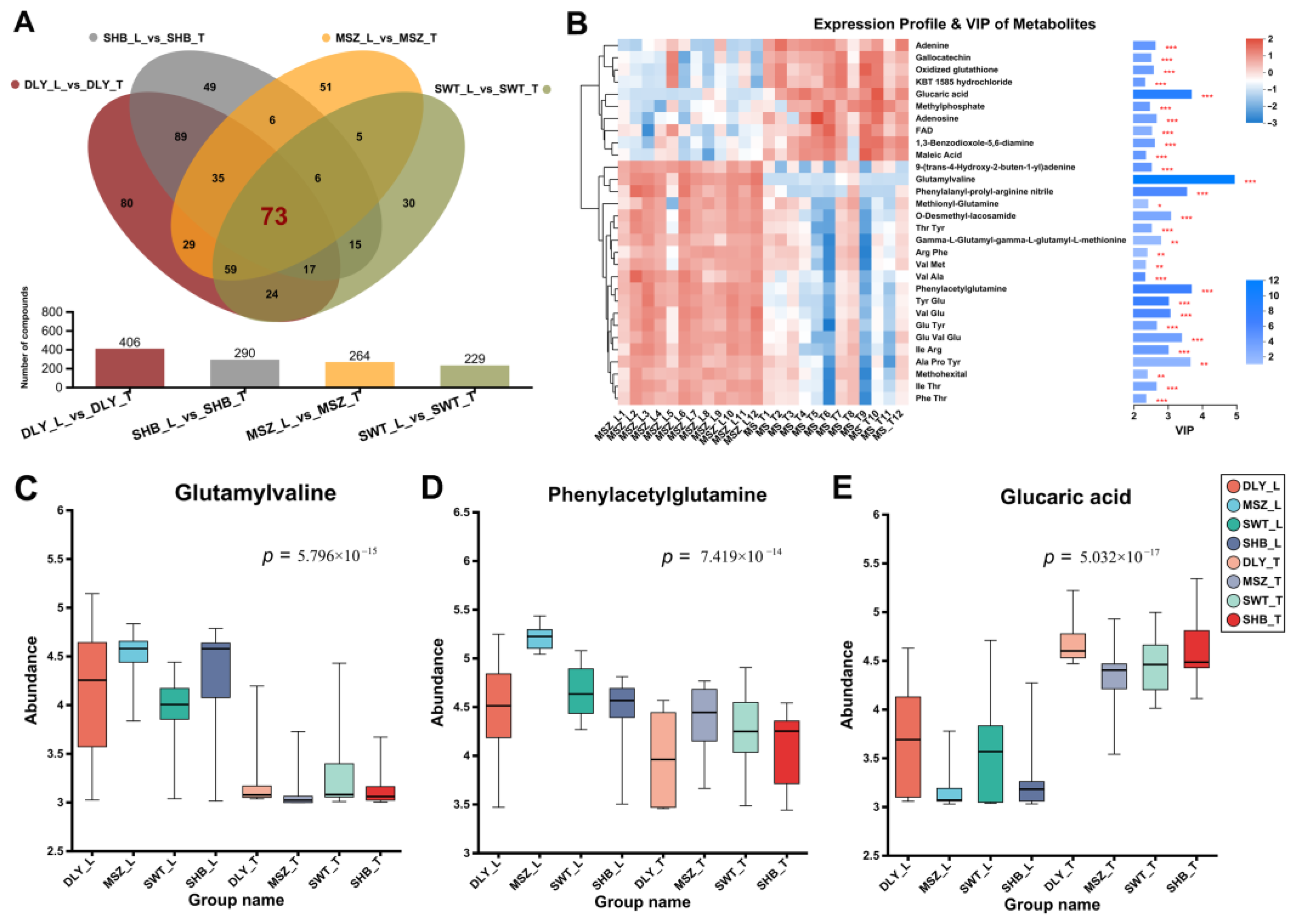
3.4. DEMs Identified between L and T Meat Parts
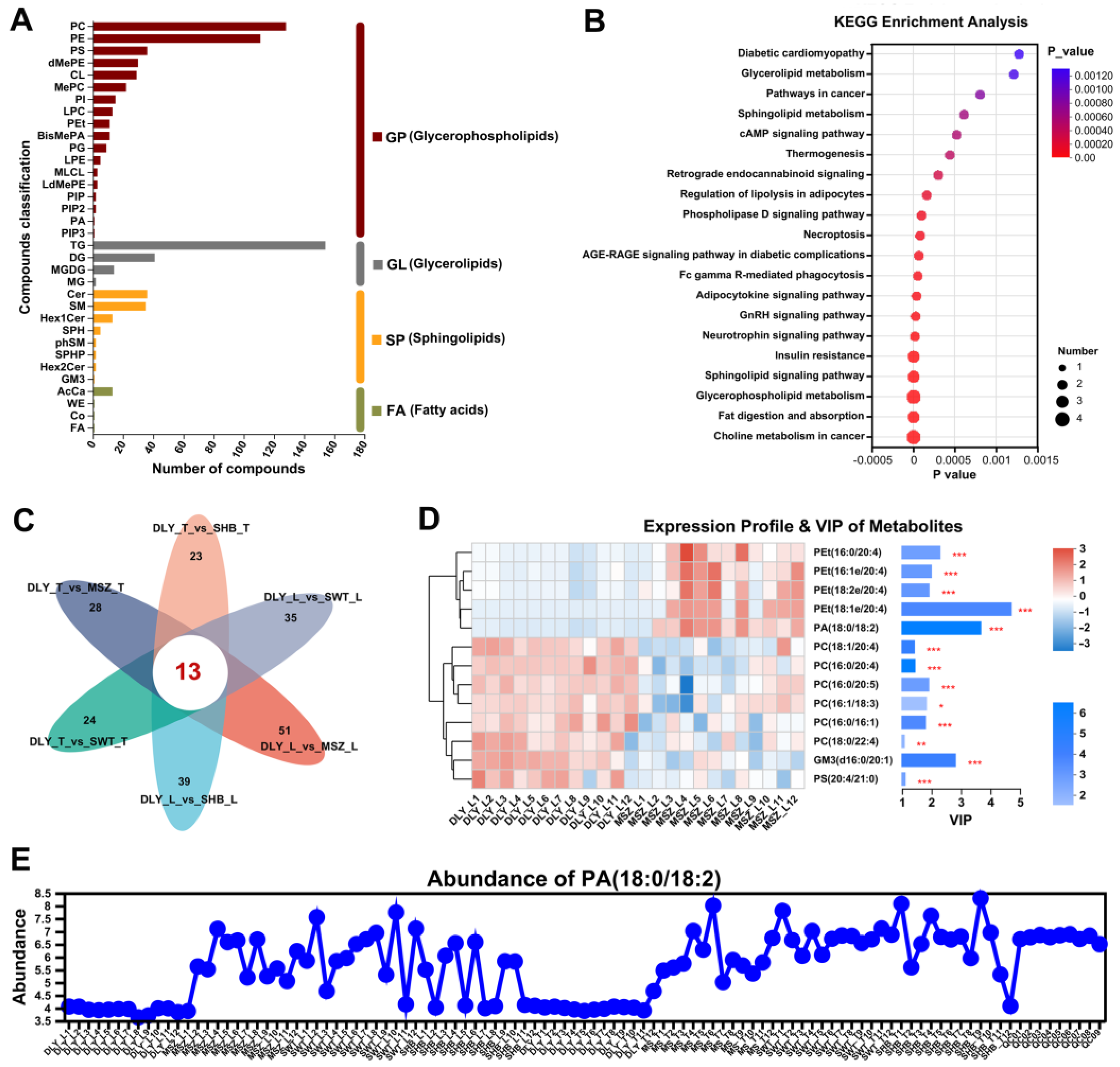
3.5. Lipidomic Analysis
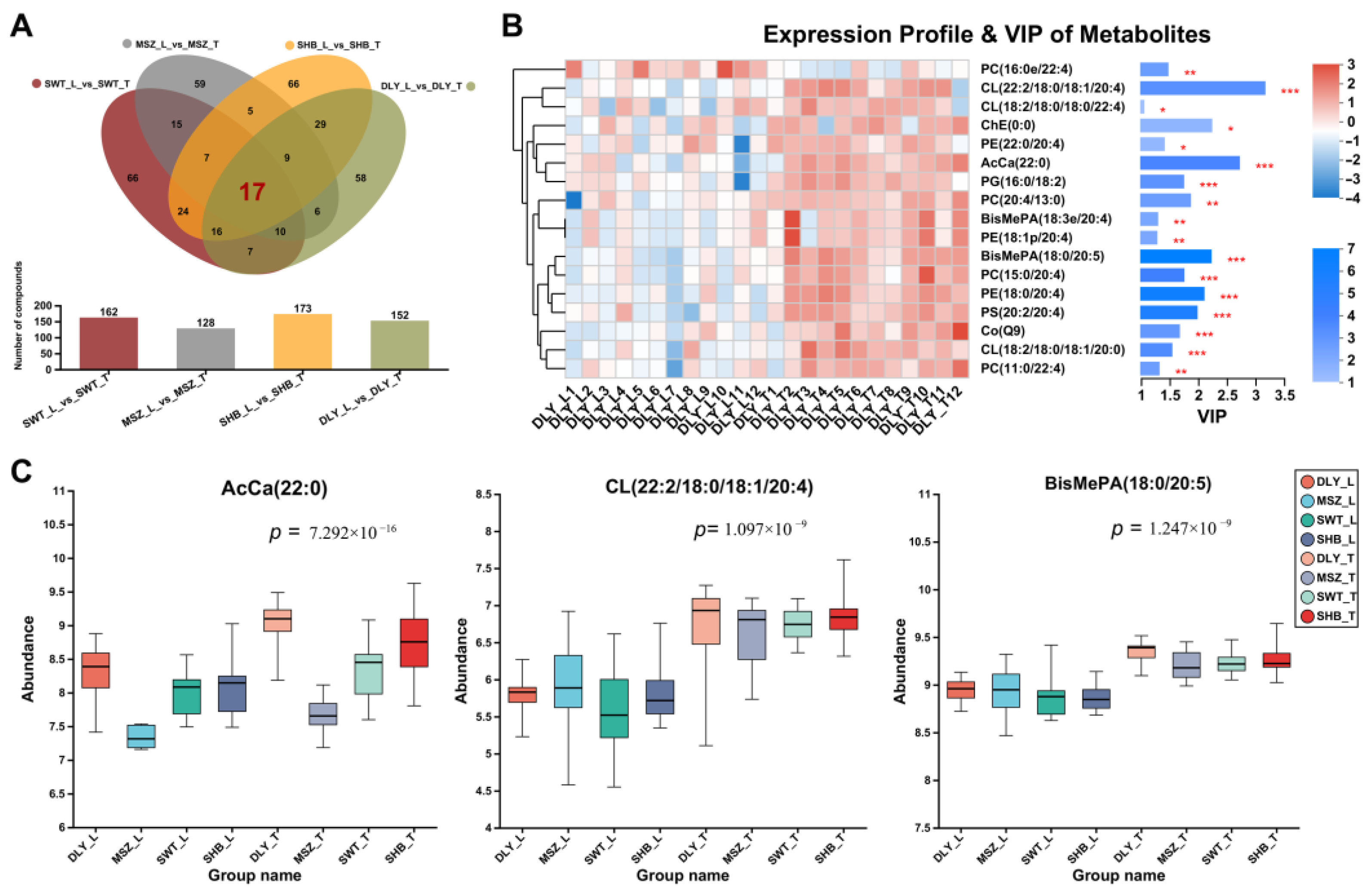
3.6. DELs Identified between L and T Meat Parts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Cheng, Y.; Xu, Y.; Hua, G.; Zheng, Z.; Li, H.; Han, L. Consumer preferences for animal welfare in China: Optimization of pork production-marketing chains. Animals 2022, 12, 3051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, X.; Jiang, G.; Tan, B.e.; Deng, J.; Yang, X.; Li, F.; Xiong, X.; Yin, Y. Effects of dietary protein/energy ratio on growth performance, carcass trait, meat quality, and plasma metabolites in pigs of different genotypes. J. Anim. Sci. Biotechnol. 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, R.; Yang, M.; Niu, N.; Wu, J.; Shu, Z.; Zhang, P.; Shi, L.; Zhao, F.; Wang, L.; et al. Metabolomics and lipidomics profiles related to intramuscular fat content and flavor precursors between Laiwu and Yorkshire pigs. Food Chem. 2023, 404, 134699. [Google Scholar] [CrossRef] [PubMed]
- Faucitano, L.; Ielo, M.C.; Ster, C.; Fiego, D.L.; Methot, S.; Saucier, L. Shelf life of pork from five different quality classes. Meat Sci. 2010, 84, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, L.; Zhang, J.; Liu, X.; Zhang, Y.; Cai, L.; Zhang, W.; Cui, L.; Yang, J.; Ji, J. A large-scale comparison of meat quality and intramuscular fatty acid composition among three Chinese indigenous pig breeds. Meat Sci. 2020, 168, 108182. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, L.; Brum, J.S. Incidence of pale, soft and exudative (PSE) pork meat in reason of extrinsic stress factors. An. Acad. Bras. Ciências 2020, 92, e20190086. [Google Scholar] [CrossRef] [PubMed]
- Adzitey, F.; Nurul, H. Pale soft exudative (PSE) and dark firm dry (DFD) meats: Causes and measures to reduce these incidences—A mini review. Int. Food Res. J. 2011, 18, 11–20. [Google Scholar]
- Choi, Y.; Kim, B. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009, 122, 105–118. [Google Scholar] [CrossRef]
- Liu, R.; Wu, G.-Y.; Li, K.-Y.; Ge, Q.-F.; Wu, M.-G.; Yu, H.; Wu, S.-L.; Bao, W.-B. Comparative study on pale, soft and exudative (PSE) and red, firm and non-exudative (RFN) pork: Protein changes during aging and the differential protein expression of the myofibrillar fraction at 1 h postmortem. Foods 2021, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shao, Y.; Liu, G.; Xing, S.; Zhang, L.; Zhu, M.; Xu, Y.; Wang, Z. Proteomics analysis as an approach to understand the formation of pale, soft, and exudative (PSE) pork. Meat Sci. 2021, 177, 108353. [Google Scholar]
- Liu, H.; He, J.; Yuan, Z.; Xie, K.; He, Z.; Zhou, X.; Wang, M.; He, J. Metabolomics Analysis Provides Novel Insights into the Difference in Meat Quality between Different Pig Breeds. Foods 2023, 12, 3476. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. N−3 fatty acids and human health: Defining strategies for public policy. Lipids 2001, 36, S83–S89. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.d.C.C.; Vicente, A.F.d.R.B. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Rong, T.; Wang, G.; Liu, Z.; Chen, W.; Li, J.; Li, J.; Ma, X. Different dietary starch sources alter the carcass traits, meat quality, and the profile of muscle amino acid and fatty acid in finishing pigs. J. Anim. Sci. Biotechnol. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, Q.; Sun, Y.; Pan, T.; Liu, S.; Miao, W.; Li, Y.; Zhou, L.; Xu, G. Lipidomic and transcriptomic analysis of the longissimus muscle of Luchuan and Duroc pigs. Front. Nutr. 2021, 8, 667622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, M.; Hou, X.; Hou, R.; Wang, L.; Shi, L.; Zhao, F.; Liu, X.; Meng, Q.; Wang, L.; et al. Characterization and difference of lipids and metabolites from Jianhe White Xiang and Large White pork by high-performance liquid chromatography–tandem mass spectrometry. Food Res. Int. 2022, 162, 111946. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, P.; Cui, Y.; Meng, Q.; Feng, Y.; Hao, Y.; Liu, J.; Piao, X.; Gu, X. Identification of metabonomics changes in longissimus dorsi muscle of finishing pigs following heat stress through lc-ms/ms-based metabonomics method. Animals 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Liu, H.; Xu, K.; Yu, R.; Oso, A.O.; Deng, J.; Yin, Y. Effects of coated cysteamine hydrochloride on muscle fiber characteristics and amino acid composition of finishing pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 1430. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Sun, L.; Pan, H.; Zhang, S.; Xu, J.; He, M.; Zhang, K.; Zhou, J.; Zhang, D.; Wu, C.; et al. Genomic Dissection through Whole-Genome Resequencing of Five Local Pig Breeds from Shanghai, China. Animals 2023, 13, 3727. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Lu, L.; Zhang, L.; Li, J.; Gu, X.; Li, J. Effects of Lactobacillus Lactis supplementation on growth performance, hematological parameters, meat quality and intestinal flora in growing-finishing pigs. Animals 2023, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Al-Dalali, S.; Zhou, H.; Xu, B. Influence of curing on the metabolite profile of water-boiled salted duck. Food Chem. 2022, 397, 133752. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, X.; Zhang, Y.; Zou, P.; Cheng, L.; Gan, R.; Li, X.; Liu, D.; Geng, F. Quantitative proteomic and metabolomic analysis of Dictyophora indusiata fruiting bodies during post-harvest morphological development. Food Chem. 2021, 339, 127884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, W.; Li, S.; Yao, S.; Qi, P.; Yang, Z.; Feng, Z.; Hou, J.; Cai, L.; Yang, M. An intelligentized strategy for endogenous small molecules characterization and quality evaluation of earthworm from two geographic origins by ultra-high performance HILIC/QTOF MS E and Progenesis QI. Anal. Bioanal. Chem. 2016, 408, 3881–3890. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E. METLIN: A technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.; Lamers, R.-J.A.; Van Der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Hrydziuszko, O.; Viant, M.R. Missing values in mass spectrometry based metabolomics: An undervalued step in the data processing pipeline. Metabolomics 2012, 8, 161–174. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Lu, X.; Lin, X.; Xu, G. A data preprocessing strategy for metabolomics to reduce the mask effect in data analysis. Front. Mol. Biosci. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. IMeta 2022, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Karaman, I. Preprocessing and pretreatment of metabolomics data for statistical analysis. In Metabolomics: From Fundamentals to Clinical Applications; Springer: Cham, Switzerland, 2017; pp. 145–161. [Google Scholar]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: A review of contemporary practice strategies and knowledge gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Prieto, B.; Eriksson, L.; Trygg, J. Variable influence on projection (VIP) for OPLS models and its applicability in multivariate time series analysis. Chemom. Intell. Lab. Syst. 2015, 146, 297–304. [Google Scholar] [CrossRef]
- Jia, A.; Xu, L.; Wang, Y. Venn diagrams in bioinformatics. Brief. Bioinform. 2021, 22, bbab108. [Google Scholar] [CrossRef] [PubMed]
- St, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar]
- Kucuk, U.; Eyuboglu, M.; Kucuk, H.O.; Degirmencioglu, G. Importance of using proper post hoc test with ANOVA. Int. J. Cardiol. 2016, 209, 346. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of Student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, W606–W612. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Wu, W.; Tang, X.; Ge, Q.; Zhan, J. Screening out important substances for distinguishing Chinese indigenous pork and hybrid pork and identifying different pork muscles by analyzing the fatty acid and nucleotide contents. Food Chem. 2021, 350, 129219. [Google Scholar] [CrossRef] [PubMed]
- Pekala, J.; Patkowska-Sokola, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochynski, S.; Librowski, T. L-carnitine-metabolic functions and meaning in humans life. Curr. Drug Metab. 2011, 12, 667–678. [Google Scholar] [CrossRef]
- Förster, L.; Indra, D.; Rosenberger, K.; Zver, L.; Hofbauer, R. L-carnitine exerts a nutrigenomic effect via direct modulation of nuclear receptor signaling in adipocytes, hepatocytes and SKMC, demonstrating its nutritional impact. Nutr. Res. 2021, 85, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; Kim, D.Y.; Han, G.D. Correlation between meat color and L-carnitine content in livestock meats. Food Sci. Biotechnol. 2009, 18, 257–261. [Google Scholar]
- Song, B.; Zheng, C.; Zheng, J.; Zhang, S.; Zhong, Y.; Guo, Q.; Li, F.; Long, C.; Xu, K.; Duan, Y.; et al. Comparisons of carcass traits, meat quality, and serum metabolome between Shaziling and Yorkshire pigs. Anim. Nutr. 2022, 8, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Losel, D.; Kalbe, C.; Rehfeldt, C. L-Carnitine supplementation during suckling intensifies the early postnatal skeletal myofiber formation in piglets of low birth weight. J. Anim. Sci. Biotechnol. 2009, 87, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Tian, Y.; Zhou, F.; Ma, J.; Xia, S.; Yang, T.; Ma, L.; Zeng, Q.; Liu, G.; et al. Obese Ningxiang pig-derived microbiota rewires carnitine metabolism to promote muscle fatty acid deposition in lean DLY pigs. Innov. 2023, 4, 100486. [Google Scholar] [CrossRef] [PubMed]
- Kuchiba-Manabe, M.; Matoba, T.; Hasegawa, K. Sensory changes in umami taste of inosine 5′-monophosphate solution after heating. J. Food Sci. 1991, 56, 1429–1432. [Google Scholar] [CrossRef]
- Subramaniyan, S.A.; Belal, S.A.; Jung, J.-H.; Jung, Y.-C.; Choi, Y.-I.; Shim, K.-S. Meat quality and physicochemical trait assessments of Berkshire and commercial 3-way crossbred pigs. Korean J. Food Sci. Anim. Resour. 2016, 36, 641. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, Y.; Cui, C.; Dong, H.; Zeng, X.; Bai, W. Umami-enhancing effect of typical kokumi-active γ-glutamyl peptides evaluated via sensory analysis and molecular modeling approaches. Food Chem. 2021, 338, 128018. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Gibney, M.J.; Brennan, L. Dietary intake patterns are reflected in metabolomic profiles: Potential role in dietary assessment studies. Am. J. Clin. Nutr. 2011, 93, 314–321. [Google Scholar] [CrossRef] [PubMed]
| Meat Quality Traits | DLY | MSZ | SWT | SHB | p-Value |
|---|---|---|---|---|---|
| IMF (%) | 3.22 ± 0.77 | 5.77 ± 0.51 | 4.74 ± 1.06 | 4.42 ± 2.29 | ** |
| Drip loss (%) | 4.76 ± 1.73 | 3.36 ± 1.09 | 5.93 ± 0.26 | 4.66 ± 1.79 | ** |
| pH | 5.78 ± 0.30 | 6.46 ± 0.27 | 5.61 ± 0.29 | 5.63 ± 0.26 | **** |
| Lightness (L*) | 15.48 ± 4.20 | 44.14 ± 2.88 | 50.21 ± 6.87 | 48.44 ± 6.56 | **** |
| Redness (a*) | 11.00 ± 1.52 | 3.81 ± 2.32 | 8.35 ± 2.64 | 6.98 ± 3.38 | * |
| Yellowness (b*) | 6.20 ± 1.71 | 9.39 ± 1.48 | 9.37 ± 2.67 | 10.67 ± 1.04 | **** |
| Water content (%) | 69.08 ± 1.47 | 69.79 ± 3.62 | 65.68 ± 6.28 | 67.50 ± 2.06 | ns |
| WHC (%) | 85.21 ± 4.28 | 91.86 ± 5.02 | 91.25 ± 6.73 | 89.65 ± 4.69 | * |
| Protein content (%) | 11.19 ± 1.79 | 14.42 ± 5.34 | 18.63 ± 2.94 | 13.10 ± 2.54 | *** |
| Shear force (kgf) | 3.35 ± 0.74 | 2.57 ± 0.31 | 4.79 ± 2.13 | 3.65 ± 0.42 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Sun, L.; Tu, W.; Cao, M.; Zhang, S.; Xu, J.; He, M.; Zhang, D.; Dai, J.; Wu, X.; et al. Characterization of Meat Metabolites and Lipids in Shanghai Local Pig Breeds Revealed by LC–MS-Based Method. Foods 2024, 13, 2327. https://doi.org/10.3390/foods13152327
Gao J, Sun L, Tu W, Cao M, Zhang S, Xu J, He M, Zhang D, Dai J, Wu X, et al. Characterization of Meat Metabolites and Lipids in Shanghai Local Pig Breeds Revealed by LC–MS-Based Method. Foods. 2024; 13(15):2327. https://doi.org/10.3390/foods13152327
Chicago/Turabian StyleGao, Jun, Lingwei Sun, Weilong Tu, Mengqian Cao, Shushan Zhang, Jiehuan Xu, Mengqian He, Defu Zhang, Jianjun Dai, Xiao Wu, and et al. 2024. "Characterization of Meat Metabolites and Lipids in Shanghai Local Pig Breeds Revealed by LC–MS-Based Method" Foods 13, no. 15: 2327. https://doi.org/10.3390/foods13152327





