Application of ATR-FTIR for Green Arabica Bean Shelf-Life Determination in Accelerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Moisture Content (MC)
2.3. Water Activity ()
2.4. Coffee Oil Extraction
2.5. Lipid Oxidation Determination
2.5.1. Peroxide Value (PV)
2.5.2. Measurement of Thiobarbituric Acid Reactive Substances (TBARS)
2.6. Shelf-Life Prediction
2.7. FTIR Spectroscopy Analysis
2.8. Statistical Analysis
3. Results and Discussion
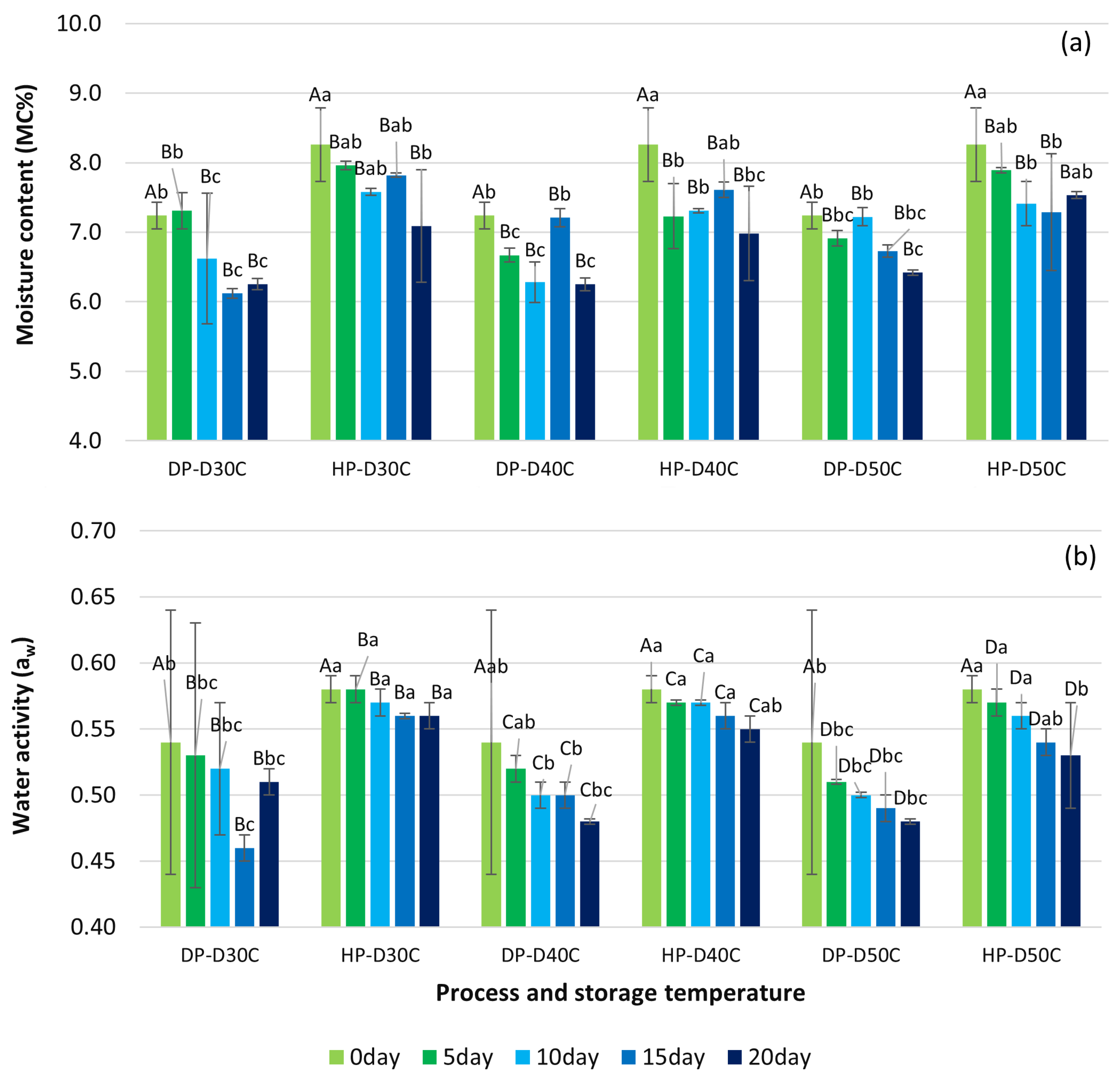
3.1. Moisture Content and Water Activity Parameter of Green Coffee Beans
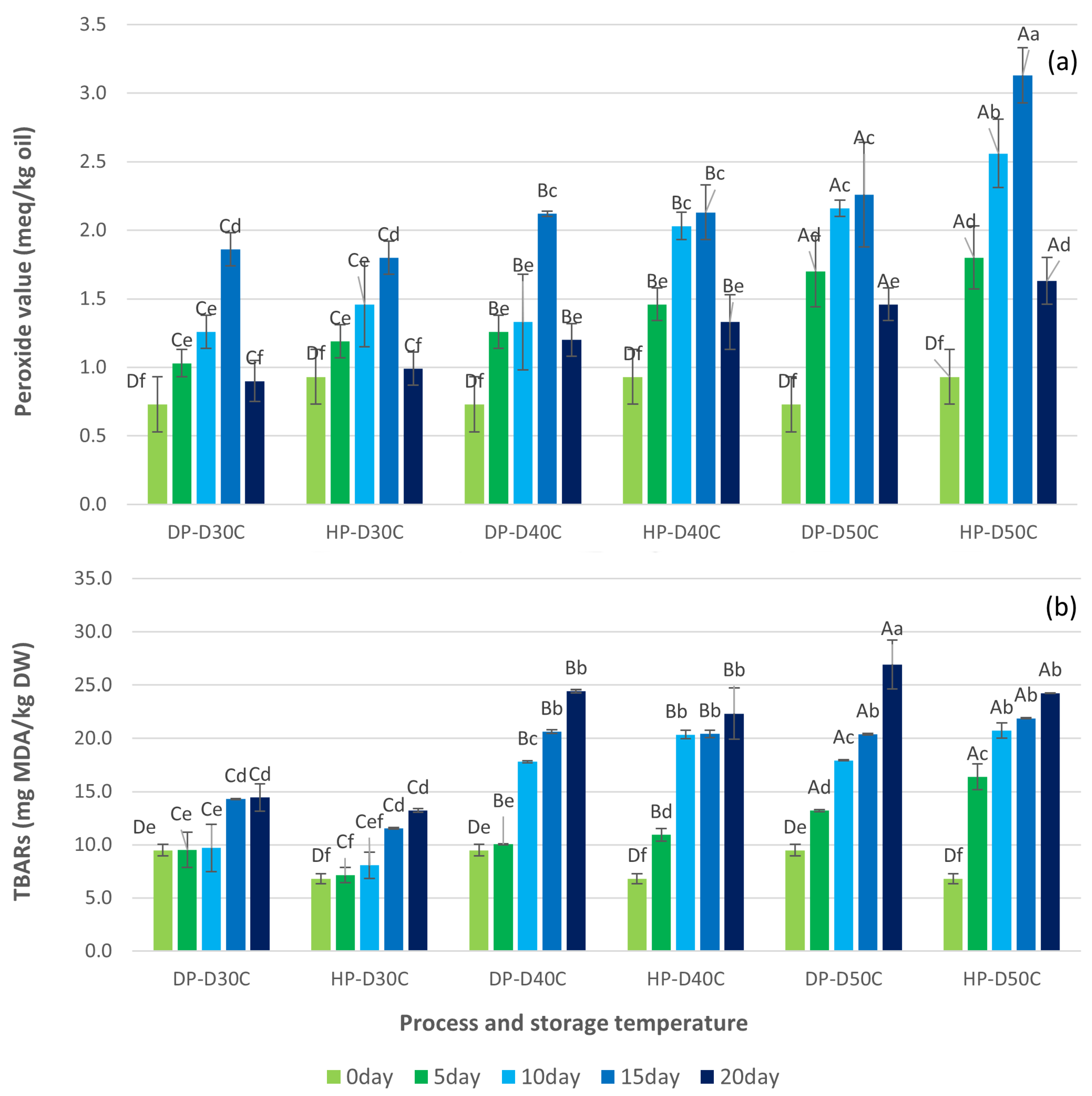
3.2. Oxidation Reactions of Green Coffee Beans
3.3. Shelf Life of Green Coffee Beans
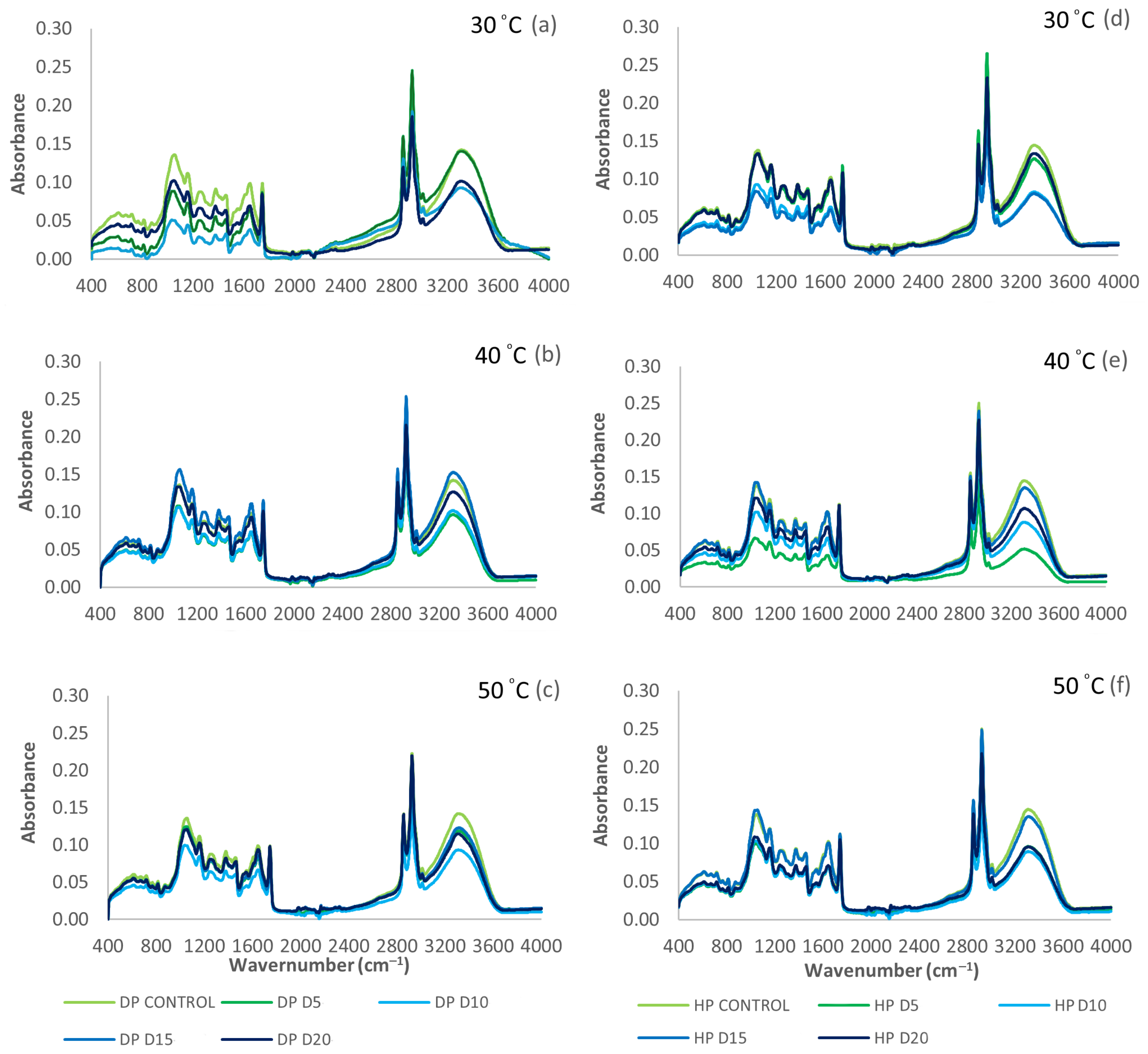
3.4. ATR-FTIR Spectra of Green Coffee Beans
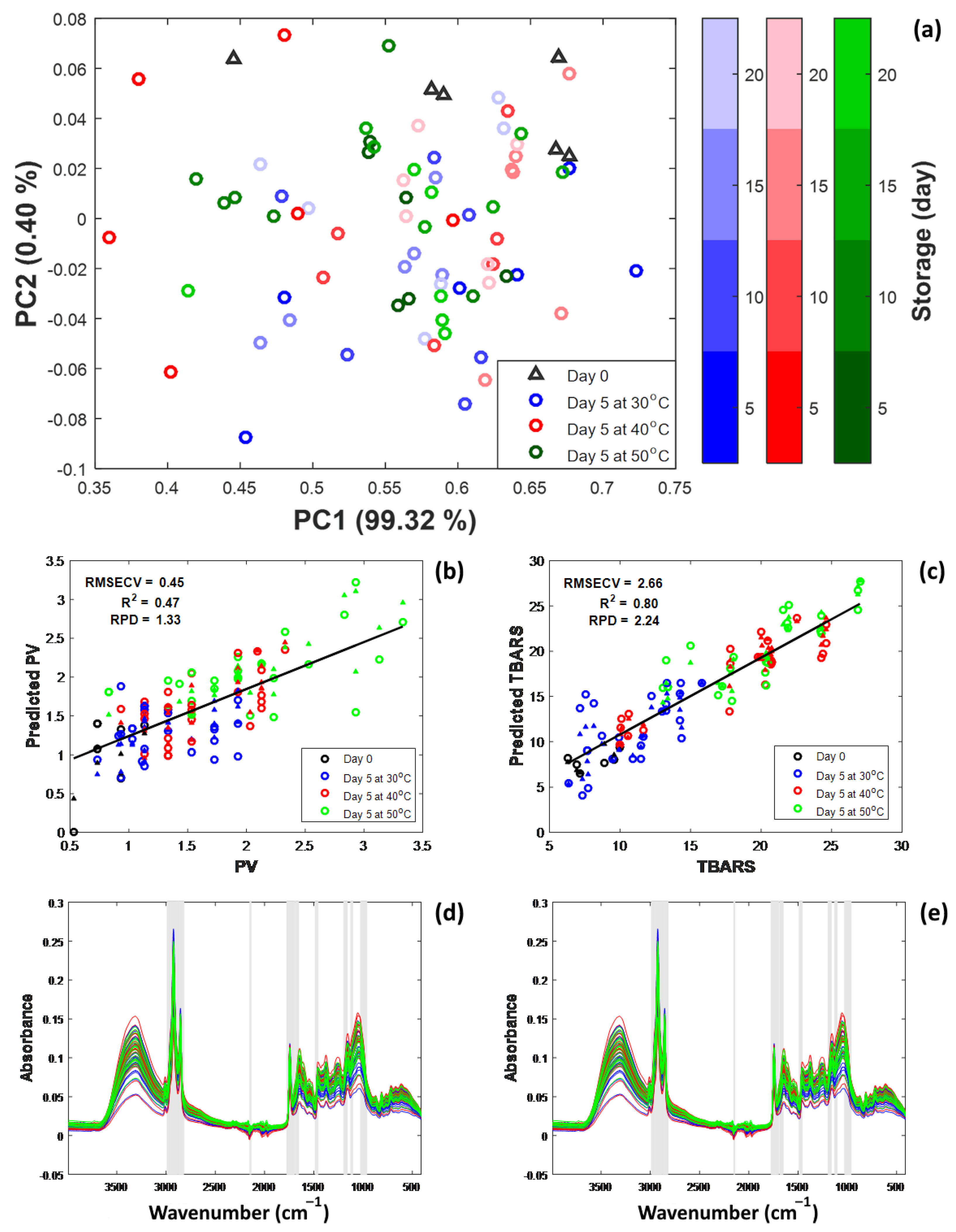
3.5. Multivariate Analysis of Coffee Shelf Life Under Accelerated Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture. Coffee: World Markets and Trade. 2023. Available online: https://apps.fas.usda.gov/psdonline/circulars/coffee.pdf (accessed on 1 February 2024).
- International Coffee Organization. Coffee Market Report September 2021. 2021. Available online: http://www.ico.org/documents/cy2020-21/cmr-0921-e.pdf (accessed on 30 September 2022).
- Kath, J.; Craparo, A.; Fong, Y.; Byrareddy, V.; Davis, A.P.; King, R.; Nguyen-Huy, T.; van Asten, P.J.A.; Marcussen, T.; Mushtaq, S.; et al. Vapour pressure deficit determines critical thresholds for global coffee production under climate change. Nat. Food 2022, 3, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Rendón, M.Y.; de Jesus Garcia Salva, T.; Bragagnolo, N. Impact of chemical changes on the sensory characteristics of coffee beans during storage. Food Chem. 2014, 147, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Aung Moon, S. Physicochemical Properties and Aromatic Profile of Arabica Coffee in Ywangan Township, Myanmar. Master’s Thesis, School of Agro-Industry, Mae Fah Luang University, Chiang Rai, Thailand, 2020. [Google Scholar]
- Haile, M.; Kang, W.H. The Role of Microbes in Coffee Fermentation and Their Impact on Coffee Quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Bruyn, F.D.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; Vuyst, L.D. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed]
- Wintgens, J.N. Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers; Wiley: Hoboken, NJ, USA, 2008; pp. 1–976. [Google Scholar] [CrossRef]
- Borém, F.M.; de Abreu, G.F.; de Carvalho Alves, A.P.; dos Santos, C.M.; Teixeira, D.E. Volatile compounds indicating latent damage to sensory attributes in coffee stored in permeable and hermetic packaging. Food Packag. Shelf Life 2021, 29, 100705. [Google Scholar] [CrossRef]
- Tripetch, P.; Borompichaichartkul, C. Effect of packaging materials and storage time on changes of colour, phenolic content, chlorogenic acid and antioxidant activity in arabica green coffee beans (Coffea arab. L. Cv. Catimor). J. Stored Prod. Res. 2019, 84, 101510. [Google Scholar] [CrossRef]
- Borém, F.M.; Ribeiro, F.C.; Figueiredo, L.P.; Giomo, G.S.; Fortunato, V.A.; Isquierdo, E.P. Evaluation of the sensory and color quality of coffee beans stored in hermetic packaging. J. Stored Prod. Res. 2013, 52, 1–6. [Google Scholar] [CrossRef]
- Ribeiro, F.C.; Borém, F.M.; Giomo, G.S.; Lima, R.R.D.; Malta, M.R.; Figueiredo, L.P. Storage of green coffee in hermetic packaging injected with CO2. J. Stored Prod. Res. 2011, 47, 341–348. [Google Scholar] [CrossRef]
- Maman, M.; Sangchote, S.; Piasai, O.; Leesutthiphonchai, W.; Sukorini, H.; Khewkhom, N. Storage fungi and ochratoxin A associated with arabica coffee bean in postharvest processes in Northern Thailand. Food Control 2021, 130, 108351. [Google Scholar] [CrossRef]
- Scheidig, C.; Czerny, M.; Schieberle, P. Changes in Key Odorants of Raw Coffee Beans during Storage under Defined Conditions. J. Agric. Food Chem. 2007, 55, 5768–5775. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef] [PubMed]
- Aung Moon, S.; Wongsakul, S.; Kitazawa, H.; Saengrayap, R. Lipid Oxidation Changes of Arabica Green Coffee Beans during Accelerated Storage with Different Packaging Types. Foods 2022, 11, 3040. [Google Scholar] [CrossRef] [PubMed]
- Zarebska, M.; Stanek, N.; Barabosz, K.; Jaszkiewicz, A.; Kulesza, R.; Matejuk, R.; Andrzejewski, D.; Biłos, Ł.; Porada, A. Comparison of chemical compounds and their influence on the taste of coffee depending on green beans storage conditions. Sci. Rep. 2022, 12, 2674. [Google Scholar] [CrossRef] [PubMed]
- Abreu, G.F.; Borém, F.M.; Oliveira, L.F.C.; Almeida, M.R.; Alves, A.P.C. Raman spectroscopy: A new strategy for monitoring the quality of green coffee beans during storage. Food Chem. 2019, 287, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P. Shelf-life prediction and testing. In Science and Technology of Enrobed and Filled Chocolate, Confectionery and Bakery Products; Elsevier: Amsterdam, The Netherlands, 2009; pp. 233–254. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Anese, M.; Nicoli, M. The Stability and Shelf Life of Coffee Products. In The Stability and Shelf Life of Food; Elsevier: Amsterdam, The Netherlands, 2016; pp. 375–398. [Google Scholar] [CrossRef]
- Anese, M.; Manzocco, L.; Nicoli, M.C. Modeling the Secondary Shelf Life of Ground Roasted Coffee. J. Agric. Food Chem. 2006, 54, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Emerging non-destructive thermal imaging technique coupled with chemometrics on quality and safety inspection in food and agriculture. Trends Food Sci. Technol. 2020, 105, 176–185. [Google Scholar] [CrossRef]
- El-Mesery, H.; Mao, H.; Abomohra, A. Applications of Non-destructive Technologies for Agricultural and Food Products Quality Inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodríguez, Y.F.; Reyes, C.A.R.; Campos, J.S.T.; Girón-Hernández, J.; Rodríguez-Gamir, J. Infrared spectroscopy coupled with chemometrics in coffee post-harvest processes as complement to the sensory analysis. LWT Food Sci. Technol. 2021, 145, 111304. [Google Scholar] [CrossRef]
- Benes, E.; Fodor, M.; Kovács, S.; Gere, A. Application of detrended fluctuation analysis and yield stability index to evaluate near infrared spectra of green and roasted coffee samples. Processes 2020, 8, 913. [Google Scholar] [CrossRef]
- Buratti, S.; Sinelli, N.; Bertone, E.; Venturello, A.; Casiraghi, E.; Geobaldo, F. Discrimination between washed Arabica, natural Arabica and Robusta coffees by using near infrared spectroscopy, electronic nose and electronic tongue analysis. J. Sci. Food Agric. 2015, 95, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodríguez, Y.; Collazos-Escobar, G.A.; Gutiérrez-Guzmán, N. ATR-FTIR for characterizing and differentiating dried and ground coffee cherry pulp of different varieties (Coffea arab. L.). Eng. Agrícola 2021, 41, 70–77. [Google Scholar] [CrossRef]
- Yusmanizar; Setiasih, I.S.; Nurjanah, S.; Muhaeimin, M.; Nurhadi, B.; Rosniawaty, S.; Munawar, A.A. Fast and Non-Destructive Prediction of Moisture Content and Chologenic Acid of Intact Coffee Beans Using Near Infrared Reflectance Spectroscopy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 506, 012033. [Google Scholar] [CrossRef]
- Wang, N.; Lim, L.T. Fourier Transform Infrared and Physicochemical Analyses of Roasted Coffee. J. Agric. Food Chem. 2012, 60, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Sahachairungrueng, W.; Meechan, C.; Veerachat, N.; Thompson, A.K.; Teerachaichayut, S. Assessing the Levels of Robusta and Arabica in Roasted Ground Coffee Using NIR Hyperspectral Imaging and FTIR Spectroscopy. Foods 2022, 11, 3122. [Google Scholar] [CrossRef] [PubMed]
- Fioresi, D.B.; Pereira, L.L.; da Silva Oliveira, E.C.; Moreira, T.R.; Ramos, A.C. Mid infrared spectroscopy for comparative analysis of fermented arabica and robusta coffee. Food Control 2021, 121, 107625. [Google Scholar] [CrossRef]
- Bona, E.; Marquetti, I.; Link, J.V.; Makimori, G.Y.F.; da Costa Arca, V.; Lemes, A.L.G.; Ferreira, J.M.G.; dos Santos Scholz, M.B.; Valderrama, P.; Poppi, R.J. Support vector machines in tandem with infrared spectroscopy for geographical classification of green arabica coffee. LWT Food Sci. Technol. 2017, 76, 330–336. [Google Scholar] [CrossRef]
- Obeidat, S.M.; Hammoudeh, A.Y.; Alomary, A.A. Application of FTIR Spectroscopy for Assessment of Green Coffee Beans According to Their Origin. J. Appl. Spectrosc. 2018, 84, 1051–1055. [Google Scholar] [CrossRef]
- Dias, R.C.E.; Valderrama, P.; Março, P.H.; dos Santos Scholz, M.B.; Edelmann, M.; Yeretzian, C. Quantitative assessment of specific defects in roasted ground coffee via infrared-photoacoustic spectroscopy. Food Chem. 2018, 255, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Franca, A.S.; Oliveira, L.S. Discrimination between defective and non-defective roasted coffees by diffuse reflectance infrared Fourier transform spectroscopy. LWT Food Sci. Technol. 2012, 47, 505–511. [Google Scholar] [CrossRef]
- Reis, N.; Botelho, B.G.; Franca, A.S.; Oliveira, L.S. Simultaneous Detection of Multiple Adulterants in Ground Roasted Coffee by ATR-FTIR Spectroscopy and Data Fusion. Food Anal. Methods 2017, 10, 2700–2709. [Google Scholar] [CrossRef]
- Raba, D.N.; Poiana, M.A.; Borozan, A.B.; Stef, M.; Radu, F.; Popa, M.V. Investigation on Crude and High-Temperature Heated Coffee Oil by ATR-FTIR Spectroscopy along with Antioxidant and Antimicrobial Properties. PLoS ONE 2015, 10, e0138080. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Lu, X.; Hu, Y.; Kitts, D.D. Application of Attenuated Total Reflectance–Fourier Transformed Infrared (ATR-FTIR) Spectroscopy To Determine the Chlorogenic Acid Isomer Profile and Antioxidant Capacity of Coffee Beans. J. Agric. Food Chem. 2016, 64, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Belchior, V.; Botelho, B.G.; Casal, S.; Oliveira, L.S.; Franca, A.S. FTIR and Chemometrics as Effective Tools in Predicting the Quality of Specialty Coffees. Food Anal. Methods 2020, 13, 275–283. [Google Scholar] [CrossRef]
- Belchior, V.; Botelho, B.G.; Oliveira, L.S.; Franca, A.S. Attenuated Total Reflectance Fourier Transform Spectroscopy (ATR-FTIR) and chemometrics for discrimination of espresso coffees with different sensory characteristics. Food Chem. 2019, 273, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Guzman, N.G.; Monje, A.F.B.; Muñoz, L.X.P. ATR-FTIR for discrimination of espresso and americano coffee pods. Coffee Sci. 2018, 13, 550. [Google Scholar] [CrossRef]
- Tandee, K.; Kittiwachana, S.; Mahatheeranont, S. Antioxidant activities and volatile compounds in longan (Dimocarpus Longan Lour.) Wine Prod. Inc. Longan Seeds. Food Chem. 2021, 348, 128921. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Valderrama, P.; Março, P.; Scholz, M.; Edelmann, M.; Yeretzian, C. Infrared-Photoacoustic Spectroscopy and Multiproduct Multivariate Calibration to Estimate the Proportion of Coffee Defects in Roasted Samples. Beverages 2023, 9, 21. [Google Scholar] [CrossRef]
- Agnoletti, B.Z.; Folli, G.S.; Pereira, L.L.; Pinheiro, P.F.; Guarçoni, R.C.; da Silva Oliveira, E.C.; Filgueiras, P.R. Multivariate calibration applied to study of volatile predictors of arabica coffee quality. Food Chem. 2022, 367, 130679. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, B.Z.; Pereira, L.L.; Alves, E.A.; Rocha, R.B.; Debona, D.G.; Lyrio, M.V.V.; Moreira, T.R.; de Castro, E.V.R.; da S. Oliveira, E.C.; Filgueiras, P.R. The terroir of Brazilian Coffea canephora: Characterization of the chemical composition. Food Res. Int. 2024, 176, 113814. [Google Scholar] [CrossRef] [PubMed]
- Official Methods of Analysis of AOAC International. Official Method 979.12. In AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Official Methods of Analysis of AOAC International. Official Method 965.33. In AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Achata, E.; Esquerre, C.; O’Donnell, C.; Gowen, A. A Study on the Application of Near Infrared Hyperspectral Chemical Imaging for Monitoring Moisture Content and Water Activity in Low Moisture Systems. Molecules 2015, 20, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Witik, R.A.; Phoutthasak, S.; Collet, A.; Wyser, Y. Shelf life prediction of oxygen-sensitive products: The influence of moisture on prediction accuracy for freeze-dried coffee. Packag. Technol. Sci. 2019, 32, 357–365. [Google Scholar] [CrossRef]
- Michalak, J.; Gujska, E.; Czarnowska, M.; Klepacka, J.; Nowak, F. Effect of Storage on Acrylamide and 5-hydroxymethylfurfural Contents in Selected Processed Plant Products with Long Shelf-life. Plant Foods Hum. Nutr. 2016, 71, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Orfanou, F.; Dermesonlouoglou, E.K.; Taoukis, P.S. Greek Coffee Quality Loss During Home Storage: Modeling the Effect of Temperature and Water Activity. J. Food Sci. 2019, 84, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Nicoli, M.C. Modeling the Effect of Water Activity and Storage Temperature on Chemical Stability of Coffee Brews. J. Agric. Food Chem. 2007, 55, 6521–6526. [Google Scholar] [CrossRef]
- Parvathy, U.; Sivaraman, G.K.; Murthy, L.N.; Visnuvinayagam, S.; Jeyakumari, A.; Ravishankar, C.N. Green coffee extract as a natural antioxidant in chill stored Indian mackerel (Rastrelliger kanagurta) Mince. Indian J. Fish. 2018, 65, 86–95. [Google Scholar] [CrossRef]
- Yoon, J.W.; Ahn, S.I.; Kim, H.N.; Park, J.H.; Park, S.Y.; Kim, J.H.; Oh, D.G.; Jhoo, J.W.; Kim, G.Y. Qualitative Characteristics and Determining Shelf-Life of Milk Beverage Product Supplemented with Coffee Extracts. Korean J. Food Sci. Anim. Resour. 2017, 37, 305–312. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Calligaris, S. Secondary Shelf Life: An Underestimated Issue. Food Eng. Rev. 2018, 10, 57–65. [Google Scholar] [CrossRef]
- Poltronieri, P.; Rossi, F. Challenges in Specialty Coffee Processing and Quality Assurance. Challenges 2016, 7, 19. [Google Scholar] [CrossRef]
- de Oliveira Junqueira, A.C.; de Melo Pereira, G.V.; Medina, J.D.C.; Alvear, M.C.R.; Rosero, R.; de Carvalho Neto, D.P.; Enríquez, H.G.; Soccol, C.R. First description of bacterial and fungal communities in Colombian coffee beans fermentation analysed using Illumina-based amplicon sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Schwan, R.F.; Ëustáquio, S.D.; Wheals, A.E. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000, 60, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Lagazio, C. Coffee brew shelf life modelling by integration of acceptability and quality data. Food Qual. Prefer. 2009, 20, 24–29. [Google Scholar] [CrossRef]
- Cincotta, F.; Tripodi, G.; Merlino, M.; Verzera, A.; Condurso, C. Variety and shelf-life of coffee packaged in capsules. LWT Food Sci. Technol. 2020, 118, 108718. [Google Scholar] [CrossRef]
- Cardelli, C.; Labuza, T. Application of Weibull Hazard Analysis to the Determination of the Shelf Life of Roasted and Ground Coffee. LWT Food Sci. Technol. 2001, 34, 273–278. [Google Scholar] [CrossRef]
- Fagan, C.C.; O’Donnell, C.P. Application of Mid-Infrared Spectroscopy to Food Processing Systems; Wiley: Hoboken, NJ, USA, 2007; pp. 119–142. [Google Scholar] [CrossRef]
- Tsiaka, T.; Kritsi, E.; Bratakos, S.M.; Sotiroudis, G.; Petridi, P.; Savva, I.; Christodoulou, P.; Strati, I.F.; Zoumpoulakis, P.; Cavouras, D.; et al. Quality Assessment of Ground Coffee Samples from Greek Market Using Various Instrumental Analytical Methods, In Silico Studies and Chemometrics. Antioxidants 2023, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, M.; Irudayaraj, J. A Rapid FTIR Spectroscopic Method for Estimation of Caffeine in Soft Drinks and Total Methylxanthines in Tea and Coffee. J. Food Sci. 2002, 67, 2507–2511. [Google Scholar] [CrossRef]

| Temperature (°C) | Process | PV | TBARS | ||
|---|---|---|---|---|---|
| Shelf Life (Days) | Shelf Life (Days) | ||||
| 30 °C | DP | 0.95 | 35.57 ± 1.69 b | 0.99 | 10.01 ± 0.27 a |
| HP | 0.89 | 51.50 ± 1.83 a | 0.99 | 8.84 ± 0.44 ab | |
| 40 °C | DP | 0.91 | 25.65 ± 1.67 c | 0.96 | 4.25 ± 0.10 c |
| HP | 0.90 | 33.88 ± 2.00 b | 0.92 | 4.19 ± 0.23 c | |
| 50 °C | DP | 0.87 | 21.10 ± 3.71 cd | 0.98 | 3.82 ± 0.10 c |
| HP | 0.99 | 15.26 ± 0.63 d | 0.93 | 3.96 ± 0.16 c | |
| Response | Samples | Processing | LVs | LOOCV | Auto-Prediction | ||||
|---|---|---|---|---|---|---|---|---|---|
| RMSECV | RPDCV | RMSE | RPD | ||||||
| PV | 78 | SNV and 1st derivative | 10 | 0.449 | 0.469 | 1.332 | 0.264 | 0.802 | 2.260 |
| TBARS | 78 | SNV and 1st derivative | 10 | 2.658 | 0.801 | 2.237 | 1.546 | 0.932 | 3.846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung Moon, S.; Wongsakul, S.; Kitazawa, H.; Kittiwachana, S.; Saengrayap, R. Application of ATR-FTIR for Green Arabica Bean Shelf-Life Determination in Accelerated Storage. Foods 2024, 13, 2331. https://doi.org/10.3390/foods13152331
Aung Moon S, Wongsakul S, Kitazawa H, Kittiwachana S, Saengrayap R. Application of ATR-FTIR for Green Arabica Bean Shelf-Life Determination in Accelerated Storage. Foods. 2024; 13(15):2331. https://doi.org/10.3390/foods13152331
Chicago/Turabian StyleAung Moon, Sai, Sirirung Wongsakul, Hiroaki Kitazawa, Sila Kittiwachana, and Rattapon Saengrayap. 2024. "Application of ATR-FTIR for Green Arabica Bean Shelf-Life Determination in Accelerated Storage" Foods 13, no. 15: 2331. https://doi.org/10.3390/foods13152331







