From Fruit to Beverage: Investigating Actinidia Species for Characteristics and Potential in Alcoholic Drink Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material and Preparation of Juices
2.2. Chemical and Nutraceutical Evaluation of Kiwifruits
2.3. Chemical Characterization of Kiwifruit Juices
2.4. Yeast Strain and Fermentation Trials
2.5. Microbial and Chemical Analysis of Kiwifruit Drinks
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Fresh Kiwifruits
3.2. Chemical Characterization of the Kiwi Juices
3.3. Aromatic Profile of the Kiwi Juices
| Compound | Perception Threshold (μg/L) | Descriptor | Tahi | Rua | SunGold |
|---|---|---|---|---|---|
| Acids | |||||
| Hexanoic acid | 420 a | Sweat | 1078.5 ± 60.1 ab | 1022.5 ± 3.5 b | 1102.5 ± 24.7 a |
| trans-2-Hexenoic acid | - | Must, fat | 94.5 ± 7.8 a | 41.5 ± 4.9 c | 58.5 ± 4.9 b |
| Total | 1173.0 ± 67.9 a | 1064.0 ± 8.5 b | 1161.0 ± 29.7 a | ||
| Alcohols | |||||
| 1-Hexanol | 1110 [38] | Resin, flower, green | 185.0 ± 5.7 a | 99.0 ± 4.2 b | 90.5 ± 3.5 b |
| 2-Hexanol | - | Resin, flower, green | 280.0 ± 31.1 b | 337.5 ± 24.7 a | 313.0 ± 2.8 ab |
| cis-3-Hexen-1-ol | 400 [37] | Grass | 277.0 ± 17.0 c | 633.5 ± 30.4 b | 681.5 ± 4.9 a |
| trans-2-Hexen-1-ol | - | Green, leaf, walnut | 86.0 ± 5.7 a | 77.5 ± 10.6 a | 63.0 ± 2.8 b |
| Homovanillic acid | - | 34.5 ± 2.1 a | 19.0 ± 4.2 b | 30.5 ± 2.1 a | |
| Benzyl alcohol | 10,000 [41] | Sweet, flower | 33.5 ± 10.6 a | 48.5 ± 9.2 a | 39.0 ± 4.2 a |
| 2-Phenylethanol | 10,000 [42] | Honey, spice, rose, lilac | 28.0 ± 12.7 a | 37.5 ± 3.5 a | 25.0 ± 4.2 a |
| Total | 924.0 ± 84.9 b | 1252.5 ± 87.0 a | 1242.5 ± 24.7 a | ||
| Aldehydes | |||||
| trans-2-Pentenal | 1500 [39] | Fruit, tomato | 237.0 ± 21.1 c | 522.5 ± 3.5 a | 420.0 ± 11.3 b |
| Hexanal | 25,000 [43] | Grass, tallow, fat | 92.0 ± 5.7 a | 62.5 ± 17.7 b | 68.0 ± 9.9 ab |
| trans-2-Hexenal | 17 [41] | Green, leaf | 100.5 ± 27.6 a | 90.5 ± 14.8 ab | 60.0 ± 14.1 b |
| Nonanal | 8 [42] | Fat, citrus, green | 81.0 ± 5.7 b | 130.5 ± 14.8 a | 49.0 ± 1.4 c |
| Total | 510.5 ± 60.1 b | 806.0 ± 50.9 a | 597.0 ± 36.8 b |
| Compound | Perception Threshold (μg/L) | Descriptor | Tahi | Rua | SunGold |
|---|---|---|---|---|---|
| Acids | |||||
| Hexanoic acid | 420 a | Sweat | 1958.0 ± 89.1 a | 832.0 ± 28.3 c | 1259.0 ± 9.9 b |
| trans-2-Hexenoic acid | - | Must, fat | 110.5 ± 14.8 a | n.d. | 23.0 ± 2.8 b |
| 4-Methyl-benzoic acid | - | - | 95.5 ± 14.8 | n.d. | n.d. |
| Total | 2164.5 ± 118.8 a | 832.0 ± 28.3 c | 1282.0 ± 12.7 b | ||
| Alcohols | |||||
| 1-Hexanol | 1110 [38] | Resin, flower, green | 685.5 ± 58.7 a | 375.0 ± 49.5 c | 483.0 ± 38.2 b |
| 2-Hexanol | - | Resin, flower, green | n.d. | n.d. | n.d. |
| cis-3-Hexen-1-ol | 400 [37] | Grass | 48.5 ± 4.9 a | 47.5 ± 3.5 a | 48.5 ± 9.2 a |
| trans-2-Hexen-1-ol | - | Green, leaf, walnut | 106.5 ± 7.8 a | 31.5 ± 4.9 c | 58.5 ± 4.9 b |
| Benzyl alcohol | 10,000 [41] | Sweet, flower | 755.0 ± 23.3 a | 382.0 ± 24.0 c | 505.5 ± 74.2 b |
| 2-Phenylethanol | 10,000 [42] | Honey, spice, rose, lilac | 21.5 ± 0.7 c | 147.5 ± 3.5 b | 168.0 ± 2.8 a |
| Total | 1590.0 ± 95.5 a | 983.5 ± 85.6 c | 1263.5 ± 129.4 b | ||
| Aldehydes | |||||
| trans-2-Pentenal | 1500 [39] | Fuit, tomato | 59.0 ± 12.7 a | 62.5 ± 17.7 a | 30.0 ± 7.1 b |
| Hexanal | 25,000 [43] | Grass, tallow, fat | 128.5 ± 4.9 a | 44.5 ± 4.9 b | 30.5 ± 3.5 c |
| Total | 187.5 ± 17.7 a | 107.0 ± 22.6 b | 60.5 ± 10.6 c |
3.4. Fermentation Trials
3.4.1. Aroma Profile
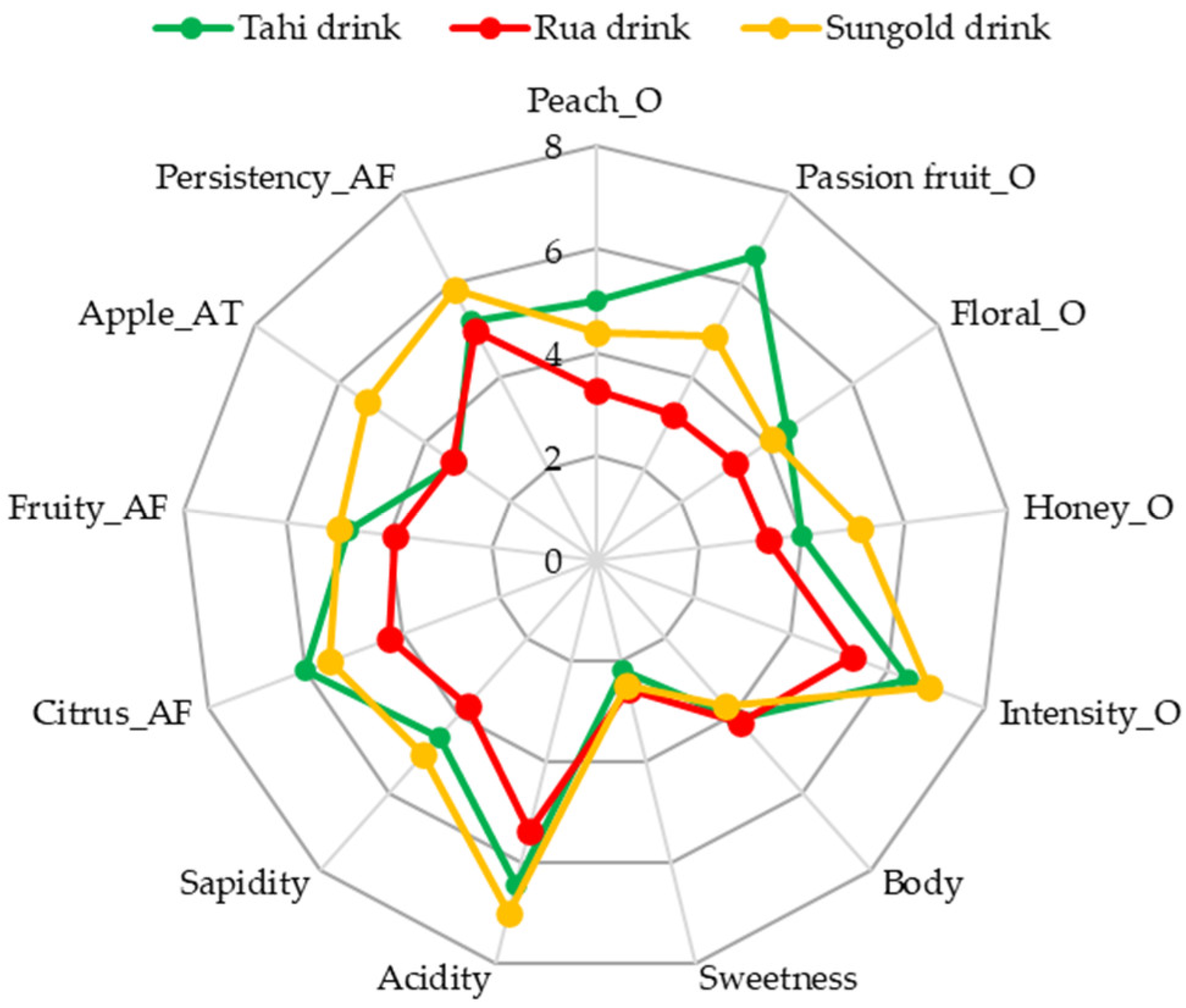
3.4.2. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giuggioli, N.R.; Baudino, C.; Briano, R.; Peano, C. Quality of packed baby kiwi cultivar ‘Hortgem Tahi®’ and ‘Hortgem Rua®’. Progr. Nutr. 2019, 21, 440–448. [Google Scholar]
- Garcia Contracting. February 2024. Available online: https://garciacontracting.co.nz/kiwifruit-varieties-in-new-zealand (accessed on 5 February 2024).
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Latocha, P.; Wołosiak, R.; Worobiej, E.; Krupa, T. Clonal differences in antioxidant activity and bioactive constituents of hardy kiwifruit (Actinidia arguta) and its year-to-year variability. J. Sci. Food Agric. 2013, 93, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Calvez, E.; Neuberger, S.; Wagner, K.; Schoedl-Hummel, K.; Debersaques, F. Management strategies for minikiwi (Actinidia arguta): Practical experiences of european growers. Acta Hortic. 2014, 1096, 451–454. [Google Scholar]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Rajoka, M.S.R.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Cossio, F.; Debersaques, F.; Latocha, P. Kiwiberry (Actinidia arguta): New Perspectives for a Great Future. Acta Hortic. 2014, 1096, 423–434. [Google Scholar] [CrossRef]
- Debersaques, F.; Mekers, O.; Decorte, J.; Van Labeke, M.C.; Schoedl-Hummel, K.; Latocha, P. Challenges faced by commercial kiwiberry (actinidia argute planch.) Production. Acta Hortic. 2014, 1096, 435–442. [Google Scholar] [CrossRef]
- Latocha, P. The nutritional and health benefits of kiwiberry (Actinidia arguta)—A Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Mumford, A. The Effect of Harvest Maturity on ‘Geneva 3’ Kiwiberry Storability, Ripening Dynamics, and Fruit Quality. Master’s Thesis, University of New Hampshire, Durham, NH, USA, 2022. [Google Scholar]
- Ritenour, M.A.; Crisosto, C.H.; Garner, D.T.; Cheng, G.W.; Zoffoli, J.P. Temperature, length of cold storage and maturity influence the ripening rate of ethylene-preconditioned kiwifruit. Postharvest Biol. Technol. 1999, 15, 107–115. [Google Scholar] [CrossRef]
- Lim, S.; Han, S.H.; Kim, J.; Lee, H.J.; Lee, J.G.; Lee, E.J. Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 2016, 190, 150–157. [Google Scholar] [CrossRef]
- Yilmaz, D.; Yildirim, I. Effects of different storage techniques on rupture properties of kiwifruits. J. Food Meas. Charact. 2016, 10, 539–545. [Google Scholar] [CrossRef]
- Oh, S.; Muneer, S.; Kwack, Y.; Shin, M.; Kim, J. Characteristic of fruit development for optimal harvest date and postharvest storability in ‘Skinny Green’ baby kiwifruit. Sci. Hortic. 2017, 222, 57–61. [Google Scholar] [CrossRef]
- Kitinoja, L.; Saran, S.; Roy, S.K.; Kader, A.A. Postharvest technology for developing countries: Challenges and opportunities in research, outreach and advocacy. J. Sci. Food Agric. 2011, 91, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Bovi, G.G.; Caleb, O.J.; Linke, M.; Rauh, C.; Mahajan, P.V. Transpiration and moisture evolution in packaged fresh horticultural produce and the role of integrated mathematical models: A review. Biosyst. Eng. 2016, 150, 24–39. [Google Scholar] [CrossRef]
- Fracassetti, D.; Bottelli, P.; Corona, O.; Foschino, R.; Vigentini, I. Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice. Metabolites. 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Soufleros, E.H.; Pissa, I.; Petridis, D.; Lygerakis, M.; Mermelas, K.; Boukouvalas, G.; Tsimitakis, E. Instrumental analysis of volatile and other compounds of Greek kiwi wine; sensory evaluation and optimisation of its composition. Food Chem. 2001, 75, 487–500. [Google Scholar] [CrossRef]
- Towantakavanit, K.; Park, Y.; Gorinstein, S. Bioactivity of wine prepared from ripened and over-ripened kiwifruit. Open Life Sci. 2011, 6, 205–215. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Rustioni, L.; Altomare, A.; Shanshiashvili, G.; Greco, F.; Buccolieri, R.; Blanco, I.; Cola, G.; Fracassetti, D. Microclimate of Grape Bunch and Sunburn of White Grape Berries: Effect onWine Quality. Foods 2023, 12, 621. [Google Scholar] [CrossRef]
- Vigentini, I.; Maghradze, D.; Petrozziello, M.; Bonello, F.; Mezzapelle, V.; Valdetara, F.; Failla, O.; Foschino, R. Indigenous Georgian Wine-Associated Yeasts and Grape Cultivars to Edit the Wine Quality in a Precision Oenology Perspective. Front. Microbiol. 2016, 7, 352. [Google Scholar] [CrossRef][Green Version]
- Mateo, J.J.; Gentilini, N.; Huerta, T.; Jimenez, M.; Di Stefano, R. Fractionation of glycoside precursors of aroma in grapes and wine. J. Chromatogr. A 1997, 778, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Petrozziello, M.; Guaita, M.; Motta, S.; Panero, L.; Bosso, A. Analytical and sensory characterization of the aroma of “Langhe D.O.C. Nebbiolo” wines: Influence of the prefermentative cold maceration with dry ice. J. Food Sci. 2011, 76, C525–C534. [Google Scholar] [PubMed]
- Fracassetti, D.; Camoni, D.; Montresor, L.; Bodon, R.; Limbo, S. Chemical Characterization and Volatile Profile of Trebbiano di Lugana Wine: A Case Study. Foods 2020, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yuan, Q.; Yang, Y.L.; Han, Q.H.; He, J.L.; Zhao, L.; Zhang, Q.; Liu, S.X.; Lin, D.R.; Wu, D.T.; et al. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules 2018, 23, 2957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative analysis of physicochemical characteristics, nutritional and functional components and antioxidant capacity of fifteen kiwifruit (Actinidia) cultivars—Comparative analysis of fifteen kiwifruit (Actinidia) cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef]
- Nardozza, S.; Boldingh, H.; Osorio, S.; Hohme, M.; Wohlers, M.; Gleave, A.; MacRae, E.; Richardson, A.; Atkinson, R.; Sulpice, R.; et al. Metabolic analysis of kiwifruit (Actinidia deliciosa) berries from extreme genotypes reveals hallmarks for fruit starch metabolism. J. Exp. Bot. 2013, 64, 5049–5063. [Google Scholar] [CrossRef] [PubMed]
- Oz, A.T.; Eris, A. Effects of controlled atmosphere storage on "Hayward" kiwifruits harvested at different TSS levels. Acta Hortic. 2010, 876, 81–84. [Google Scholar] [CrossRef]
- Hall, A.J.; Richardson, A.C.; Snelgar, W.P. Modelling fruit development in ‘Hayward’ kiwifruit. Acta Hortic. 2006, 707, 41–47. [Google Scholar] [CrossRef]
- Fisk, C.; McDaniel, M.; Strik, B.; Zhao, Y. Physocochemical, sensory and nutritive qualities of hardy kiwifruit (Actinidia arguta `Ananasnaya`) as affected by harvest maturity and storage. J. Food Sci. Sens. Nutr. Qual. Foods 2006, 71, 3. [Google Scholar]
- Sun, T.; Simon, P.; Tanumihardjo, S. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef]
- Jeffery, P.B.; Banks, N.H. Firmness-temperature coefficient of kiwifruit. N. Z. J. Crop Hortic. Sci. 1994, 22, 97–101. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Ferreira, A.; Barbosa, C.; Lage, P.; Mendes-Faia, A. The impact of nitrogen on yeast fermentation and wine quality. Ciénc. Téc. Vitiv. 2011, 26, 17–32. [Google Scholar]
- Li, H.; Cao, S.; Liu, Z.; Li, N.; Xu, D.; Yang, Y.; Mo, H.; Hu, L. Rapid assessment of ready-to-eat Xuxiang kiwifruit quality based on chroma recognition and GC-MS analysis. LWT 2023, 182, 114796. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Viario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odour Thresholds, Odour Qualities and Retention Indices of Keyfood Odorants; Deutsche forschungsanstalt fur Lebensmittelchemie and Institut fur Lebensmittelchemie der Technischen Universitat Munchen: Garching, Germany, 1998. [Google Scholar]
- Coralia, V.G.; Quek, S.W.; Stevenson, R.J.; Winz, R.A. Characterization of the Bound Volatile Extract from Baby Kiwi (Actinidia arguta). J. Agric. Food Chem. 2011, 59, 8358–8365. [Google Scholar]
- Odor & Flavor Detection Thresholds in Water (In Parts per Billion). 2024. Available online: http://www.leffingwell.com/odorthre.htm (accessed on 11th March 2024).
- Gamero, A.; Ferreira, V.; Pretorius, I.S.; Querol, A. Wine, beer and cider: Unrevelling the aroma profile. In Molecular Mechanisms in Yeast Carbon Metabolism; Piskur, J., Compagno, C., Eds.; Springer: Berlin/Hidelberg, Germany, 2014; pp. 261–297. [Google Scholar]
- Zheng, L. Intensity of odor and sensory irritation as a function of hexanal concentration and inter-presentation intervals: An exploratory study. Percept. Mot. Ski. 2010, 111, 210–228. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic fruit wine production via reciprocal selection of juice and non-Saccharomyces species. Food Microbiol. 2019, 79, 66–74. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii contribution in mixed brewing fermentations with different Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 259, 7–13. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; Gonzalez, C.; Calderon, F.; Suarez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1977, 14, 199–203. [Google Scholar] [CrossRef]
- Casadey, R.; Challier, C.; Senz, A.; Criado, S. Antioxidant ability of tyrosol and derivative-compounds in the presence of O2(1Îg)-species. Studies of synergistic antioxidant effect with commercial antioxidants. Food Chem. 2019, 285, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, J.; Yang, Y.; Deng, J.; Zhu, K.; Yi, Y.; Tang, J.; Jiang, X.; Zhu, C.; Laghi, L. Effects of S. cerevisiae strains on the sensory characteristics and flavor profile of kiwi wine based on E-tongue, GC-IMS and 1H-NMR. LWT 2023, 185, 115193. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Effect of sequential fermentation with four non-Saccharomyces and Saccharomyces cerevisiae on nutritional characteristics and flavor profiles of kiwi wines. J. Food Compos. Anal. 2022, 109, 104480. [Google Scholar] [CrossRef]
- Huang, D.; Ren, X.; Zhong, Y.; Liu, Y.; Jiang, J.; Song, Y.; Xin, L.; Lu, Y.; Qin, Y. Effect of multi-step chaptalisation on physicochemical properties, potentially harmful alcohols, nutritional composition and volatile profiles of kiwi wine. Int. J. Food Sci. Technol. 2023, 58, 3715–3726. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Ong, P.K.; Acree, T.E. Similarities in the aroma chemistry of Gewürztraminer variety wines and lychee (Litchi chinesis sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef]
- Moreno, J.A.; Zea, L.; Moyano, L.; Medina, M. Aroma compounds as markers of the changes in sherry wines subjected to biological ageing. Food Control 2005, 16, 333–338. [Google Scholar] [CrossRef]


| Fresh Kiwifruit cv | TSS (°Brix) | Acidity (%) | Hardness (N) | Dry Matter (%) | Total Phenolic Index (mg/100 g Gallic Acid Equivalents) | Antioxidant Capacity (mmol Fe2+/kg) |
|---|---|---|---|---|---|---|
| Tahi | 11.7 ± 0.9 ab | 2.0 ± 0.2 ab | 4.98 ± 0.8 b | 18.5 ± 0.9 ab | 187.09 ± 13.89 ab | 45.09 ± 1.99 b |
| Rua | 10.3 ± 1.1 b | 2.2 ± 0.3 a | 3.57 ± 0.5 b | 19.8 ± 1.3 b | 209.36 ± 12.36 a | 57.98 ± 2.98 a |
| SunGold | 12.9 ± 0.6 a | 1.5 ± 0.2 b | 6.45 ± 0.9 a | 22.4 ± 0.7 a | 168.25 ± 19.69 b | 29.87 ± 1.89 c |
| Tahi | Rua | SunGold | |
|---|---|---|---|
| Sugars (g/L) | 102.63 ± 7.49 b | 140.80 ± 7.42 a | 141.47 ± 0.40 a |
| Readily assimilable nitrogen (mg N/L) | 121 ± 4.84 b | 199 ± 7.96 a | 204 ± 9.18 a |
| pH | 3.38 ± 0.01 c | 3.64 ± 0.02 a | 3.60 ± 0.02 b |
| Titratable Acidity (g citric acid/L) | 11.45 ± 0.34 c | 12.59 ± 0.37 b | 14.19 ± 0.38 a |
| Malic Acid (g/L) | 1.92 ± 0.04 a | 1.74 ± 0.04 b | 1.19 ± 0.02 c |
| Citric Acid (g/L) | 7.94 ± 0.16 a | 5.26 ± 0.11 c | 6.18 ± 0.12 b |
| Succinic Acid (g/L) | 0.46 ± 0.02 a | 0.31 ± 0.01 b | n.d. |
| Tahi | Rua | SunGold | |
|---|---|---|---|
| Sugars (g/L) | 0.15 ± 0.03 b | 0.20 ± 0.01 a | 0.13 ± 0.00 b |
| pH | 3.32 ± 0.03 c | 3.46 ± 0.01 b | 3.78 ± 0.09 a |
| Total Acidity (g citric acid/L) | 14.71 ± 0.58 b | 11.67 ± 0.47 c | 16.75 ± 0.67 a |
| Ethanol (g/L) | 6.46 ± 1.47 a | 7.90 ± 1.04 a | 8.85 ± 1.06 a |
| Glycerol (g/L) | 2.08 ± 0.21 a | 2.09 ± 0.21 a | 1.85 ± 0.13 a |
| Malic Acid (g/L) | 2.19 ± 0.29 a | 2.31 ± 0.27 ab | 1.77 ± 0.22 b |
| Lactic Acid (g/L) | n.d. | n.d. | n.d. |
| Acetic Acid (g/L) | 0.21 ± 0.03 a | 0.24 ± 0.06 a | 0.63 ± 0.37 a |
| Citric Acid (g/L) | 8.96 ± 0.50 a | 6.66 ± 0.75 b | 8.86 ± 1.30 a |
| Succinic Acid (g/L) | 0.91 ± 0.11 a | 0.90 ± 0.02 a | 0.94 ± 0.13 a |
| Compound | Perception Threshold (μg/L) | Descriptor | Tahi | Rua | SunGold |
|---|---|---|---|---|---|
| Acids | |||||
| Isobutyric acid | 2300 [52] | Rancid, butter, cheese | 512.5 ± 17.7 a | 321.5 ± 27.6 c | 413.5 ± 2.1 b |
| Hexanoic acid | 420 [52] | Sweat | 2092.0 ± 48.1 c | 3138.5 ± 116.7 a | 2773.5 ± 111.0 b |
| Butyric acid | 240 [41] | Sweat, rancid, cheese | 448.0 ± 5.7 a | 333.5 ± 12.0 b | 310.0 ± 7.1 c |
| 2-Methylbutanoic acid | 33 [42] | Cheese, sweat | 157.0 ± 4.2 a | 352.0 ± 9.9 b | 161.0 ± 2.8 a |
| 2-Ethylbutanedioic acid | - | - | 385.0 ± 9.9 a | 234.5 ± 3.5 b | 143.5 ± 10.6 c |
| Octanoic acid | 500 [52] | Cheese, sweat | 3232.0 ± 5.7 b | 4036.5 ± 21.9 a | 2326.0 ± 26.9 c |
| Decanoic acid | 1000 [42] | Rancid, fat | 410.5 ± 14.8 b | 412.5 ± 12.0 b | 498.5 ± 4.9 a |
| Decenoic acid | 2 [42] | Fat | 249.5 ± 2.1 a | 202.5 ± 3.5 c | 225.5 ± 7.8 b |
| Total | 7486.5 ± 108.2 b | 9031.5 ± 207.2 a | 6851.5 ± 173.2 c | ||
| Alcohols | |||||
| 1-Hexanol | 1110 [38] | Resin, flower, green | 1120.0 ± 43.8 a | 2325.0 ± 35.4 b | 1138.0 ± 159.8 a |
| 2-Hexanol | - | Resin, flower, green | 72.5 ± 3.5 b | 21.5 ± 4.9 c | 92.5 ± 10.6 a |
| 3-Ethoxy-1-propanol | - | Fruit | n.d. | 23.5 ± 2.1. | 9.5 ± 3.5. |
| cis-3-Hexen-1-ol | 400 [37] | Grass | 27.5 ± 6.4 a | 23.5 ± 6.4 a | 25.0 ± 1.4 a |
| trans-2-Hexenol | - | Green, leaf, walnut | 26.5 ± 19.1 b | 22.5 ± 3.5 b | 92.5 ± 10.6 a |
| trans-3-Hexenol | Moss, fresh | 621.0 ± 2.8 a | 413.0 ± 2.8 c | 522.0 ± 9.9 b | |
| Decanol | Fat | 42.5 ± 3.5 ab | 47.5 ± 3.5 a | 37.0 ± 2.8 b | |
| Isoamyl alcohol | 30,000 [42] | Spirit, alcoholic | 8003.0 ± 25.5 a | 5140.5 ± 29.0 c | 7082.5 ± 60.1 b |
| 2,3-Butanediol | - | Fruit, onion | 1810.0 ± 21.2 a | 1762.5 ± 16.3 b | 1293.5 ± 12.0 c |
| 2-Phenylethanol | 10,000 [42] | Honey, spice, rose, lilac | 34,999 ± 9 c | 70,193 ± 16 a | 61,565 ± 622 b |
| Benzyl alcohol | 10,000 [41] | Sweet, flower | 92.5 ± 3.5 a | 59.0 ± 5.7 b | 87.0 ± 2.8 a |
| Methionol | - | Sweet, potato | 27± 13.4 c | 349.0 ± 9.9 a | 187.0 ± 18.4 b |
| p-Tyrosol | - | - | 1240.5 ± 6.4 c | 2018.5 ± 4.9 b | 2097.0 ± 11.3 a |
| Total | 47,090 ± 152 c | 82,400 ± 136 a | 72,132 ± 152 b | ||
| Aldehydes | |||||
| Phenylethanal | 1 [37] | Honey, sweet, hawthorn | 20.5 ± 0.7 a | 11.0 ± 1.4 c | 15.0 ± 1.4 b |
| Methyl benzenaldehyde | - | - | 108.0 ± 4.2 b | 113.5 ± 16.3 b | 155.0 ± 4.2 a |
| Total | 128.5 ± 4.9 b | 124.5 ± 17.7 b | 170.0 ± 5.7 a | ||
| Esters | |||||
| Isoamyl acetate | 12,270 [42] | Banana | 413.0 ± 11.3 a | 237.0 ± 21.1 c | 333.5 ± 26.2 b |
| Ethyl acetate | 7500 [42] | Pineapple | n.d. | 52.5 ± 3.5 b | 77.5 ± 10.6 a |
| Ethyl butyrate | 1 [41] | Apple | 110.0 ± 14.1 a | 55.0 ± 7.1 c | 86.0 ± 1.4 b |
| Ethyl isobutyrate | 15 [53] | Sweet, rubber | 47.5 ± 10.6 a | 22.5 ± 3.5 b | 25.0 ± 7.1 b |
| Ethyl hexanoate | 14 [54] | Apple, peach | 406.0 ± 8.5 a | 188.0 ± 19.8 c | 310.0 ± 7.1 b |
| Ethyl octanoate | 2 [55] | Fruit, fat | 412.5 ± 17.7 c | 510.0 ± 7.1 b | 606.0 ± 8.5 a |
| Ethyl succinate | 655.5 ± 7.8 a | 439.5 ± 12.0 c | 564.5 ± 13.4 b | ||
| Phenylethyl acetate | 250 [42] | Rose, honey, tobacco | 250.0 ± 4.2 a | 202.0 ± 4.2 a | 147.5 ± 6.4 b |
| Diethyl malate | - | Brown sugar, sweet | 129.0 ± 1.4 a | 109.5 ± 6.4 b | 128.5 ± 4.9 a |
| Ethyl hydrogen succinate | - | Wine, fruit | 5557.5 ± 10.6 c | 6926.5 ± 149.2 a | 5938.5 ± 160.5 b |
| Hexyl acetate | 2 [41] | Fruit | 140.0 ± 14.1 b | 165.0 ± 49.5 ab | 225.0 ± 34.5 a |
| γ-Butyrolactone | - | Caramel, sweet | 30.0 ± 2.8 c | 89.5 ± 4.9 a | 50.0 ± 7.1 b |
| α-Methyl-γ-butyrolactone | - | Woody | 137.5 ± 4.9 a | 104.0 ± 5.7 b | 81.5 ± 4.9 c |
| Total | 8253.5 ± 108.2 b | 9155.0 ± 294.2 a | 8573.5 ± 293.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Canito, A.; Altomare, A.; Giuggioli, N.; Foschino, R.; Fracassetti, D.; Vigentini, I. From Fruit to Beverage: Investigating Actinidia Species for Characteristics and Potential in Alcoholic Drink Production. Foods 2024, 13, 2380. https://doi.org/10.3390/foods13152380
Di Canito A, Altomare A, Giuggioli N, Foschino R, Fracassetti D, Vigentini I. From Fruit to Beverage: Investigating Actinidia Species for Characteristics and Potential in Alcoholic Drink Production. Foods. 2024; 13(15):2380. https://doi.org/10.3390/foods13152380
Chicago/Turabian StyleDi Canito, Alessandra, Alessio Altomare, Nicole Giuggioli, Roberto Foschino, Daniela Fracassetti, and Ileana Vigentini. 2024. "From Fruit to Beverage: Investigating Actinidia Species for Characteristics and Potential in Alcoholic Drink Production" Foods 13, no. 15: 2380. https://doi.org/10.3390/foods13152380
APA StyleDi Canito, A., Altomare, A., Giuggioli, N., Foschino, R., Fracassetti, D., & Vigentini, I. (2024). From Fruit to Beverage: Investigating Actinidia Species for Characteristics and Potential in Alcoholic Drink Production. Foods, 13(15), 2380. https://doi.org/10.3390/foods13152380









