Antioxidant Activity of Extracts of Balloon Flower Root (Platycodon grandiflorum), Japanese Apricot (Prunus mume), and Grape (Vitis vinifera) and Their Effects on Beef Jerky Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Balloon Flower Root Extract (BFE), Japanese Apricot Extract (JAE), and Grape Extract (GE)
2.2. Preparation of Beef Jerky
2.3. Meat Color and Sensory Evaluation
2.4. Volatile Basic Nitrogen (VBN), Lipid Peroxide, and Microbial Count during Storage
2.5. Total Polyphenol and Flavonoid Content of BFE, JAE, and GEs
2.6. DPPH Radical Scavenging Activity and ABTS Radical Scavenging Activity
2.7. Ferric-Reducing Antioxidant Power (FRAP)
2.8. Statistical Analysis
3. Results and Discussion
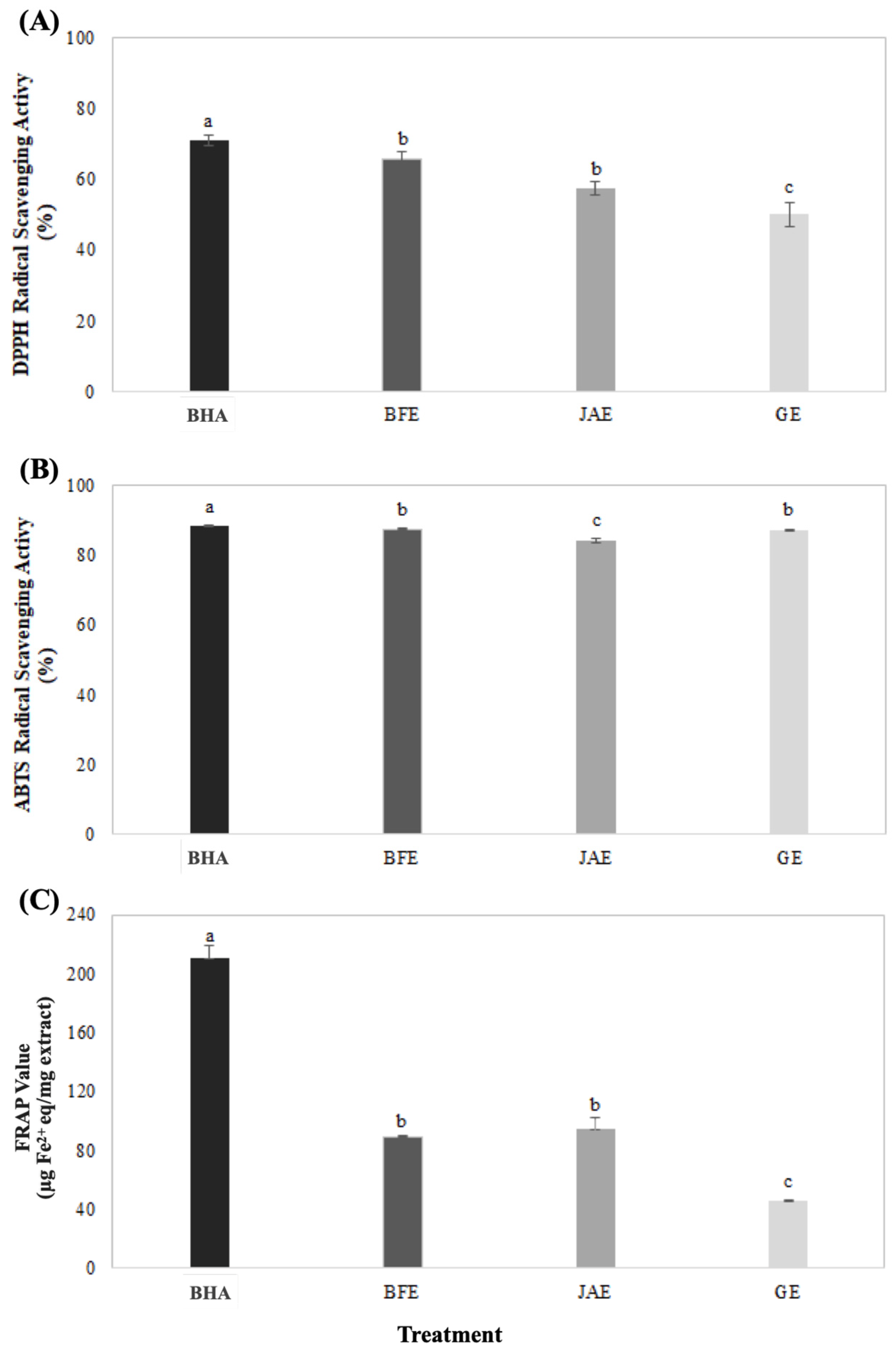
3.1. Total Polyphenol, Flavonoid Content, and Antioxidant Activity of Extracts
3.2. Yield of Jerky with Added Extracts
3.3. Meat Color of Jerky with Added Extracts
3.4. The TBARS, VBN and Microbial Values of Jerky with Added Extracts
3.5. The Sensory Quality of Jerky with Added Extracts
3.6. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Meat Market in South Korea—Statistics & Facts. Available online: https://www.statista.com/topics/5758/meat-industry-in-south-korea/ (accessed on 13 July 2024).
- Troy, D.J.; Kerry, J.P. Consumer perception and the role of science in the meat industry. Meat Sci. 2010, 86, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Seong, P.N.; Kim, J.H.; Cho, S.H.; Kang, G.H.; Park, B.Y.; Park, K.M.; Kim, D.H.; Jeong, D.W. The effects of marinating with commercial vinegars on the quality characteristics of biceps femoris muscle on Hanwoo. Ann. Anim. Resour. Sci. 2012, 23, 26–32. [Google Scholar]
- Ku, S.K.; Kim, H.J.; Yu, S.C.; Jeon, K.H.; Kim, Y.B. Effects of injection and tumbling methods on the meat properties of marinated beef. Food Sci. Anim. Resour. 2013, 33, 244–250. [Google Scholar] [CrossRef]
- Park, S.; Shim, Y.S.; Jeong, S.; Lee, H.S.; Kim, J.C. Investigation of quality properties of commercial jerky from Korean market for establishment of quality parameters. J. East Asian Soc. Diet. Life 2016, 26, 230–236. [Google Scholar] [CrossRef]
- Lim, D.G.; Kim, J.J.; Seo, K.S.; Nam, K.C. Effect of natural antioxidant extracted from Citrus junos Seib or Prunus mume on the quality traits of sun-dried Hanwoo beef jerky. Korean J. Agric. Sci. 2012, 39, 243–253. [Google Scholar]
- Cho, E.J.; Lee, J.E. The effect of addition of kinds of sugar and drying method on quality and storage characteristics of beef jerky. Korean J. Food Sci. Technol. 2000, 16, 511–520. [Google Scholar]
- Mailloux, R.J. An update on mitochondrial reactive oxygen species production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of reactive oxygen species by mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial management of reactive oxygen species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Nilsson, R.; Liu, N.A. Nuclear DNA damages generated by reactive oxygen molecules (ROS) under oxidative stress and their relevance to human cancers, including ionizing radiation-induced neoplasia part I: Physical, chemical and molecular biology aspects. Radiat. Med. Prot. 2020, 1, 140–152. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.H.; Oh, S.W. Grapefruit seed extract as a natural food antimicrobial: A review. Food Bioprocess Technol. 2021, 14, 626–633. [Google Scholar] [CrossRef]
- Youn, D.H.; Moon, Y.H.; Jung, I.C. Changes in quality of pork patty containing red wine during cold storage. J. Life Sci. 2007, 17, 91–96. [Google Scholar] [CrossRef][Green Version]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M.; Duran, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, H.G. Effect of starter culture and temperature on the flavor and sensory characteristics of dry-cured ham. Food Sci. Anim. Resour. 2024, 44, 570–585. [Google Scholar] [CrossRef]
- Gómez-Sierra, T.; Hernández-Cruz, E.Y.; Ortega-Lozano, A.J.; Jiménez-Uribe, A.P.; Chaverri, J.P.; Medina-Reyes, E.I. Toxicological aspects of natural food additives. In Natural Additives in Foods; Springer International Publishing: Cham, Switzerland, 2022; pp. 303–323. [Google Scholar]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosladis, I.; Fletouris, D.J. Effect of rosemary extract, chitosan and α-totopherol on lipid oxidation and color stability during frozen storage of beef burgers. Meat Sci. 2007, 75, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.H.; Hong, S.; Lim, M.H.; Hong, S. Availability of Opuntia ficus-indica var. saboten stem extracts as a natural preservative. Asian J. Beauty Cosmetol. 2016, 14, 449–461. [Google Scholar] [CrossRef]
- Oh, K.Y.; Ahn, S.C.; Oh, S.M. A Study of shelf-life and antimicrobial activity on putrefactive microorganisms related to soybean curd of Persicaria hydropiper L. extracts. Culin. Sci. Hosp. Res. 2016, 22, 198–211. [Google Scholar]
- Seleshe, S.; Ameer, A.; Kang, S.N. Exploration of the antioxidant chemical constituents and antioxidant performance of various solvent extracts of eighteen plants. Prev. Nutr. Food Sci. 2022, 27, 212–222. [Google Scholar] [CrossRef]
- Lim, H.J.; Kim, G.D.; Jung, E.Y.; Seo, H.W.; Joo, S.T.; Jin, S.K.; Yang, H.S. Effect of curing time on the physicochemical and sensory properties of beef jerky replaced salt with soy sauce, red pepper paste and soybean paste. Asian-Australas. J. Anim Sci. 2014, 27, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.N. Ethanol extracts from mistletoe (Viscum album L.) act as natural antioxidants and antimicrobial agents in uncooked pork patties during refrigerated storage. Asian-Australas. J. Anim. Sci. 2016, 29, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Witte, V.C.; Krause, G.F.; Bailey, M.E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/STAT User’s Guide, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2019. [Google Scholar]
- Kim, S.B.; Jo, Y.H.; Liu, Q.; Ahn, J.H.; Hong, I.P.; Han, S.M.; Lee, M.K. Optimization of extraction condition of bee pollen using response surface methodology: Correlation between anti-melanogenesis, antioxidant activity, and phenolic content. Molecules 2015, 20, 19764–19774. [Google Scholar] [CrossRef] [PubMed]
- Seleshe, S.; Kang, S.N. Antioxidant and antimicrobial activities of food byproducts from cassava, peanut pea flour and MSG-CMS and its characteristics in meat patty as a food preservative. J. Agri. Life Sci. 2019, 53, 141–151. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Sampson, J.; Bramley, P.M.; Holloway, D.E. Why do we expect carotenoids to be antioxidants in vivo. Free Radic. Res. 1997, 26, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Valantina, R.S.; Neelamegam, P. Selective ABTS and DPPH-radical scavenging activity of peroxide from vegetable oils. Int. Food Res. J. 2015, 22, 289. [Google Scholar]
- Micklander, E.; Peshlov, B.; Purslow, P.P.; Engelsen, S.B. NMR-Cooking: Monitoring the changes in meat during cooking by low-Field 1H NMR. Trends Food Sci. Technol. 2002, 13, 341–346. [Google Scholar] [CrossRef]
- Winger, R.J.; Fennema, O. Tenderness and water holding properties of beef muscle as influenced by freezing and subsequent storage at −3 or 15 °C. J. Food Sci. 1976, 41, 1433–1438. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Tomasevic, I.; Djekic, I.; Furnols, M.F.; Terjung, N.; Lorenzo, J.M. Recent advances in meat color research. Curr. Opin. Food Sci. 2021, 41, 81–87. [Google Scholar] [CrossRef]
- Ojha, K.S.; Granato, D.; Rajuria, G.; Barba, F.J.; Kerry, J.P.; Tiwari, B.K. Application of chemometrics to assess the influence of ultrasound frequency, Lactobacillus sakei culture and drying on beef jerky manufacture: Impact on amino acid profile, organic acids, texture and colour. Food Chem. 2018, 239, 544–550. [Google Scholar] [CrossRef] [PubMed]
- King, J.N.; Whyte, R. Does it look cooked? A review of factors that influence cooked meat color. J. Food Sci. 2006, 71, R31–R40. [Google Scholar]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, E.D.A.; Morton, J.D.; Bhat, Z.F.; Kong, L. Meat color: Factors affecting color stability. Ref. Modul. Food Sci. 2019, 2, 202–210. [Google Scholar]
- Jo, S.C.; Nam, K.C.; Min, B.R.; Ahn, D.U.; Cho, S.H.; Park, W.P.; Lee, S.C. Antioxidant activity of Prunus mume extract in cooked chicken breast meat. Int. J. Food Sci. Technol. 2006, 41, 15–19. [Google Scholar] [CrossRef]
- Rojas, M.C.; Brewer, M.S. Effect of natural antioxidants on oxidative stability of frozen, vacuum-packaged beef and pork. J. Food Qual. 2008, 31, 173–188. [Google Scholar] [CrossRef]
- Sasse, A.; Colindres, P.; Brewer, M.S. Effect of natural and synthetic antioxidants on the oxidative stability of cooked, frozen pork patties. J. Food Sci. 2009, 74, S30–S35. [Google Scholar] [CrossRef]
- Luo, H.; Lin, S.; Ren, F.; Wu, L.; Chen, L.; Sun, Y. Antioxidant and antimicrobial capacity of Chinese medicinal herb extracts in raw sheep meat. J. Food Prot. 2007, 70, 1440–1445. [Google Scholar] [CrossRef]
- Kulkarni, S.; DeSantos, F.A.; Kattamuri, S.; Rossi, S.J.; Brewer, M.S. Effect of grape seed extract on oxidative, color and sensory stability of a pre-cooked, frozen, re-heated beef sausage model system. Meat Sci. 2011, 88, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Lorenzo, J.M.; Amado, I.R.; Franco, D. Effect of addition of green tea, chestnut and grape extract on the shelf-life pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Abdul, M.M.Y.; Khatib, A.; Abdull, R.A.F. Comparative analysis of the chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Neupane, P.; Lamichhane, J. Estimation of total phenolic content, total flavonoid content and antioxidant capacities of five medicinal plants from Nepal. Vegetos 2020, 33, 360–366. [Google Scholar] [CrossRef]
- Kainama, H.; Fatmawati, S.; Santoso, M.; Papilaya, P.M.; Ersam, T. The relationship of free radical scavenging and total phenolic and flavonoid contents of Garcinia lasoar PAM. Pharm. Chem. J. 2020, 53, 1151–1157. [Google Scholar] [CrossRef]
- Othman, A.; Mukhtar, N.J.; Ismail, N.S.; Chang, S.K. Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants. Int. Food Res. 2014, 21, 759–766. [Google Scholar]
| Ingredients | Treatments 1 | ||||
|---|---|---|---|---|---|
| PS | VE | BFE | JAE | GE | |
| Beef | 84.000 | 84.000 | 84.000 | 84.000 | 84.000 |
| Soy sauce | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Sodium nitrite | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| Ascorbic acid | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 |
| Salt | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 |
| Sodium polyphosphate | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 |
| Sugar | 4.570 | 4.570 | 4.565 | 4.570 | 4.565 |
| Beef powder | 0.560 | 0.560 | 0.560 | 0.560 | 0.560 |
| Bulgogi spice | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Black pepper | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 |
| Sodium L-glutamate | 0.460 | 0.460 | 0.460 | 0.460 | 0.460 |
| Onion powder | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 |
| Garlic powder | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 |
| Sausage spice | 0.230 | 0.230 | 0.230 | 0.230 | 0.230 |
| D-sorbitol | 1.500 | 1.500 | 1.500 | 1.500 | 1.500 |
| Water | 2.602 | 2.652 | 2.657 | 2.652 | 2.657 |
| Total | 99.900 | 99.950 | 99.950 | 99.950 | 99.950 |
| PS | 0.100 | NA | NA | NA | NA |
| VE | NA | 0.050 | NA | NA | NA |
| BFE | NA | NA | 0.050 | NA | NA |
| JAE | NA | NA | NA | 0.050 | NA |
| GE | NA | NA | NA | NA | 0.050 |
| Extracts 1 | TPC 2 (mg CAT eq/g Extract) 3 | TFC 2 (mg RUT eq/g Extract) 3 |
|---|---|---|
| BFE | 6.85 ± 0.19 a | 10.52 ± 0.22 a |
| JAE | 1.75 ± 0.12 c | 5.41 ± 0.35 b |
| GE | 2.86 ± 0.25 b | 2.83 ± 1.07 c |
| SEM 4 | 0.20 | 0.66 |
| Treatments 1 | Yield (%) |
|---|---|
| PS | 42.11 ± 1.61 |
| VE | 40.67 ± 0.97 |
| BFE | 41.04 ± 2.08 |
| JAE | 39.51 ± 1.14 |
| GE | 43.60 ± 0.62 |
| SEM 2 | 1.38 |
| Color 1 | Storage (Days) | Treatments 2 | SEM 3 | ||||
|---|---|---|---|---|---|---|---|
| PS | VE | BFE | JAE | GE | |||
| L* | 1 | 40.81 ± 1.45 aA | 39.07 ± 1.95 bcA | 39.32 ± 1.05 abA | 35.89 ± 0.62 dA | 37.51 ± 0.64 cdA | 1.25 |
| 30 | 26.81 ± 3.08 B | 26.18 ± 1.15 B | 26.97 ± 1.20 B | 24.42 ± 1.96 B | 24.94 ± 1.63 B | 1.93 | |
| a* | 1 | 10.04 ± 1.31 aB | 10.37 ± 1.39 aB | 8.26 ± 1.03 bcB | 7.19 ± 0.86 cB | 9.43 ± 0.62 abB | 1.08 |
| 30 | 18.84 ± 2.37 aA | 16.15 ± 2.38 bA | 19.63 ± 1.71 aA | 16.22 ± 1.46 bA | 13.21 ± 1.14 cA | 1.88 | |
| b* | 1 | 5.73 ± 0.98 aB | 5.37 ± 1.66 aB | 3.60 ± 0.55 bB | 3.20 ± 0.62 bB | 3.61 ± 0.15 bB | 0.94 |
| 30 | 12.88 ± 2.06 bA | 7.91 ± 0.40 cA | 14.80 ± 0.32 aA | 8.01 ± 1.43 cA | 8.53 ± 1.14 cA | 1.50 | |
| Trait | Storage (Days) | Treatments 1 | SEM 2 | ||||
|---|---|---|---|---|---|---|---|
| PS | VE | BFE | JAE | GE | |||
| TBARS | 1 | 0.272 ± 0.001 aB | 0.263 ± 0.002 bB | 0.255 ± 0.001 cB | 0.263 ± 0.002 bB | 0.261 ± 0.002 bB | 0.00 |
| 30 | 0.371 ± 0.004 cA | 0.368 ± 0.003 cA | 0.381 ± 0.001 bA | 0.427 ± 0.005 aA | 0.371 ± 0.005 cA | 0.01 | |
| VBN | 1 | 9.34 ± 0.81 bB | 12.89 ± 0.49 a | 13.63 ± 0.32 a | 10.27 ± 0.81 bB | 12.14 ± 1.62 aB | 0.92 |
| 30 | 11.86 ± 0.61 dA | 12.51 ± 0.16 c | 14.47 ± 0.43 b | 15.13 ± 0.28 aA | 15.22 ± 0.32 aA | 0.29 | |
| Trait | Storage (Days) | Treatments 1 | SEM 2 | ||||
|---|---|---|---|---|---|---|---|
| PS | VE | BFE | JAE | GE | |||
| TPC | 1 | 1.23 ± 0.09 A | ND | ND | ND | 1.19 ± 0.11 A | 0.02 |
| 30 | ND B | ND | ND | ND | ND B | NA | |
| E. coli | 1 | ND | ND | ND | ND | ND | NA |
| 30 | ND | ND | ND | ND | ND | NA | |
| Sensory Quality | Storage (Days) | Treatments 1 | SEM 2 | ||||
|---|---|---|---|---|---|---|---|
| PS | VE | BFE | JAE | GE | |||
| Color | 1 | 3.50 ± 0.50 | 3.60 ± 0.53 A | 3.43 ± 0.40 B | 3.27 ± 0.25 | 3.07 ± 0.12 A | 0.39 |
| 30 | 3.77 ± 0.25 b | 3.20 ± 0.17 cdB | 4.20 ± 0.17 aA | 3.57 ± 0.25 bc | 2.97 ± 0.15 dB | 0.20 | |
| Flavor | 1 | 3.87 ± 0.32 | 3.77 ± 0.25 | 4.13 ± 0.21 | 4.00 ± 0.10 | 3.93 ± 0.15 B | 0.22 |
| 30 | 3.90 ± 0.10 b | 3.45 ± 0.10 c | 4.27 ± 0.25 a | 4.03 ± 0.06 ab | 4.10 ± 0.17 abA | 0.20 | |
| Off-flavor | 1 | 1.03 ± 0.06 | 1.03 ± 0.06 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.04 |
| 30 | 1.07 ± 0.06 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.03 ± 0.06 | 1.03 ± 0.06 | 0.05 | |
| Juiciness | 1 | 3.70 ± 0.17 | 3.67 ± 0.29 | 3.60 ± 0.17 | 3.37 ± 0.12 | 3.57 ± 0.21 | 0.20 |
| 30 | 3.60 ± 0.17 | 3.60 ± 0.10 | 3.70 ± 0.36 | 3.83 ± 0.29 | 3.60 ± 0.17 | 0.24 | |
| Tenderness | 1 | 3.20 ± 0.17 bc | 2.97 ± 0.15 c | 3.20 ± 0.26 bc | 3.47 ± 0.15 b | 3.83 ± 0.15 a | 0.18 |
| 30 | 3.13 ± 0.15 | 3.43 ± 0.40 | 3.57 ± 0.40 | 3.10 ± 0.10 | 3.30 ± 0.20 | 0.28 | |
| Overall acceptability | 1 | 3.30 ± 0.20 c | 3.40 ± 0.20 bc | 4.33 ± 0.15 a | 3.70 ± 0.26 bc | 3.73 ± 0.25 b | 0.22 |
| 30 | 3.23 ± 0.20 b | 3.43 ± 0.38 b | 4.46 ± 0.21 a | 3.53 ± 0.45 b | 3.76 ± 0.21 b | 0.31 | |
| TFC | DPPH | ABTS | FRAP | TBARS | |
|---|---|---|---|---|---|
| TPC | –0.54 * | 0.93 ** | 0.68 ** | 0.25 * | 0.67 ** |
| TFC | 0.65 ** | 0.58 ** | 0.69 ** | 0.17 * | |
| DPPH | 0.23 * | 0.39 * | 0.75 ** | ||
| ABTS | 0.29 | 0.40 * | |||
| FRAP | 0.29 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.J.; Yim, D.G.; Reaney, M.J.T.; Kim, Y.J.; Shim, Y.Y.; Kang, S.N. Antioxidant Activity of Extracts of Balloon Flower Root (Platycodon grandiflorum), Japanese Apricot (Prunus mume), and Grape (Vitis vinifera) and Their Effects on Beef Jerky Quality. Foods 2024, 13, 2388. https://doi.org/10.3390/foods13152388
Kim BJ, Yim DG, Reaney MJT, Kim YJ, Shim YY, Kang SN. Antioxidant Activity of Extracts of Balloon Flower Root (Platycodon grandiflorum), Japanese Apricot (Prunus mume), and Grape (Vitis vinifera) and Their Effects on Beef Jerky Quality. Foods. 2024; 13(15):2388. https://doi.org/10.3390/foods13152388
Chicago/Turabian StyleKim, Beom Joon, Dong Gyun Yim, Martin J. T. Reaney, Young Jun Kim, Youn Young Shim, and Suk Nam Kang. 2024. "Antioxidant Activity of Extracts of Balloon Flower Root (Platycodon grandiflorum), Japanese Apricot (Prunus mume), and Grape (Vitis vinifera) and Their Effects on Beef Jerky Quality" Foods 13, no. 15: 2388. https://doi.org/10.3390/foods13152388
APA StyleKim, B. J., Yim, D. G., Reaney, M. J. T., Kim, Y. J., Shim, Y. Y., & Kang, S. N. (2024). Antioxidant Activity of Extracts of Balloon Flower Root (Platycodon grandiflorum), Japanese Apricot (Prunus mume), and Grape (Vitis vinifera) and Their Effects on Beef Jerky Quality. Foods, 13(15), 2388. https://doi.org/10.3390/foods13152388






