Effects of Tea Seed Oil Extracted by Different Refining Temperatures on the Intestinal Microbiota of High-Fat-Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil Refining Procedures
2.3. Fatty Acid Determination
2.4. Determination of Active Ingredients
2.4.1. Total Polyphenols
2.4.2. Tocopherols and Squalene
2.4.3. Sterols and Carotenoids
2.5. Physical and Chemical Property Analysis
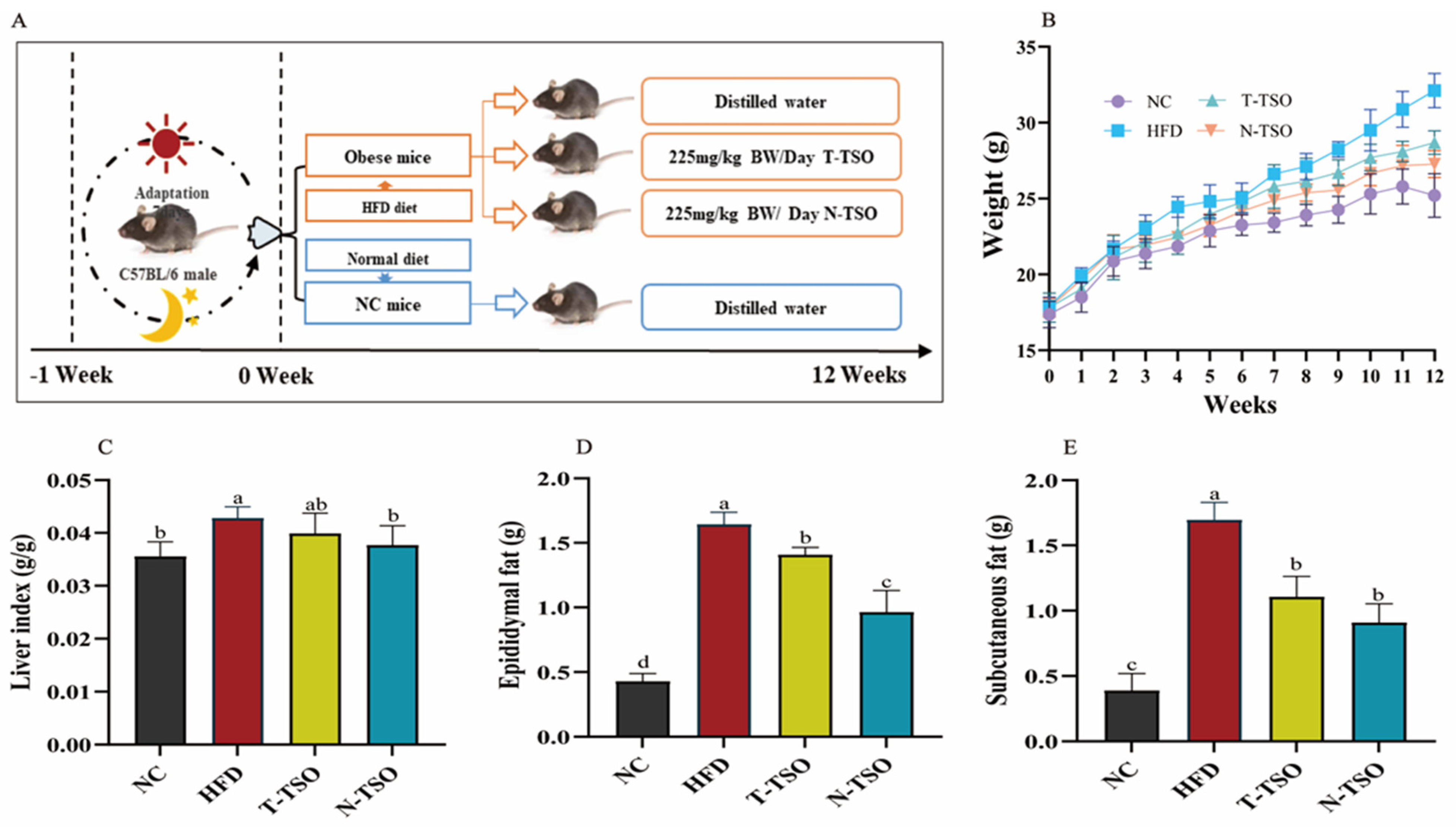
2.6. Animal Experimental Design
2.7. Measurement of Serum Biochemical Indicators
2.8. H&E Staining of Liver Tissue and Epididymal Fat
2.9. RT-PCR Analysis
2.10. 16 S rRNA Sequencing for Gut Microbiota
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Refining on Oil Physicochemical Properties
3.2. Effect of Different Refined TSO on BW and Fat Content in Mice
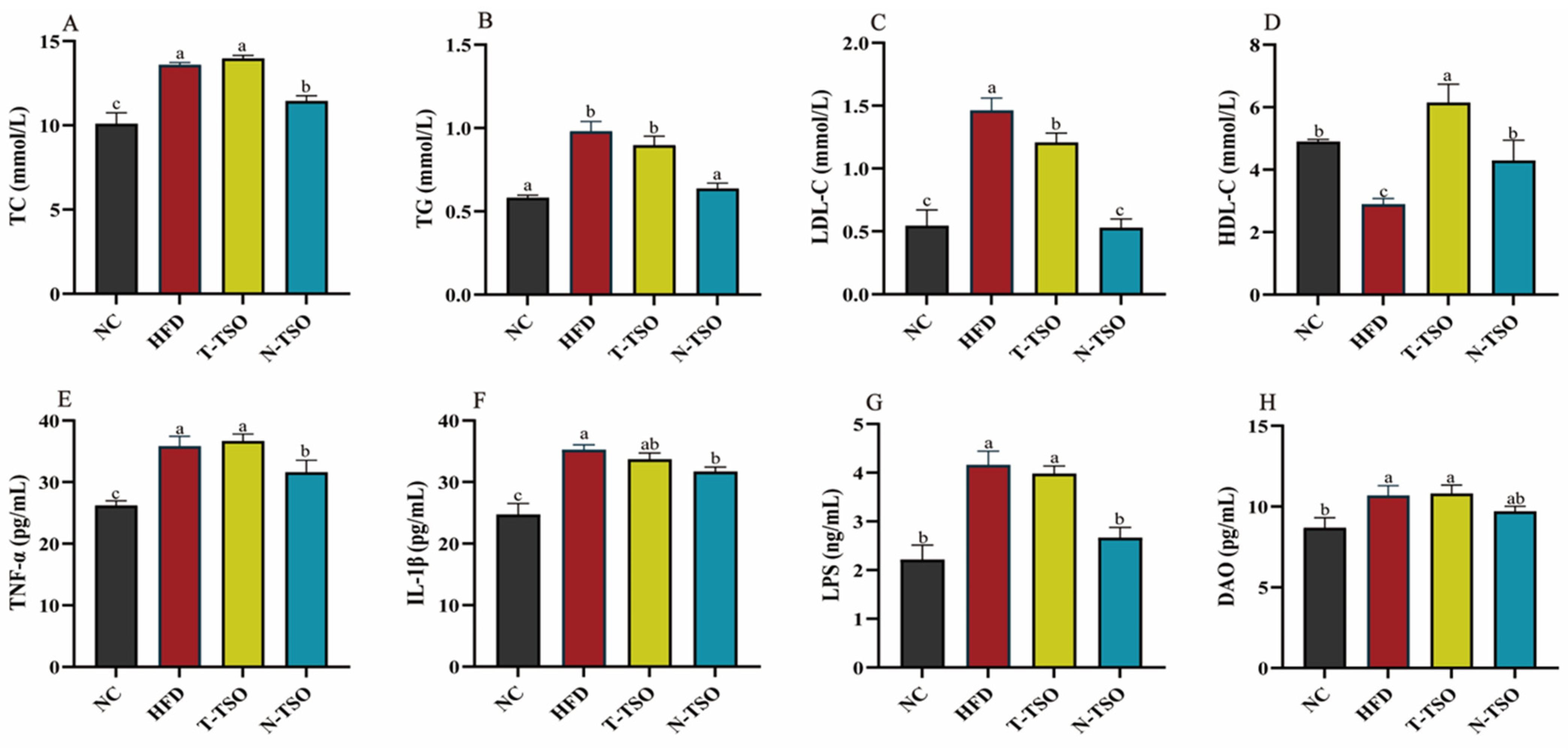
3.3. Effect of Different Refined TSO on Dyslipidemia in Obese Mice
3.4. Effect of Different Refined TSO on Inflammation in Obese Mice
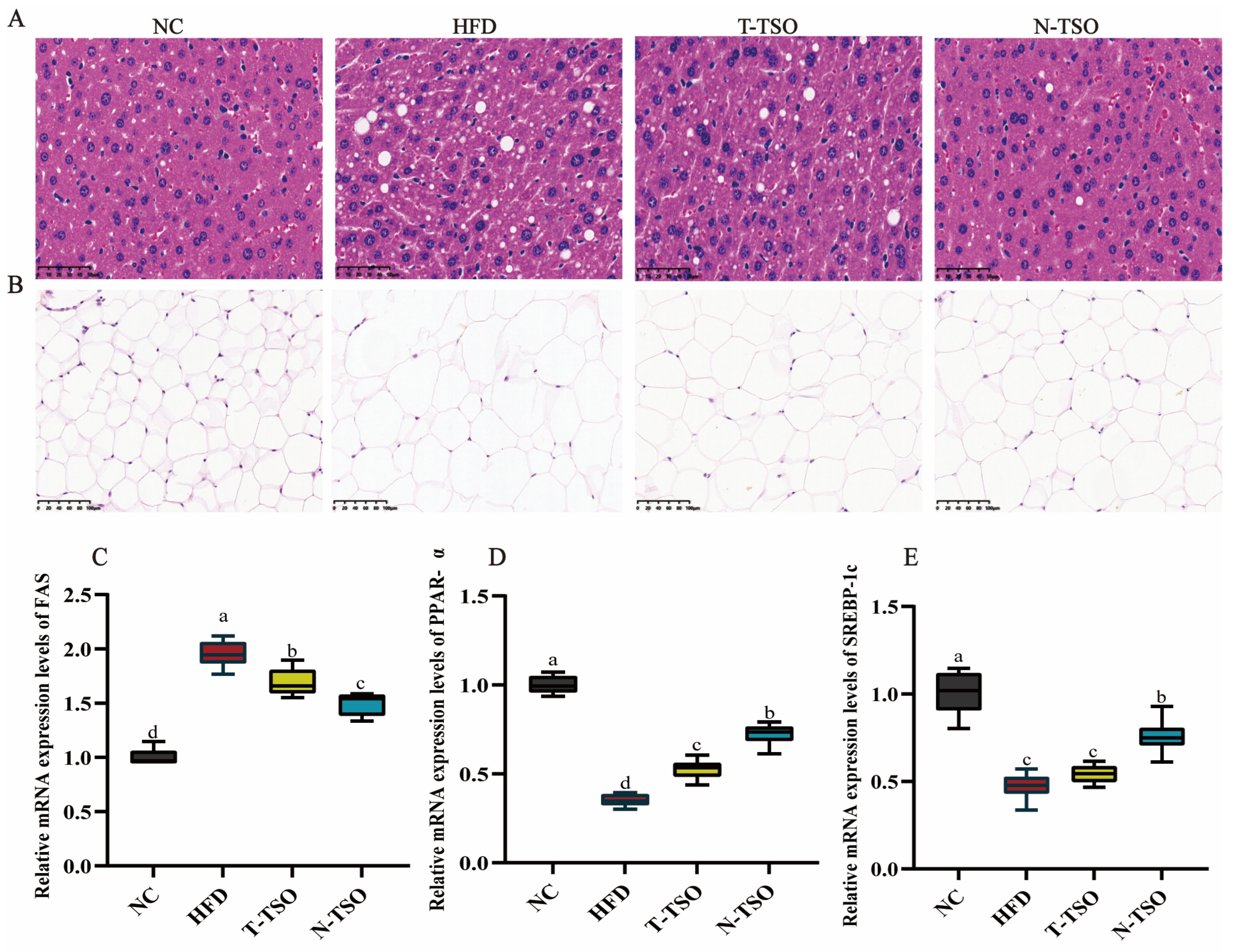
3.5. Observation of Adipose Tissue Morphology
3.6. Effect of Different Refined TSO on Lipid Metabolism Genes
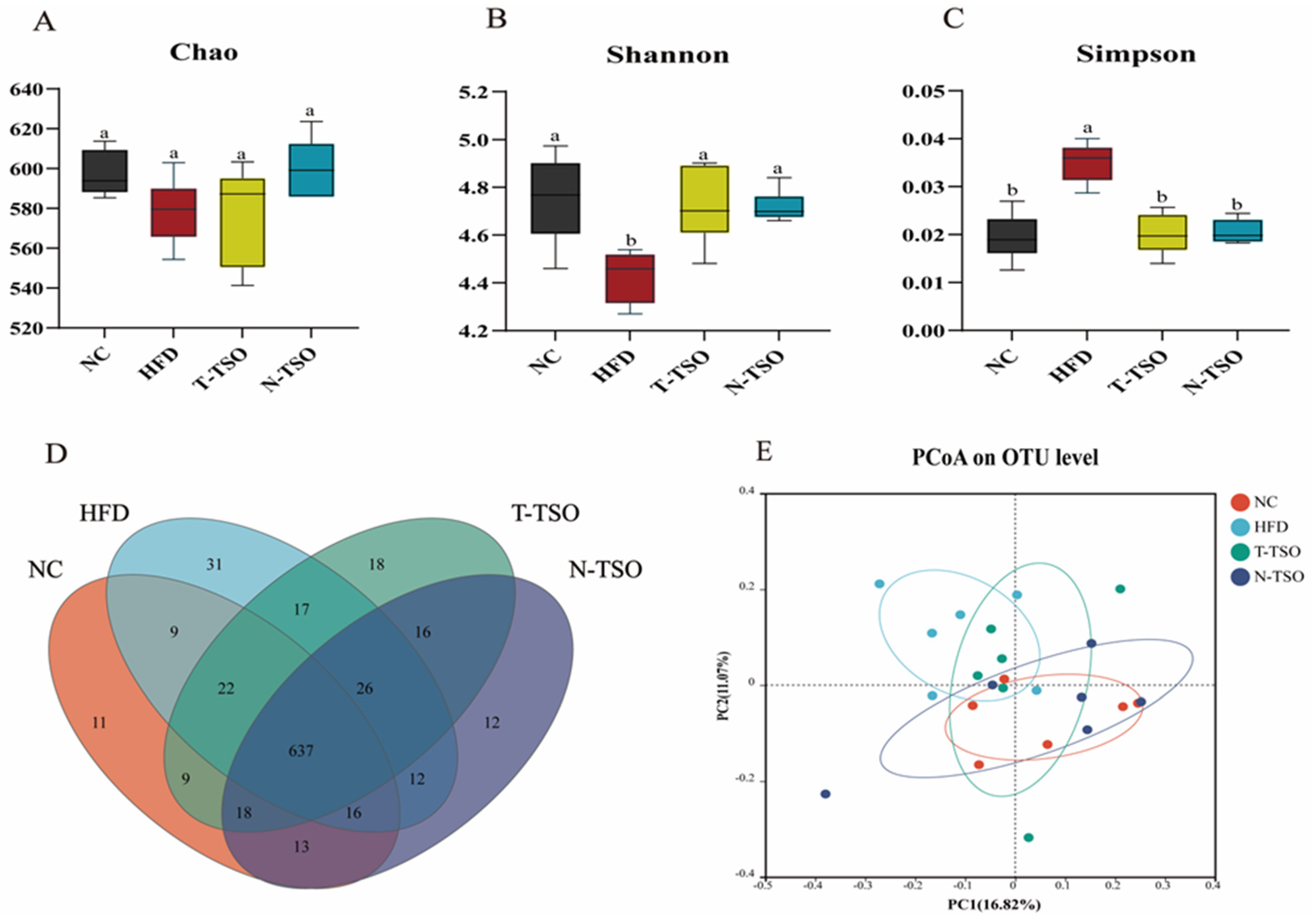
3.7. Effect of Different Refined TSO on Intestinal Microbiota of Obese Mice
3.8. Microbial Composition Analyses at the Phylum Level
3.9. Microbial Composition Analyses at the Genus Level
3.10. Correlation between Obesity-Related Indicators and Gut Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golden, A. Obesity’s Impact. Nurs. Clin. N. Am. 2021, 56, xiii–xiv. [Google Scholar] [CrossRef] [PubMed]
- FAO. Overweight and obesity impact to surpass $4 trillion by 2035. In World Food Regulation Review; FAO: Rome, Italy, 2023. [Google Scholar]
- Tang, C.; Wang, Y.; Xu, Z.; Chen, D.; Xu, J.; Yang, D.; Zhang, L.; Liu, J.; Kan, J. The relationships between high-fat diet and metabolic syndrome: Potential mechanisms. Food Biosci. 2024, 59, 104261. [Google Scholar] [CrossRef]
- Sun, S.; Cao, C.; Li, J.; Meng, Q.; Cheng, B.; Shi, B.; Shan, A. Lycopene Modulates Placental Health and Fetal Development Under High-Fat Diet During Pregnancy of Rats. Mol. Nutr. Food Res. 2021, 65, e2001148. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Zhou, X.; Wang, W.; Zhou, Y.; Zhou, X.; Deng, S.; Zheng, B.; Wen, Z. Effect of Antarctic krill phospholipid (KOPL) on high fat diet-induced obesity in mice. Food Res. Int. 2021, 148, 110456. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Baretić, M. Obesity drug therapy. Minerva Endocrinol. 2013, 38, 245–254. [Google Scholar] [PubMed]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Q.; Lu, H.; Jiang, C.; Hu, W.; Yu, S.; Xiang, X.; Tan, C.P.; Feng, Y.; Zhang, J.; et al. Antidiabetic effect of sciadonic acid on type 2 diabetic mice through activating the PI3K-AKT signaling pathway and altering intestinal flora. Front. Nutr. 2022, 9, 1053348. [Google Scholar] [CrossRef]
- He, L.; Zhou, G.Y.; Zhang, H.Y.; Liu, J.A. Research progress on the health function of tea oil. J. Med. Plants Res. 2011, 5, 485–489. [Google Scholar]
- Wang, X.; Zeng, Q.; Verardo, V.; Contreras, M.d.M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “FancyTiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.T.; Tung, Y.T.; Wu, C.C.; Tu, P.S.; Yen, G.C. Camellia Oil ( Camellia oleifera Abel.) Modifies the Composition of Gut Microbiota and Alleviates Acetic Acid-Induced Colitis in Rats. J. Agric. Food Chem. 2018, 66, 7384–7392. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Hsu, Y.J.; Chien, Y.W.; Huang, C.C.; Huang, W.C.; Chiu, W.C. Tea Seed Oil Prevents Obesity, Reduces Physical Fatigue, and Improves Exercise Performance in High-Fat-Diet-Induced Obese Ovariectomized Mice. Molecules 2019, 24, 980. [Google Scholar] [CrossRef] [PubMed]
- Natolino, A.; Da Porto, C.; Scalet, M. Broken and Intact Cell Model for supercritical carbon dioxide extraction of tea Camellia sinensis (L) seed oil. J. Supercrit. Fluids 2022, 180, 105422. [Google Scholar] [CrossRef]
- Wei, J.; Chen, L.; Qiu, X.; Hu, W.; Sun, H.; Chen, X.; Bai, Y.; Gu, X.; Wang, C.; Chen, H.; et al. Optimizing refining temperatures to reduce the loss of essential fatty acids and bioactive compounds in tea seed oil. Food Bioprod. Process. 2015, 94, 136–146. [Google Scholar] [CrossRef]
- GB 5009.168-2016; National Standard for Food Safety Determination of Fatty Acids in Foods. Standards Press of China: Beijing, China, 2016.
- Wang, X.; Zeng, Q.; del Mar Contreras, M.; Wang, L. Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef] [PubMed]
- LS/T 6119-2017; Determination of Polyphenols in Vegetable Oils. Standards Press of China: Beijing, China, 2018.
- Li, Z.; Wang, W.; Liu, X.; Qi, S.; Lan, D.; Wang, Y. Effect of different degumming processes on the retention of bioactive components, acylglycerol and phospholipid composition of rapeseed oil. Process Biochem. 2023, 133, 190–199. [Google Scholar] [CrossRef]
- GB/T 35026-2018; Tea Camellia Seed Oil. Standards Press of China: Beijing, China, 2018.
- Aldamarany, W.A.S.; Taocui, H.; Liling, D.; Mei, H.; Yi, Z.; Zhong, G. Perilla, sunflower, and tea seed oils as potential dietary supplements with anti-obesity effects by modulating the gut microbiota composition in mice fed a high-fat diet. Eur. J. Nutr. 2023, 62, 2509–2525. [Google Scholar] [CrossRef]
- David, E.M.; Pacharinsak, C.; Jampachaisri, K.; Hagan, L.; Marx, J.O. Use of Ketamine or Xylazine to Provide Balanced Anesthesia with Isoflurane in C57BL/6J Mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2022, 61, 457–467. [Google Scholar] [CrossRef]
- Nakatsu, N.; Igarashi, Y.; Aoshi, T.; Hamaguchi, I.; Saito, M.; Mizukami, T.; Momose, H.; Ishii, K.J.; Yamada, H. Isoflurane is a suitable alternative to ether for anesthetizing rats prior to euthanasia for gene expression analysis. J. Toxicol Sci. 2017, 42, 491–497. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, C.; Lu, H.; Zhou, T.; Hu, W.; Ping Tan, C.; Feng, Y.; Shen, G.; Xiang, X.; Chen, L. Camellia oil alleviates DSS-induced colitis in mice by regulating the abundance of intestinal flora and suppressing the NF-κB signaling pathway. J. Funct. Foods 2023, 108, 105777. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, L.; Wang, R.; Chen, Y.; Deng, S.; Shen, G.; Liu, S.; Xiang, X. Hypoglycemic Mechanism of Tegillarca granosa Polysaccharides on Type 2 Diabetic Mice by Altering Gut Microbiota and Regulating the PI3K-Akt Signaling Pathway. Food Sci. Hum. Wellness 2023, 13, 842–855. [Google Scholar] [CrossRef]
- Sahari, M.A.; Ataii, D.; Hamedi, M. Characteristics of tea seed oil in comparison with sunflower and olive oils and its effect as a natural antioxidant. J. Am. Oil Chem. Soc. 2004, 81, 585–588. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Chen, H.; Qian, L.; Xu, P. Fatty acid composition and antioxidant activity of tea (Camellia sinensis L.) seed oil extracted by optimized supercritical carbon dioxide. Int. J. Mol. Sci. 2011, 12, 7708–7719. [Google Scholar] [CrossRef]
- Zhuo-Ting, Y.; Xian-Yan, L.; Xin-Chu, W. Comparison of physicochemical properties and fatty acids composition of tea seed oil and camellia oleifera seed oil. Sci. Technol. Food Ind. 2011, 12, 156–162. [Google Scholar] [CrossRef]
- Morrison, M.C.; Mulder, P.; Stavro, P.M.; Suárez, M.; Arola-Arnal, A.; van Duyvenvoorde, W.; Kooistra, T.; Wielinga, P.Y.; Kleemann, R. Replacement of Dietary Saturated Fat by PUFA-Rich Pumpkin Seed Oil Attenuates Non-Alcoholic Fatty Liver Disease and Atherosclerosis Development, with Additional Health Effects of Virgin over Refined Oil. PLoS ONE 2015, 10, e0139196. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shang, K.; Lin, C.; Wang, C.; Shi, X.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Gao, J.; Ma, L.; Ma, J.; Xia, S.; Gong, S.; Yin, Y.; Chen, Y. Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids. Nutrients 2022, 14, 2424. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Z.; Song, H.; Hu, H.; Ma, S.; Tao, Y.; Hao, Z.; Feng, X.; Pan, Y.; Gong, S.; et al. Recent advances in tea seeds (Camellia Sinensis (L.) O. Kuntze): Active ingredients, health effects, and potential applications. Trends Food Sci. Technol. 2023, 141, 104192. [Google Scholar] [CrossRef]
- Amin, M.N.; Hussain, M.S.; Sarwar, M.S.; Rahman Moghal, M.M.; Das, A.; Hossain, M.Z.; Chowdhury, J.A.; Millat, M.S.; Islam, M.S. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, N.; Chen, Z.; Li, K.; Qiao, D.; Zhao, S.; Zhang, B. Ingesting retrograded rice (Oryza sativa) starch relieves high-fat diet induced hyperlipidemia in mice by altering intestinal bacteria. Food Chem. 2023, 426, 136540. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-virgin olive oil and the gut-brain axis: Influence on gut microbiota, mucosal immunity, and cardiometabolic and cognitive health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef]
- Weng, G.; Duan, Y.; Zhong, Y.; Song, B.; Zheng, J.; Zhang, S.; Yin, Y.; Deng, J. Plant Extracts in Obesity: A Role of Gut Microbiota. Front. Nutr. 2021, 8, 727951. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Z.P.; Liu, C.Q.; Qi, L.; Sheng, Y.; Zou, D.J. Modulation of the gut microbiome: A systematic review of the effect of bariatric surgery. Eur. J. Endocrinol. 2017, 178, 43–56. [Google Scholar] [CrossRef]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef]
- Lyu, Q.; Deng, H.; Wang, S.; El-Seedi, H.; Cao, H.; Chen, L.; Teng, H. Dietary supplementation with casein/cyanidin-3-O-glucoside nanoparticles alters the gut microbiota in high-fat fed C57BL/6 mice. Food Chem. 2023, 412, 135494. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Song, C.; Li, L.; Wang, T.; Hu, J.; Zhu, L.; Yue, T. Lactobacillus alleviated obesity induced by high-fat diet in mice. J. Food Sci. 2021, 86, 5439–5451. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T.; et al. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. iScience 2021, 24, 103342. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef]
- Xiang, Z.; Olabisi Oluwabukola, C.; Eagle, S.H.C.; Kaili, F.; Harry, C.H.L.; Yi-Xiang, W.; Anthony, W.H.C.; Hong, W.; Xiaoyong, Y.; Joseph, J.Y.S.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761. [Google Scholar] [CrossRef]


| Parameter | Crude | N-TSO | T-TSO | CNS-TSO |
|---|---|---|---|---|
| Refractive index (n40) | 1.478 ± 0.124 | 1.470 ± 0.137 | 1.468 ± 0.116 | 1.462–1.472 |
| Acid value (mg KOH/g) | 4.1 ± 0.04 | 0.21 ± 0.02 | 0.14 ± 0.06 | <0.8 |
| Iodine value (g/100 g) | 87.5 ± 1.2 | 88.1 ± 1.4 | 89.2 ± 1.1 | 74–95 |
| Peroxide value (mmol kg−1) | 8.97 ± 0.64 | 0.85 ± 0.10 | 0.57 ± 0.08 | <6.00 |
| Moisture and volatile matter (%) | 0.22 ± 0.07 | 0.07 ± 0.01 | 0.05 ± 0.01 | <0.10 |
| Insoluble impurities (%) | 0.18 ± 0.02 | 0.04 ± 0.01 | 0.02 ± 0.01 | <0.05 |
| Phospholipids (%) | 0.275 ± 0.02 | 0.017 ± 0.002 | 0.007 ± 0.001 | <0.02 |
| Fatty acid composition | ||||
| SFA (%) | ||||
| C14:0 | 0.18 ± 0.03 | 0.17 ± 0.02 | 0.18 ± 0.01 | <0.5 |
| C16:0 | 15.18 ± 0.03 | 15.06 ± 0.01 | 15.11 ± 0.01 | 13.0–18.0 |
| C18:0 | 2.71 ± 0.01 | 2.63 ± 0.01 | 2.67 ± 0.02 | 2.0–6.0 |
| C20:0 | 0.089 ± 0.001 | 0.085 ± 0.001 | 0.082 ± 0.001 | NR |
| C22:0 | 0.044 ± 0.001 | 0.041 ± 0.001 | 0.042 ± 0.001 | NR |
| C24:0 | 0.051 ± 0.001 | 0.048 ± 0.001 | 0.049 ± 0.002 | NR |
| Total | 18.25 ± 0.073 | 18.03 ± 0.043 | 18.13 ± 0.044 | NR |
| MUFA (%) | ||||
| C16:1 | 0.38 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.02 | NR |
| C18:1 | 57.96 ± 0.21 | 57.16 ± 0.18 | 57.06 ± 0.23 | 50.0–68.0 |
| C20:1 | 0.70 ± 0.002 | 0.68 ± 0.001 | 0.69 ± 0.002 | NR |
| C22:1 | 0.26 ± 0.004 | 0.25 ± 0.003 | 0.25 ± 0.004 | NR |
| C24:1 | 0.067 ± 0.003 | 0.063 ± 0.001 | 0.065 ± 0.002 | NR |
| Total | 59.37 ± 0.23 | 58.51 ± 0.20 | 59.44 ± 0.26 | NR |
| PUFA (%) | ||||
| C18:2 | 21.13 ± 0.007 | 21.01 ± 0.002 | 21.06 ± 0.004 | 15.0–35.0 |
| C18:3 | 0.59 ± 0.039 | 0.58 ± 0.028 | 0.57 ± 0.031 | 0.2–2.0 |
| C20:2 | 0.014 ± 0.001 | 0.013 ± 0.002 | 0.013 ± 0.001 | <1.5 |
| Total | 21.73 ± 0.047 | 21.60 ± 0.032 | 21.64 ± 0.036 | NR |
| Parameter | Crude | N-TSO | T-TSO | CNS-TSO |
|---|---|---|---|---|
| Total Polyphenols (mg/100 g) | 36.57 ± 0.34 a | 29.73 ± 0.23 b | 7.50 ± 0.43 c | NR |
| Total tocopherol (mg/100 g) | 41.68 ± 0.35 a | 35.38 ± 0.45 b | 10.32 ± 0.24 c | NR |
| Sterols (mg/100 g) | 177.21 ± 4.38 a | 158.77 ± 4.84 b | 44.68 ± 3.45 c | NR |
| Squalene (mg/100 g) | 23.86 ± 0.18 a | 20.04 ± 0.58 b | 6.41 ± 0.48 c | NR |
| Carotenoids (mg/100 g) | 21.69 ± 0.22 a | 15.19 ± 0.44 b | 3.85 ± 0.43 c | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Jiang, Q.; Lu, H.; Jiang, C.; Hu, W.; Liu, H.; Xiang, X.; Tan, C.P.; Zhou, T.; Shen, G. Effects of Tea Seed Oil Extracted by Different Refining Temperatures on the Intestinal Microbiota of High-Fat-Diet-Induced Obese Mice. Foods 2024, 13, 2352. https://doi.org/10.3390/foods13152352
Chen L, Jiang Q, Lu H, Jiang C, Hu W, Liu H, Xiang X, Tan CP, Zhou T, Shen G. Effects of Tea Seed Oil Extracted by Different Refining Temperatures on the Intestinal Microbiota of High-Fat-Diet-Induced Obese Mice. Foods. 2024; 13(15):2352. https://doi.org/10.3390/foods13152352
Chicago/Turabian StyleChen, Lin, Qihong Jiang, Hongling Lu, Chenkai Jiang, Wenjun Hu, Hanxiao Liu, Xingwei Xiang, Chin Ping Tan, Tianhuan Zhou, and Guoxin Shen. 2024. "Effects of Tea Seed Oil Extracted by Different Refining Temperatures on the Intestinal Microbiota of High-Fat-Diet-Induced Obese Mice" Foods 13, no. 15: 2352. https://doi.org/10.3390/foods13152352
APA StyleChen, L., Jiang, Q., Lu, H., Jiang, C., Hu, W., Liu, H., Xiang, X., Tan, C. P., Zhou, T., & Shen, G. (2024). Effects of Tea Seed Oil Extracted by Different Refining Temperatures on the Intestinal Microbiota of High-Fat-Diet-Induced Obese Mice. Foods, 13(15), 2352. https://doi.org/10.3390/foods13152352







