Pectins Rich in RG-I Extracted from Watermelon Peel: Physicochemical, Structural, Emulsifying, and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pectin Extraction and Purification
2.3. Physicochemical Property of Pectin
2.4. Structural Characterization of Pectin
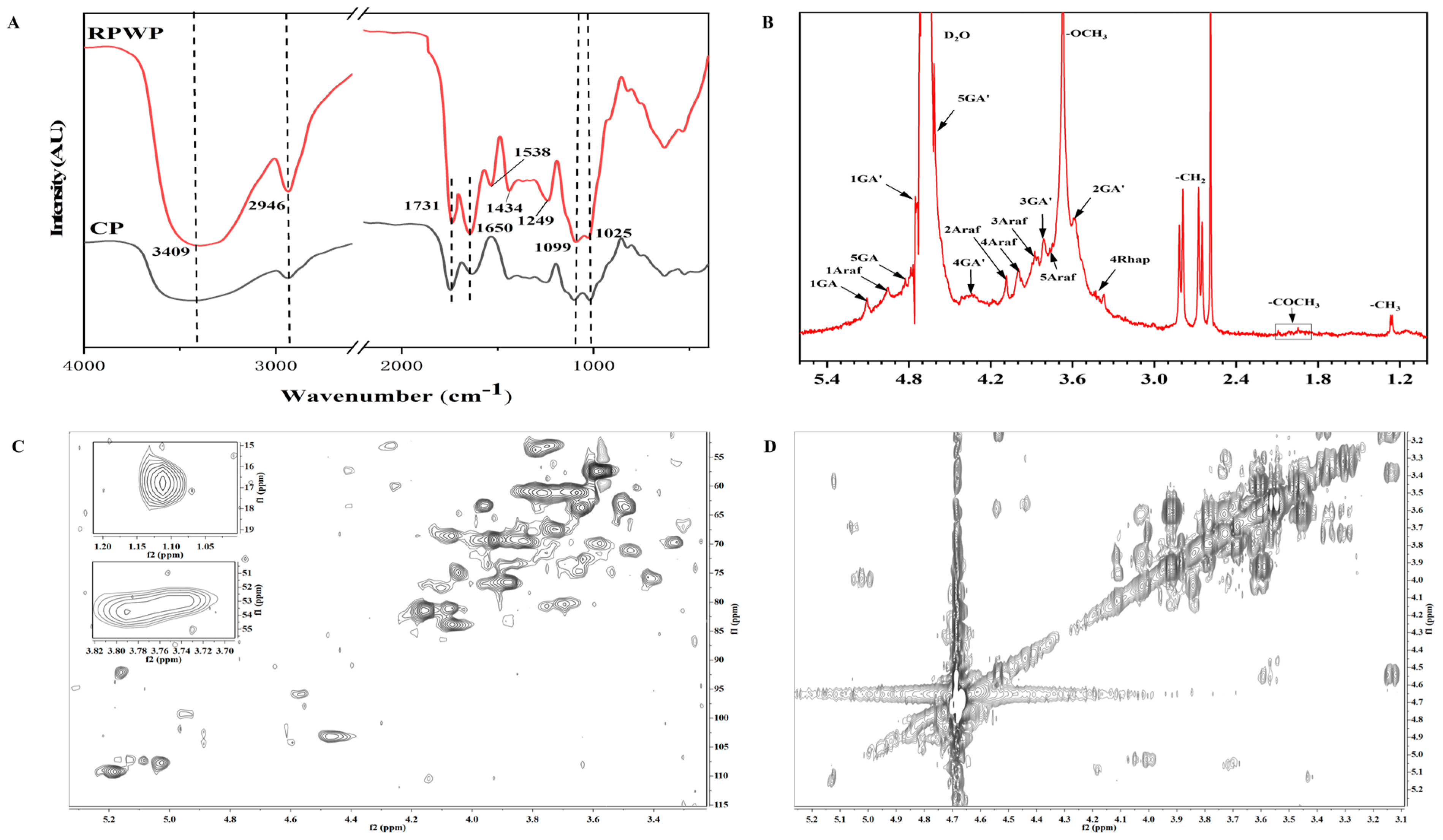
2.4.1. Fourier Transform Infrared Spectroscopy
2.4.2. Monosaccharides and Molecular Weight Distribution Analysis
2.4.3. NMR Spectroscopy Analysis
2.4.4. Glycosidic Linkage Analysis
2.5. Three-Phase Contact Angle
2.6. Preparation of Emulsion
2.6.1. Emulsifying Properties
2.6.2. Emulsion Droplet Size Distribution and Optical Microscopy Observation
2.7. Rheological Properties
2.8. Antioxidant Capacities
2.9. Statistical Analysis
3. Results and Discussions
3.1. Chemical Composition and Molecular Weight
3.2. Glycosidic Linkage Analysis
3.3. FTIR and NMR Analysis
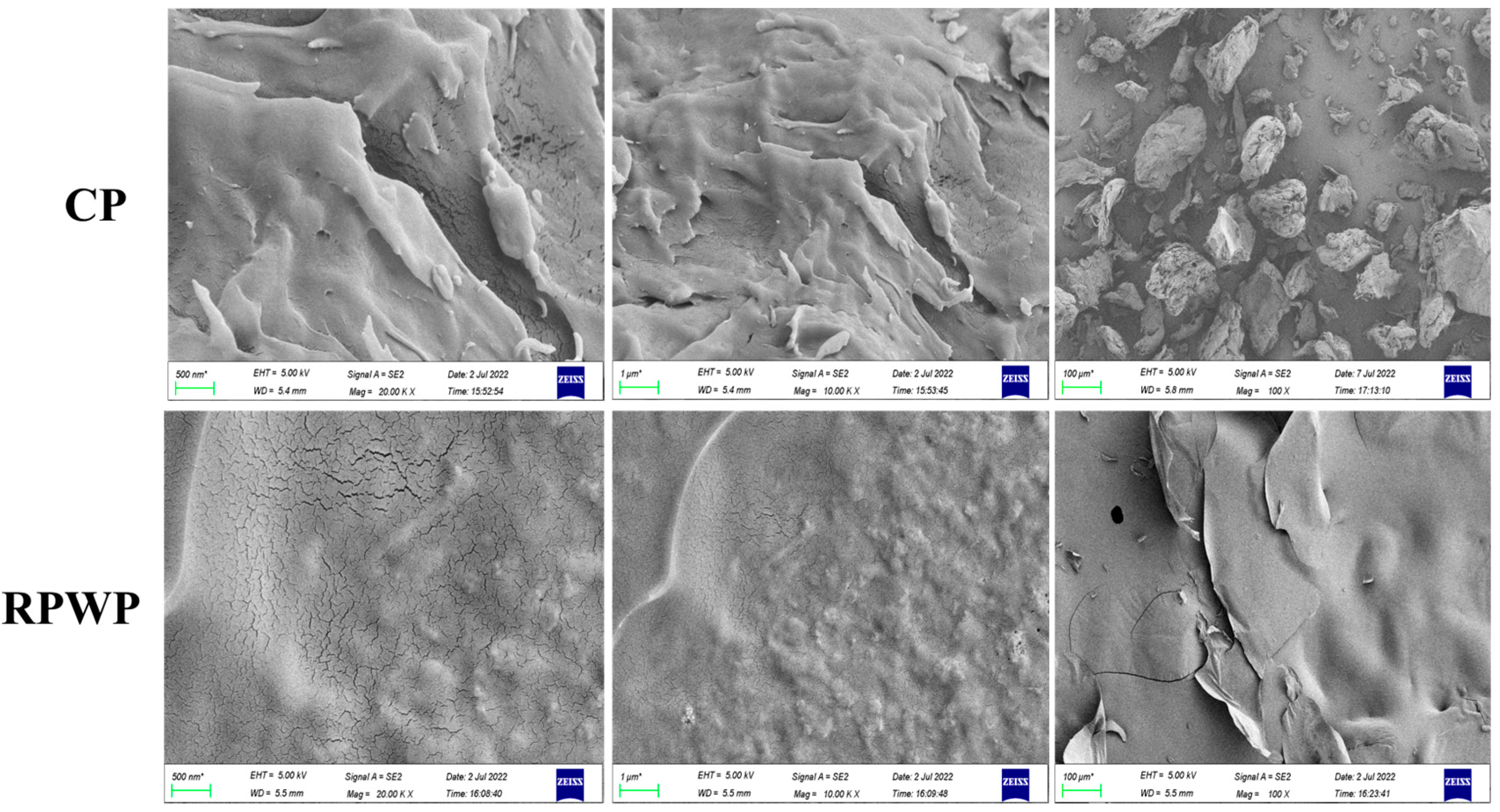
3.4. Wettability and Scanning Electron Microscopy (SEM) Analysis of RPWP and CP
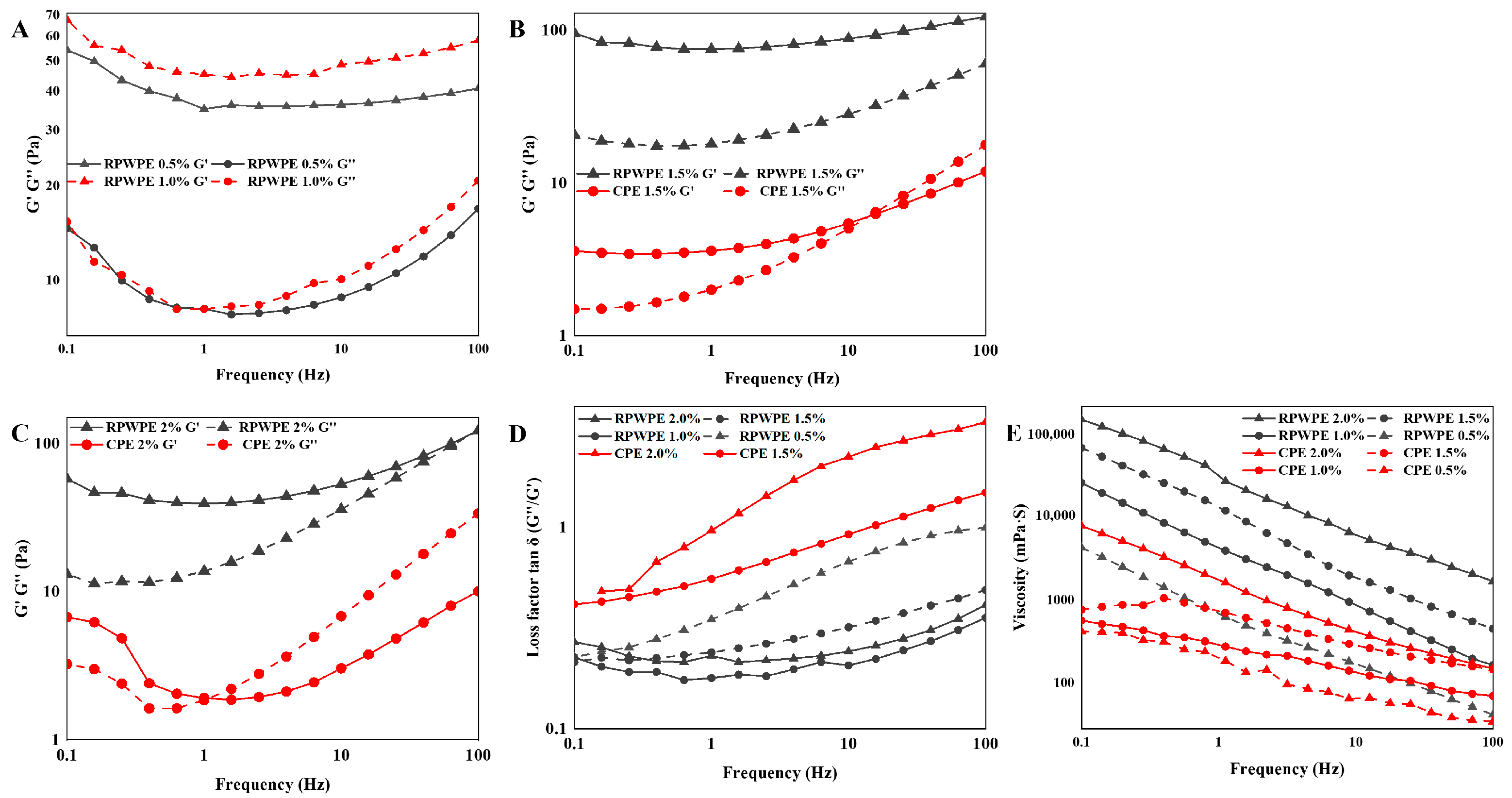
3.5. Analysis of Rheological Properties
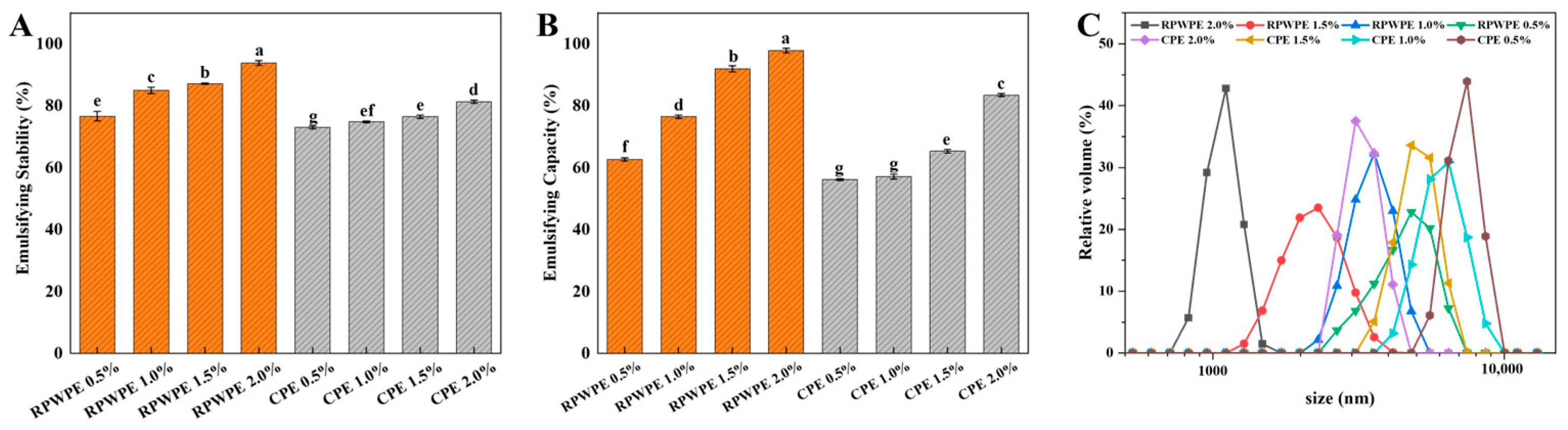
3.6. Emulsifying Properties of RPWP
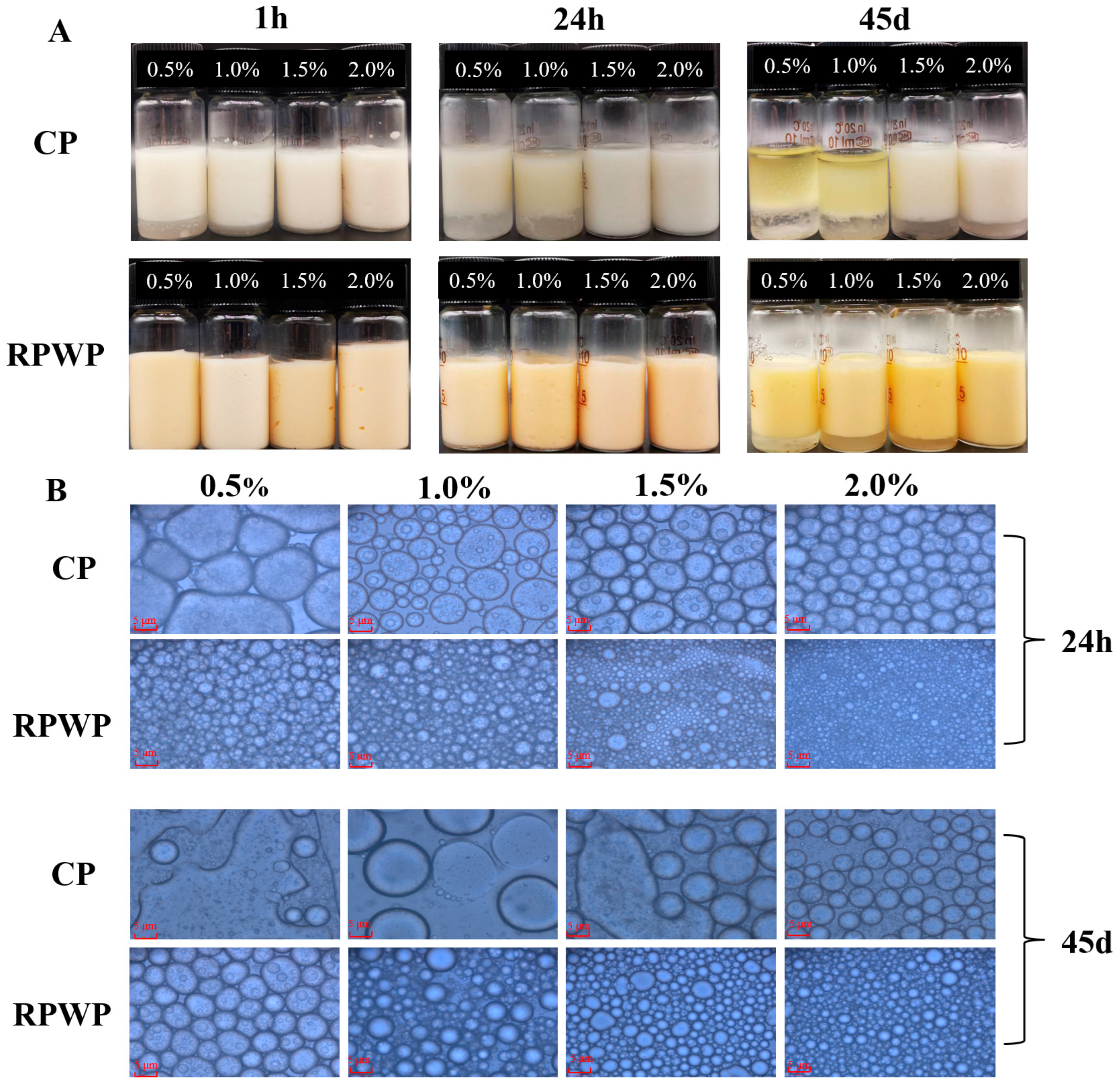
The Visual Appearance, Microstructure, and Particle Size Distribution of Emulsions
3.7. Analysis of Emulsion Rheological Properties
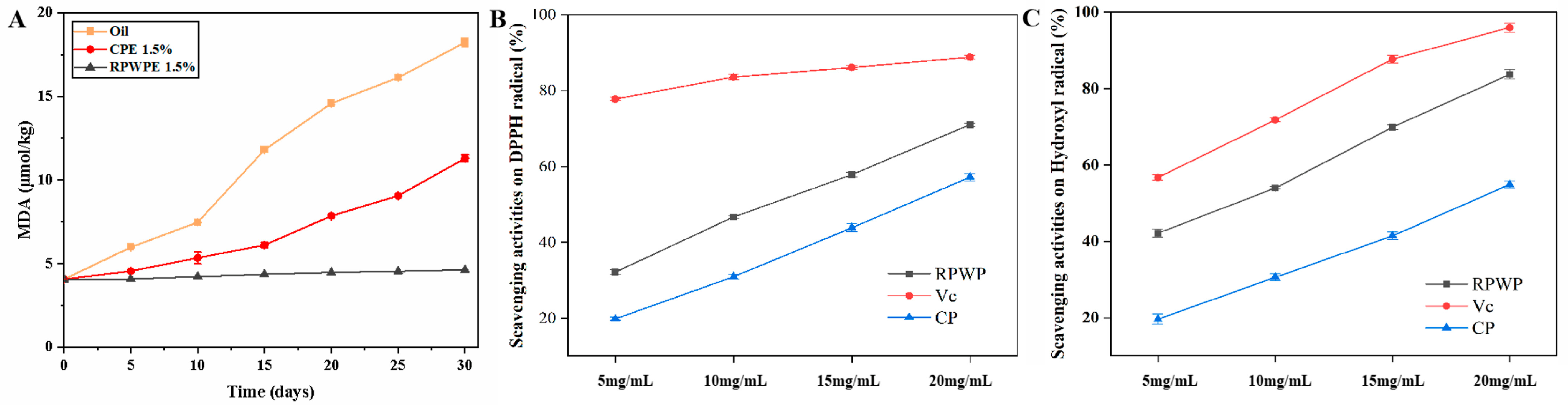
3.8. Antioxidant Ability Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Cai, J. Preparation of branched RG-I-rich pectin from red dragon fruit peel and the characterization of its probiotic properties. Carbohyd. Polym. 2023, 299, 120144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Q.; Chen, J.L.; Zhang, H.; Wu, D.M.; Ye, X.Q.; Linardt, R.J.; Chen, S.G. Gelling mechanism of RG-I enriched citrus pectin: Role of arabinose side-chains in cation-and acid-induced gelation. Food Hydrocoll. 2020, 101, 105536. [Google Scholar] [CrossRef]
- Zhang, S.; Waterhouse, G.I.; Du, Y.; Fu, Q.; Sun, Y.; Wu, P.; Ai, S.; Sun-Waterhouse, D.X. Structural, rheological and emulsifying properties of RG-I enriched pectins from sweet and sour cherry pomaces. Food Hydrocoll. 2023, 139, 108442. [Google Scholar] [CrossRef]
- Hu, W.; Cheng, H.; Wu, D.; Chen, J.; Ye, X.; Chen, S. Enhanced extraction assisted by pressure and ultrasound for targeting RG-I enriched pectin from citrus peel wastes: A mechanistic study. Food Hydrocoll. 2022, 133, 107778. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Yan, L.; Li, S.; Ye, X.; Liu, D.; Ding, T.; Linhardt, R.J.; Orfila, C. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocoll. 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Zhang, S.; Waterhouse, G.I.; Xu, F.; He, Z.; Du, Y.; Lian, Y.; Wu, P.; Sun-Waterhouse, D. Recent advances in utilization of pectins in biomedical applications: A review focusing on molecular structure-directing health-promoting properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 3386–3419. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, C.; Ye, R.; Tang, H.; Jiang, L.; Liu, Y. Development of active packaging with chitosan, guar gum and watermelon rind extract: Characterization, application and performance improvement mechanism. Int. J. Biol. Macromol. 2023, 227, 711–725. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, S.; Lin, J.; Zheng, H.; Lei, H.; Yu, Q.; Jiang, W.J.F.C. Active film preparation using pectin and polyphenols of watermelon peel and its applications for super-chilled storage of chilled mutton. Carbohyd. Polym. 2023, 417, 135838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, Z.; Cheng, Y.; Xu, F.; Cheng, X.; Wu, P. Physicochemical characterization and emulsifying properties evaluation of RG-I enriched pectic polysaccharides from Cerasus humilis. Carbohyd. Polym. 2021, 260, 117824. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yang, B.; Kealey, K.; Chen, J.; Solval, K.M. Developing microencapsulated powders containing polyphenols and pectin extracted from Georgia-grown pomegranate peels. LWT 2022, 154, 112644. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohyd. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Méndez, D.A.; Martínez-Abad, A.; Martínez-Sanz, M.; López-Rubio, A.; Fabra, M.J. Tailoring structural, rheological and gelling properties of watermelon rind pectin by enzymatic treatments. Food Hydrocoll. 2023, 135, 108119. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Zhang, H.; Liu, Y.; Zhu, C. Effect of different processing methods of hawthorn on the properties and emulsification performance of hawthorn pectin. Carbohyd. Polym. 2022, 298, 120121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.-M.; Xie, A.-J.; Wang, X.-D.; Zhu, C.-Y.; Qin, G.-Y. Chinese quince (Chaenomeles sinensis) seed gum: Structural characterization. Food Hydrocoll. 2018, 75, 237–245. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Xu, F.; Cheng, Y.; Waterhouse, G.I.; Sun-Waterhouse, D.X.; Wu, P. Enhancing the performance of konjac glucomannan films through incorporating zein–pectin nanoparticle-stabilized oregano essential oil Pickering emulsions. Food Hydrocoll. 2022, 124, 107222. [Google Scholar] [CrossRef]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohyd. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, S.; Zhou, T.; Waterhouse, G.I.; Du, Y.; Sun-Waterhouse, D.X.; Wu, P. Green approaches for dietary fibre-rich polysaccharide production from the cooking liquid of Adzuki beans: Enzymatic extraction combined with ultrasonic or high-pressure homogenisation. Food Hydrocoll. 2022, 130, 107679. [Google Scholar] [CrossRef]
- Méndez, D.A.; Fabra, M.J.; Martínez-Abad, A.; Μartínez-Sanz, Μ.; Gorria, M.; López-Rubio, A.J.F.H. Understanding the different emulsification mechanisms of pectin: Comparison between watermelon rind and two commercial pectin sources. Food Hydrocoll. 2021, 120, 106957. [Google Scholar] [CrossRef]
- Li, Z.; Xi, J.; Chen, H.; Chen, W.; Chen, W.; Zhong, Q.; Zhang, M. Effect of glycosylation with apple pectin, citrus pectin, mango pectin and sugar beet pectin on the physicochemical, interfacial and emulsifying properties of coconut protein isolate. Food. Res. Int. 2022, 156, 111363. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, A.-Q.; Yin, J.-Y.; Nie, S.-P. Structural characteristics of three pectins isolated from white kidney bean. Int. J. Biol. Macromol. 2021, 182, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.; Lee, R.; Fréchet, J. One-step synthesis of hyperbranched dendritic polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Tang, W.; Liu, D.; Wang, J.-Q.; Huang, X.-J.; Yin, J.-Y.; Geng, F.; Nie, S.P. Isolation and structure characterization of a low methyl-esterified pectin from the tuber of Dioscorea opposita Thunb. Food Chem. 2021, 359, 129899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fu, Q.; Li, H.; Wu, P.; Waterhouse, G.I.; Li, Y.; Ai, S. A pectocellulosic bioplastic from fruit processing waste: Robust, biodegradable, and recyclable. Chem Eng. J. 2023, 463, 142452. [Google Scholar] [CrossRef]
- Dias, I.P.; Barbieri, S.F.; Fetzer, D.E.L.; Corazza, M.L.; Silveira, J.L. Effects of pressurized hot water extraction on the yield and chemical characterization of pectins from Campomanesia xanthocarpa Berg fruits. Int. J. Biol. Macromol. 2020, 146, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Z.; Zhao, H.; Liu, M.; Lin, C.; Li, L.; Ma, B.J. Pectin polysaccharide from Flos Magnoliae (Xin Yi, Magnolia biondii Pamp. flower buds): Hot-compressed water extraction, purification and partial structural characterization. Food Hydrocoll. 2022, 122, 107061. [Google Scholar] [CrossRef]
- Barbieri, S.F.; da Costa Amaral, S.; Ruthes, A.C.; de Oliveira Petkowicz, C.L.; Kerkhoven, N.C.; da Silva, E.R.A.; Silveira, J.L.M.J. Pectins from the pulp of gabiroba (Campomanesia xanthocarpa Berg): Structural characterization and rheological behavior. Carbohyd. Polym. 2019, 214, 250–258. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S.J.F.H. Physiochemical, rheological and emulsifying properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, conventional enzymatic, and alkaline de-esterification: A comparison study. Food Hydrocoll. 2019, 93, 146–155. [Google Scholar] [CrossRef]
- Hua, X.; Xu, S.; Wang, M.; Chen, Y.; Yang, H.; Yang, R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017, 232, 443–449. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Zhang, J.; Jiang, Y.; Li, D. Pectin extracted from dragon fruit Peel: An exploration as a natural emulsifier. Int. J. Biol. Macromol. 2022, 221, 976–985. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, X.; Yang, W.; Fu, Q.; Su, F.; Wu, P.; Li, Y.; Wang, F.; Li, H.; Ai, S. Converting fruit peels into biodegradable, recyclable and antimicrobial eco-friendly bioplastics for perishable fruit preservation. Bioresour. Technol. 2024, 4, 131074. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, S.; Sun-Waterhouse, D.; Zhou, T.; Xu, F.; Waterhouse, G.I.; Wu, P. Physicochemical, structural and emulsifying properties of RG-I enriched pectin extracted from unfermented or fermented cherry pomace. Food Chem. 2023, 405, 134985. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohyd. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Kavya, M.; Jacob, A.R.; Nisha, P. Pectin emulsions and emulgels: Bridging the correlation between rheology and microstructure. Food Hydrocoll. 2023, 143, 108868. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Gao, Z.; Xiang, W.; Wu, Y.; Hu, B.; Ni, X.; Nishinari, K.; Fang, Y. Influence of interfacial properties/structure on oxygen diffusion in oil-in-water emulsions. Food Res. Int. 2023, 170, 112973. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, S.; Waterhouse, G.I.; Zhou, T.; Du, Y.; Sun-Waterhouse, D.; Wu, P. Yeast fermentation of apple and grape pomaces affects subsequent aqueous pectin extraction: Composition, structure, functional and antioxidant properties of pectins. Food Hydrocoll. 2022, 133, 107945. [Google Scholar] [CrossRef]

| Samples | RPWP | CP |
|---|---|---|
| Molecular weight | ||
| Mw (kDa) | 1991 ± 1.1 | 576 ± 0.45 |
| Mn (kDa) | 1025 ± 1.14 | 324 ± 0.26 |
| Mw/Mn | 1.94 ± 0.00 | 1.78 ± 0.00 |
| Monosaccharide composition | ||
| Fuc (mol%) | 0.6 ± 0.05 | 0.3 ± 0.09 |
| Rha (mol %) | 2.9 ± 0.12 | 6.2 ± 0.22 |
| Ara (mol %) | 30.7 ± 0.12 | 2.5 ± 0.26 |
| Gal (mol %) | 29.6 ± 0.39 | 20.1 ± 0.5 |
| Glc (mol %) | 3.4 ± 0.09 | 6.5 ± 1.71 |
| Xyl (mol %) | 2.3 ± 0.94 | 13.2 ± 0.41 |
| Man (mol %) | 0.8 ± 0.03 | 0 |
| GalA (mol %) | 27.9 ± 0.51 | 50.5 ± 0.94 |
| Sugar molar ratios | ||
| HG (%) | 24.97 ± 0.447 | 44.27 ± 0.92 |
| RG-I (%) | 66.17 ± 0.37 | 35.00 ± 0.92 |
| Rha/GalA | 0.11 ± 0.40 | 0.12 ± 0.00 |
| (Ara + Gal)/Rha | 20.59 ± 0.99 | 3.65 ± 0.15 |
| GalA/(Gal + Ara + Rha + Xyl) | 0.43 ± 0.12 | 1.20 ± 0.01 |
| Protein (%) | 6.1 ± 0.48 | 2.13 ± 0.28 |
| Total polyphenol (%) | 2.4 ± 0.08 | 2.58 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Cheng, X.; Du, Y.; Tang, P.; Chen, L.; Chen, W.; Zheng, Z. Pectins Rich in RG-I Extracted from Watermelon Peel: Physicochemical, Structural, Emulsifying, and Antioxidant Properties. Foods 2024, 13, 2338. https://doi.org/10.3390/foods13152338
Ma X, Cheng X, Du Y, Tang P, Chen L, Chen W, Zheng Z. Pectins Rich in RG-I Extracted from Watermelon Peel: Physicochemical, Structural, Emulsifying, and Antioxidant Properties. Foods. 2024; 13(15):2338. https://doi.org/10.3390/foods13152338
Chicago/Turabian StyleMa, Xiaojun, Xinxin Cheng, Yuyi Du, Peiyao Tang, Liangxiao Chen, Wei Chen, and Zhenjia Zheng. 2024. "Pectins Rich in RG-I Extracted from Watermelon Peel: Physicochemical, Structural, Emulsifying, and Antioxidant Properties" Foods 13, no. 15: 2338. https://doi.org/10.3390/foods13152338







