Comprehensive Study of Total Nitrogen Content and Microfluidic Profiles in Additive-Enriched Plant-Based Drinks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant-Based Milk Alternative Samples
2.2. Microchip Gel Electrophoresis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Total Nitrogen Content Comparison
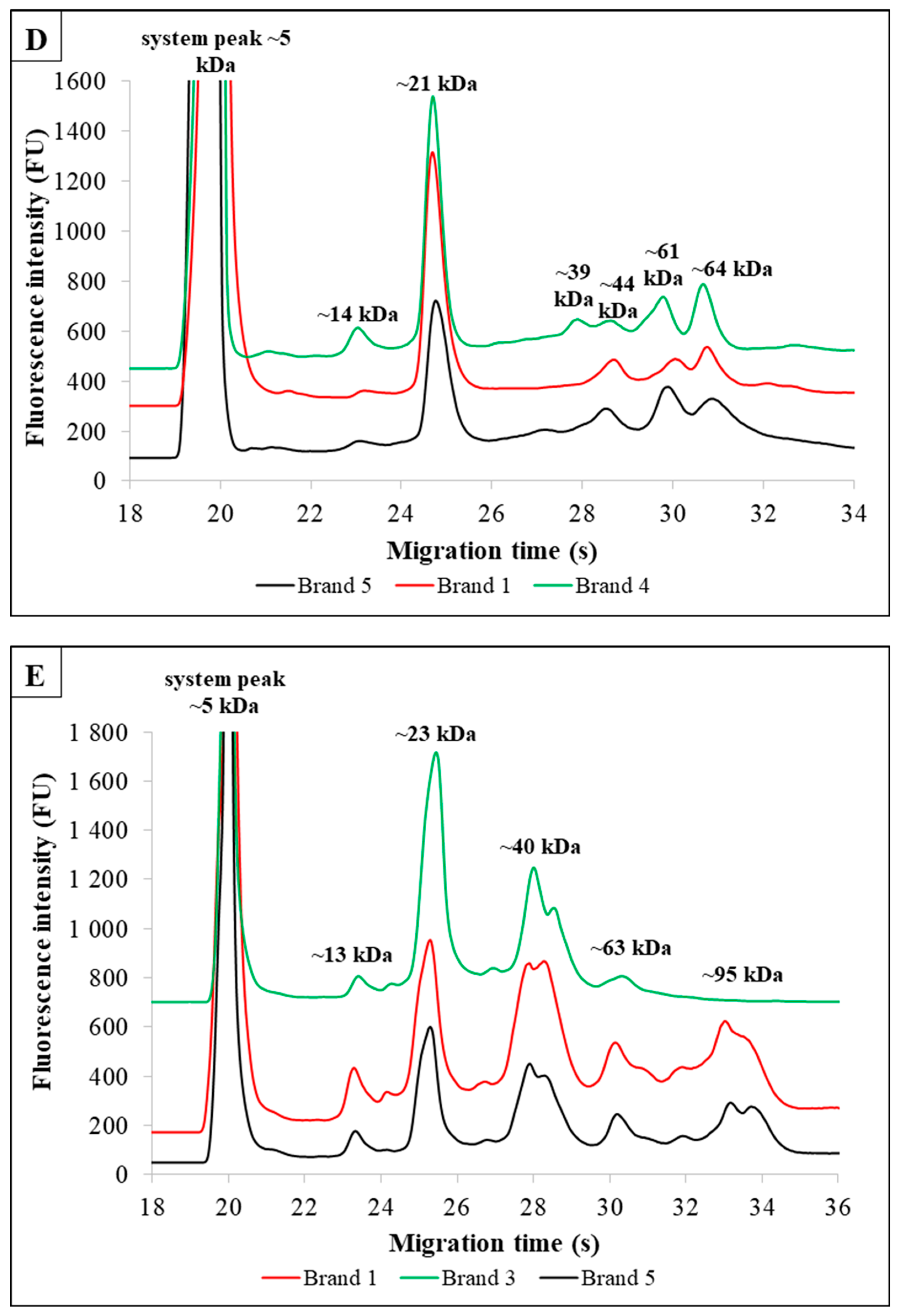
3.2. Plant-Based Milk Alternatives Enriched With Additives
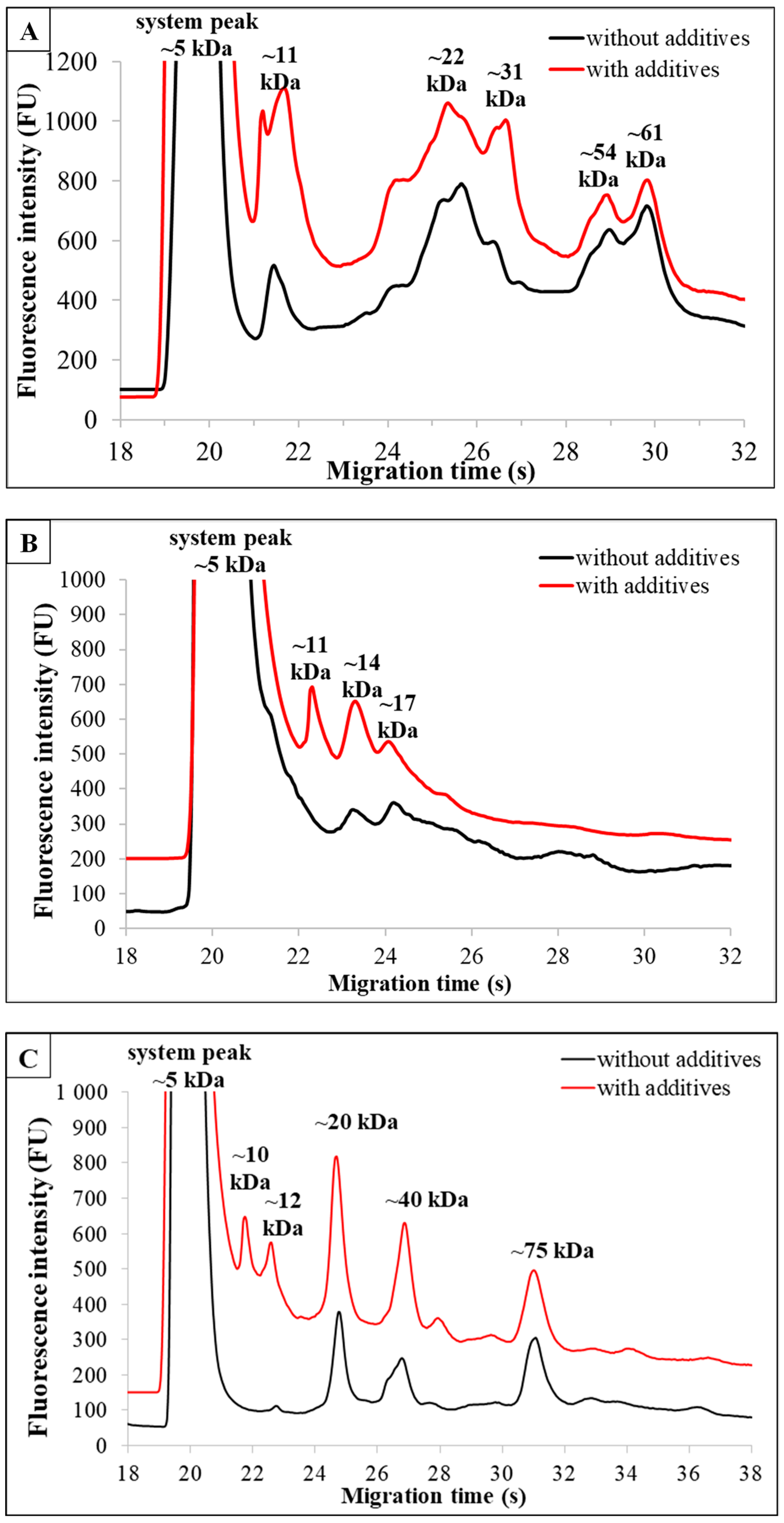
3.3. Comparison of Non-Additive and Additive-Enriched Products of the Same Type of Plant-Based Milk Alternatives
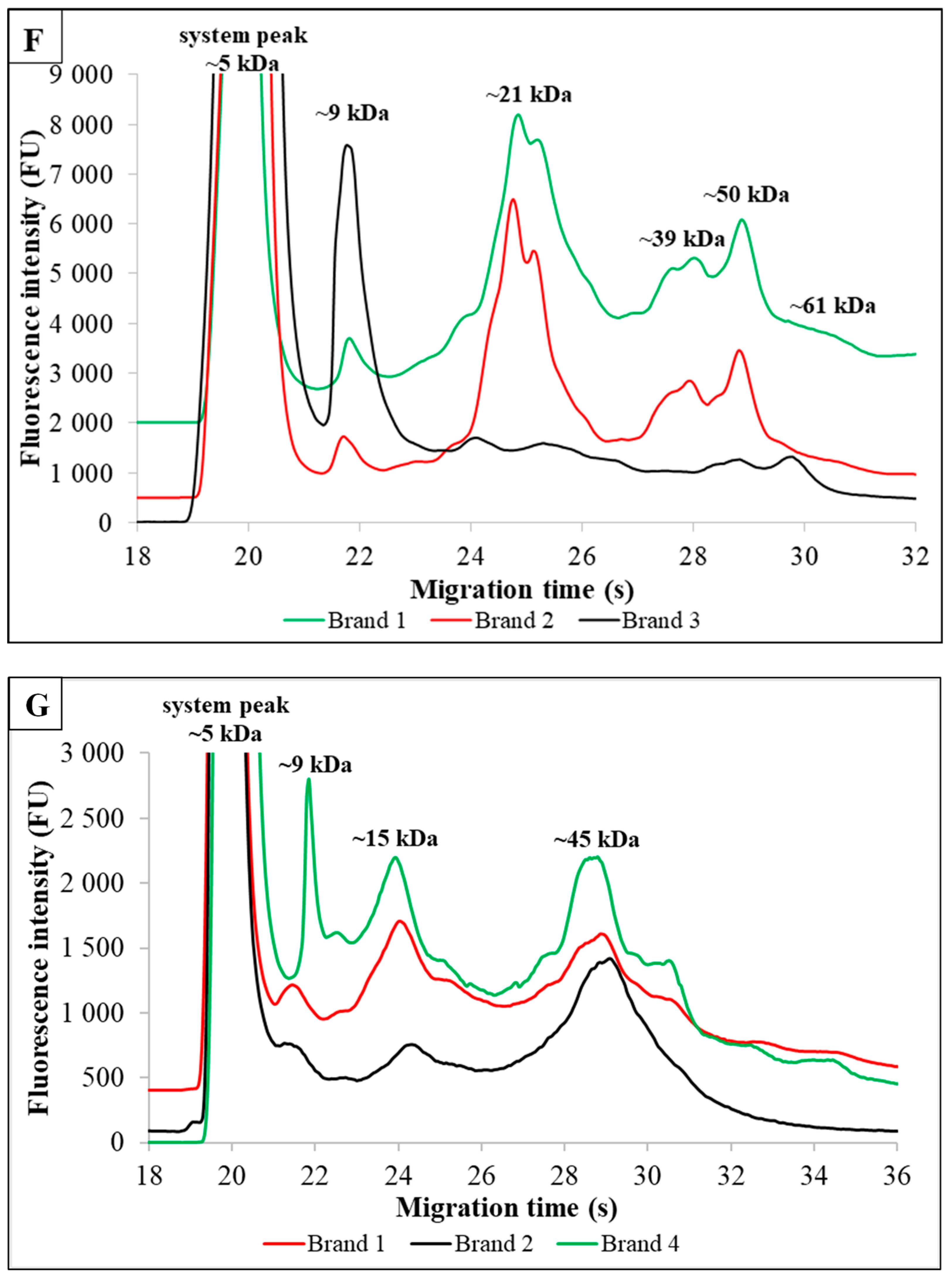
3.4. Special Plant-Based Milk Alternatives Enriched With Additives
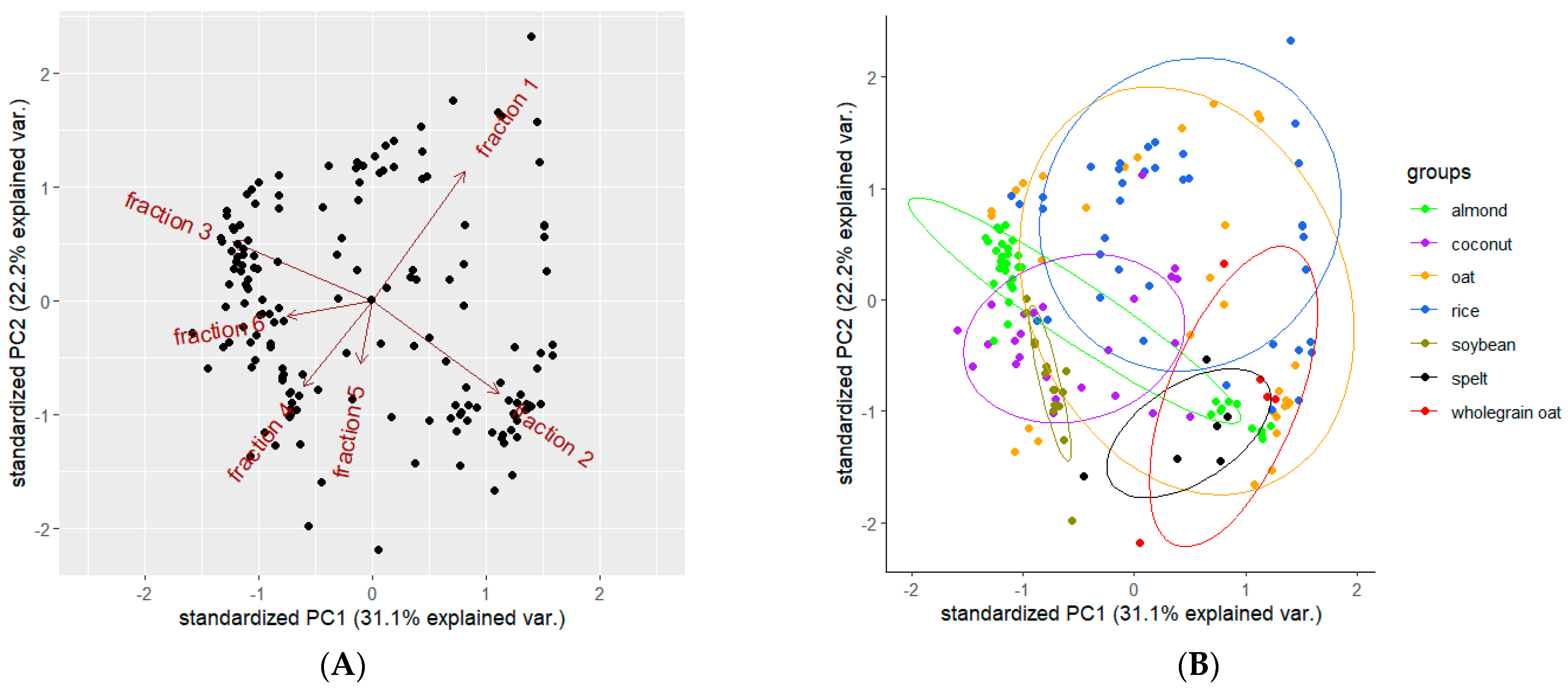
3.5. Statistical Diversity in PBMAs Enriched With Additives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahern, N.; Arendt, E.K.; Sahin, A.W. Protein Soft Drinks: A Retail Market Analysis and Selected Product Characterization. Beverages 2023, 9, 73. [Google Scholar] [CrossRef]
- Su, W.; Zhang, Y.Y.; Li, S.; Sheng, J. Consumers’ Preferences and Attitudes towards Plant-Based Milk. Foods 2023, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Salvaleda-Mateu, M.; Rodríguez-Varela, C.; Labarta, E. Do Popular Diets Impact Fertility? Nutrients 2024, 16, 1726. [Google Scholar] [CrossRef]
- Silva, J.C.D.e.; Anghinoni, I.C.A.B.; Gomes, M.B. Plant-Based Food for the Prevention of Type 2 Diabetes: Scoping Review. Nutrients 2024, 16, 1671. [Google Scholar] [CrossRef]
- Escobar-Sáez, D.; Montero-Jiménez, L.; García-Herrera, P.; Sánchez-Mata, M.C. Plant-based drinks for vegetarian or vegan toddlers: Nutritional evaluation of commercial products, and review of health benefits and potential concerns. Food Res. Int. 2022, 160, 111646. [Google Scholar] [CrossRef]
- Lo Turco, V.; Sgrò, B.; Albergamo, A.; Nava, V.; Rando, R.; Potortì, A.G.; Di Bella, G. Assessment of the Accuracy of Nutrition Label and Chemical Composition of Plant-Based Milks Available on the Italian Market. Foods 2023, 12, 3207. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition Through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef]
- Hu, G.-G.; Liu, J.; Wang, Y.-H.; Yang, Z.-N.; Shao, H.-B. Applications of Plant Protein in the Dairy Industry. Foods 2022, 11, 1067. [Google Scholar] [CrossRef]
- Cecchi, S.; Di Stante, S.; Belcastro, S.; Bertuzzi, V.; Cardillo, A.; Diotallevi, L.; Grabocka, X.; Kulurianu, H.; Martello, M.; Nastasi, V.; et al. Supplemented Very Low Protein Diet (sVLPD) in Patients with Advanced Chronic Renal Failure: Clinical and Economic Benefits. Nutrients 2023, 15, 3568. [Google Scholar] [CrossRef]
- Gonzalez, P.; Lozano, P.; Solano, F. Unraveling the Metabolic Hallmarks for the Optimization of Protein Intake in Pre-Dialysis Chronic Kidney Disease Patients. Nutrients 2022, 14, 1182. [Google Scholar] [CrossRef]
- Garneata, L.; Mocanu, C.-A.; Simionescu, T.P.; Mocanu, A.E.; Dragomir, D.R.; Mircescu, G. Low Protein Diet Reduces Proteinuria and Decline in Glomerular Filtration Rate in Advanced, Heavy Proteinuric Diabetic Kidney Disease. Nutrients 2024, 16, 1687. [Google Scholar] [CrossRef]
- The European Parliament. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers (ver. 01/01/2018); Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Xiao, X.; Zou, P.-R.; Hu, F.; Zhu, W.; Wei, Z.-J. Updates on Plant-Based Protein Products as an Alternative to Animal Protein: Technology, Properties, and Their Health Benefits. Molecules 2023, 28, 4016. [Google Scholar] [CrossRef] [PubMed]
- Fructuoso, I.; Romão, B.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Zandonadi, R.P. An Overview on Nutritional Aspects of Plant-Based Beverages Used as Substitutes for Cow’s Milk. Nutrients 2021, 13, 2650. [Google Scholar] [CrossRef]
- Roland, I.S.; Aguilera-Toro, M.; Nielsen, S.D.-H.; Poulsen, N.A.; Larsen, L.B. Processing-Induced Markers in Proteins of Commercial Plant-Based Drinks in Relation to Compositional Aspects. Foods 2023, 12, 3282. [Google Scholar] [CrossRef]
- Gravel, A.; Dubois-Laurin, F.; Turgeon, S.L.; Doyen, A. The role of the 7S/11S globulin ratio in the gelling properties of mixed β-lactoglobulin/pea proteins systems. Food Hydrocoll. 2024, 156, 110273. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Blazek, V.; Caldwell, R.A. Comparison of SDS gel capillary electrophoresis with microfluidic lab-on-a-chip technology to quantify relative amounts of 7S and 11S proteins from 20 soybean cultivars. Int. J. Food Sci. Technol. 2009, 44, 2127–2134. [Google Scholar] [CrossRef]
- Hernández, H.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and Healthier Dairy Products through the Addition of Microalgae: A Review. Foods 2022, 11, 755. [Google Scholar] [CrossRef]
- Cavallo, G.; Lorini, C.; Garamella, G.; Bonaccorsi, G. Seaweeds as a “Palatable” Challenge between Innovation and Sustainability: A Systematic Review of Food Safety. Sustainability 2021, 13, 7652. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Liu, C.-m.; Peng, Q.; Zhong, J.-z.; Liu, W.; Zhong, Y.-j.; Wang, F. Molecular and Functional Properties of Protein Fractions and Isolate from Cashew Nut (Anacardium occidentale L.). Molecules 2018, 23, 393. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Singh, T.K.; Stockmann, R.; Jegasothy, H.; Buckow, R. Quality Attributes of Ultra-High Temperature-Treated Model Beverages Prepared with Faba Bean Protein Concentrates. Foods 2021, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Quezada, C.; Urra, M.; Mella, C.; Zúñiga, R.N.; Troncoso, E. Plant-Based Oil-in-Water Food Emulsions: Exploring the Influence of Different Formulations on Their Physicochemical Properties. Foods 2024, 13, 513. [Google Scholar] [CrossRef]
- Borsatto, J.V.B.; Maciel, E.V.S.; Cifuentes, A.; Lanças, F.M. Online Extraction Followed by LC–MS/MS Analysis of Lipids in Natural Samples: A Proof-of-Concept Profiling Lecithin in Seeds. Foods 2023, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Balogh-Hartmann, F.; Páger, C.; Bufa, A.; Madarászné Horváth, I.; Verzár, Z.; Marosvölgyi, T.; Makszin, L. Microfluidic Analysis for the Determination of Protein Content in Different Types of Plant-Based Drinks. Molecules 2023, 28, 6684. [Google Scholar] [CrossRef]
- Agilent Technologies, Inc. Quantification Strategies Using the High Sensitivity Protein 250 Assay for the Agilent 2100 Bioanalyzer Technical Note 5989-8941EN. In 5989-8941EN; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2016. [Google Scholar]
- Olías, R.; Delgado-Andrade, C.; Padial, M.; Marín-Manzano, M.C.; Clemente, A. An Updated Review of Soy-Derived Beverages: Nutrition, Processing, and Bioactivity. Foods 2023, 12, 2665. [Google Scholar] [CrossRef]




| (A) | ||||
| Product | Measured TNg/100 mL | Nominal TP *g/100 mL | p-Value | Protein and NPN Additives |
| Oat (n = 96) | 2.0 (1.1–6.4) | 0.3–1.0 | <0.001 | Sunflower oil, soluble maize grits, chicory root fiber, pea protein, lecithin, safflower oil, B vitamins, sea red algae, marine algae, gellan gum |
| Rice (n = 120) | 0.7 (0.2–1.2) | 0.1–0.5 | <0.001 | Sunflower oil, rapeseed lecithin, gellan gum safflower oil, B vitamins, red algae, marine algae |
| Coconut (n = 66) | 1.8 (1.2–4.4) | 0.0–1.1 | <0.001 | Grape juice concentrate, sunflower lecithin, lecithins, B vitamins, soybean, rice, guargum, gellan gum, xanthan gum |
| Soybean (n = 42) | 3.7 (1.8–8.3) | 2.8–3.4 | 0.227 | Marine algae, red algae, aroma, apple juice, B vitamins, gellan gum |
| Almond (n = 78) | 8.8 (6.1–12.2) | 0.0–0.8 | <0.001 | Sunflower lecithin, carob meal, maize maltodextrin, gellan gum, guargum, maltodextrin, B vitamins, sunflower oil |
| Wholegrain oat (n = 18) | 8.5 (3.8–9.0) | 0.6–0.8 | 0.080 | sunflower oil, marine algae |
| Spelt (n = 24) | 1.5 (0.7–3.5) | 0.7–0.8 | 0.116 | sunflower oil |
| (B) | ||||
| Hazelnut (n = 6) | 5.0 (2.4–10.5) | 0.4 | 0.018 | carob flour, gellan gum, sunflower lecithin, B vitamins |
| Cashew (n =6) | 5.2 (1.9–14.5) | 0.5 | 0.026 | carob flour, gellan gum, sunflower lecithin, B vitamins |
| Wholegrain spelt (n = 6) | 2.8 (2.3–2.9) | 0.0 | 0.014 | sunflower oil |
| Quinoa (n = 6) | 1.0 (0.7–1.5) | 1.1 | 0.746 | rice, sunflower oil, safflower oil |
| Brazil nut (n = 6) | 8.3 (5.1–15.1) | 0.7 | 0.026 | rice starch, locust bean gum, sunflower lecithin |
| Brown rice (n = 12) | 0.9 (0.3–2.1) | <0.5 | 0.122 | sunflower oil, safflower oil |
| Buckwheat rice (n = 6) | 0.8 (0.6–1.4) | 0.8 | 0.916 | rice, sunflower oil, safflower oil |
| Millet (n = 6) | 0.9 (0.8–1.2) | 0.7 | 0.812 | sunflower oil |
| Hemp (n = 6) | 8.6 (6.9–14.2) | 0.5 | 0.012 | rosemary extract |
| Oat–flax–hemp (n = 6) | 6.7 (4.3–8.5) | 0.7 | 0.028 | oat, 4% hemp paste, linseed oil |
| Pea (n = 6) | 5.5 (2.1–8.1) | 1.4 | 0.113 | sunflower oil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogh-Hartmann, F.; Páger, C.; Bufa, A.; Sipos, Z.; Dávidovics, A.; Verzár, Z.; Marosvölgyi, T.; Makszin, L. Comprehensive Study of Total Nitrogen Content and Microfluidic Profiles in Additive-Enriched Plant-Based Drinks. Foods 2024, 13, 2329. https://doi.org/10.3390/foods13152329
Balogh-Hartmann F, Páger C, Bufa A, Sipos Z, Dávidovics A, Verzár Z, Marosvölgyi T, Makszin L. Comprehensive Study of Total Nitrogen Content and Microfluidic Profiles in Additive-Enriched Plant-Based Drinks. Foods. 2024; 13(15):2329. https://doi.org/10.3390/foods13152329
Chicago/Turabian StyleBalogh-Hartmann, Fruzsina, Csilla Páger, Anita Bufa, Zoltán Sipos, Anna Dávidovics, Zsófia Verzár, Tamás Marosvölgyi, and Lilla Makszin. 2024. "Comprehensive Study of Total Nitrogen Content and Microfluidic Profiles in Additive-Enriched Plant-Based Drinks" Foods 13, no. 15: 2329. https://doi.org/10.3390/foods13152329







