The Preventive Effect of Low-Molecular Weight Oyster Peptides on Lipopolysaccharide-Induced Acute Colitis in Mice by Modulating Intestinal Microbiota Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of the Basic Properties of Substances
2.3. Peptides Sequencing
2.4. Animals and Experimental Design
2.5. Routine Blood Analysis
2.6. Serum Parameters Analysis
2.7. Histopathological Observation
2.8. Detection of Anti-Oxidant Properties
2.9. Gut Microbiota Analysis
2.10. Faecal SCFAs Measurement
2.11. Statistical Analysis of Data
3. Results and Discussion
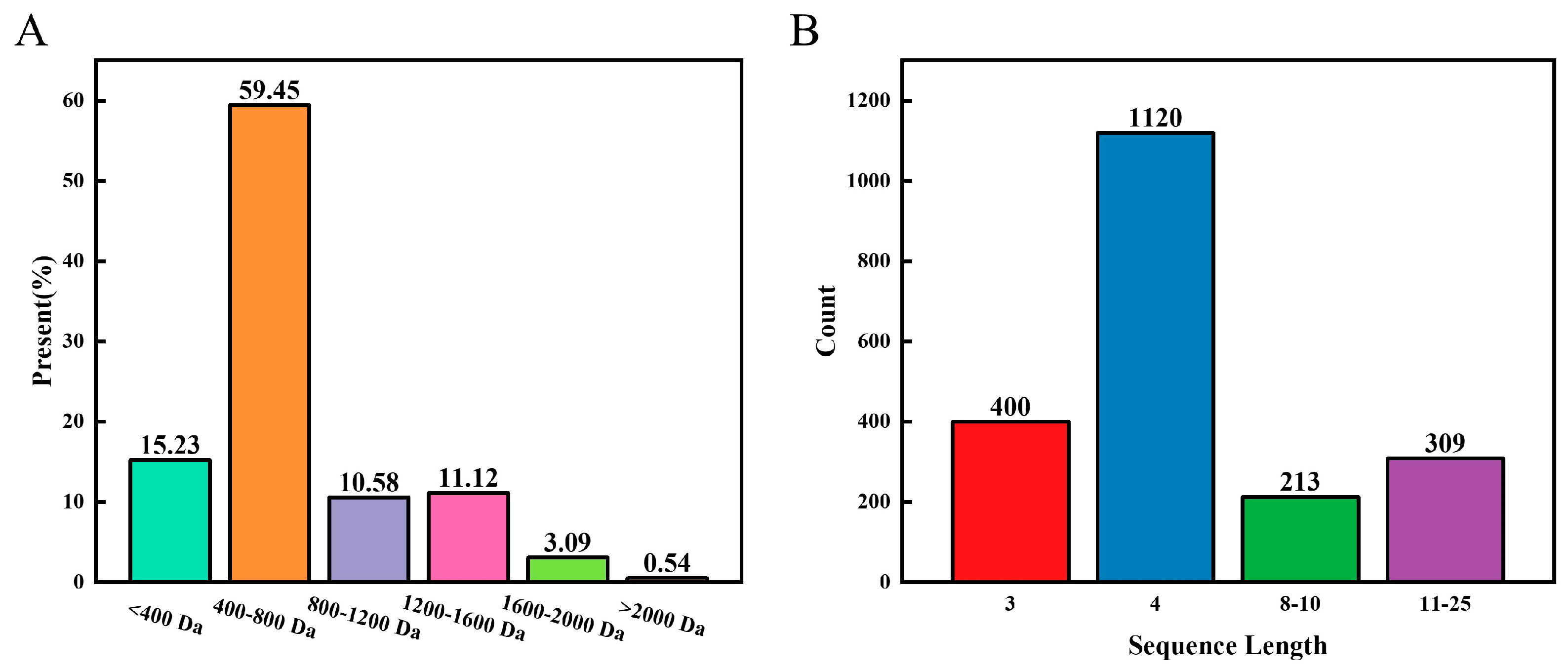
3.1. Characteristic Analysis of the LOPs
3.2. Peptide Sequence
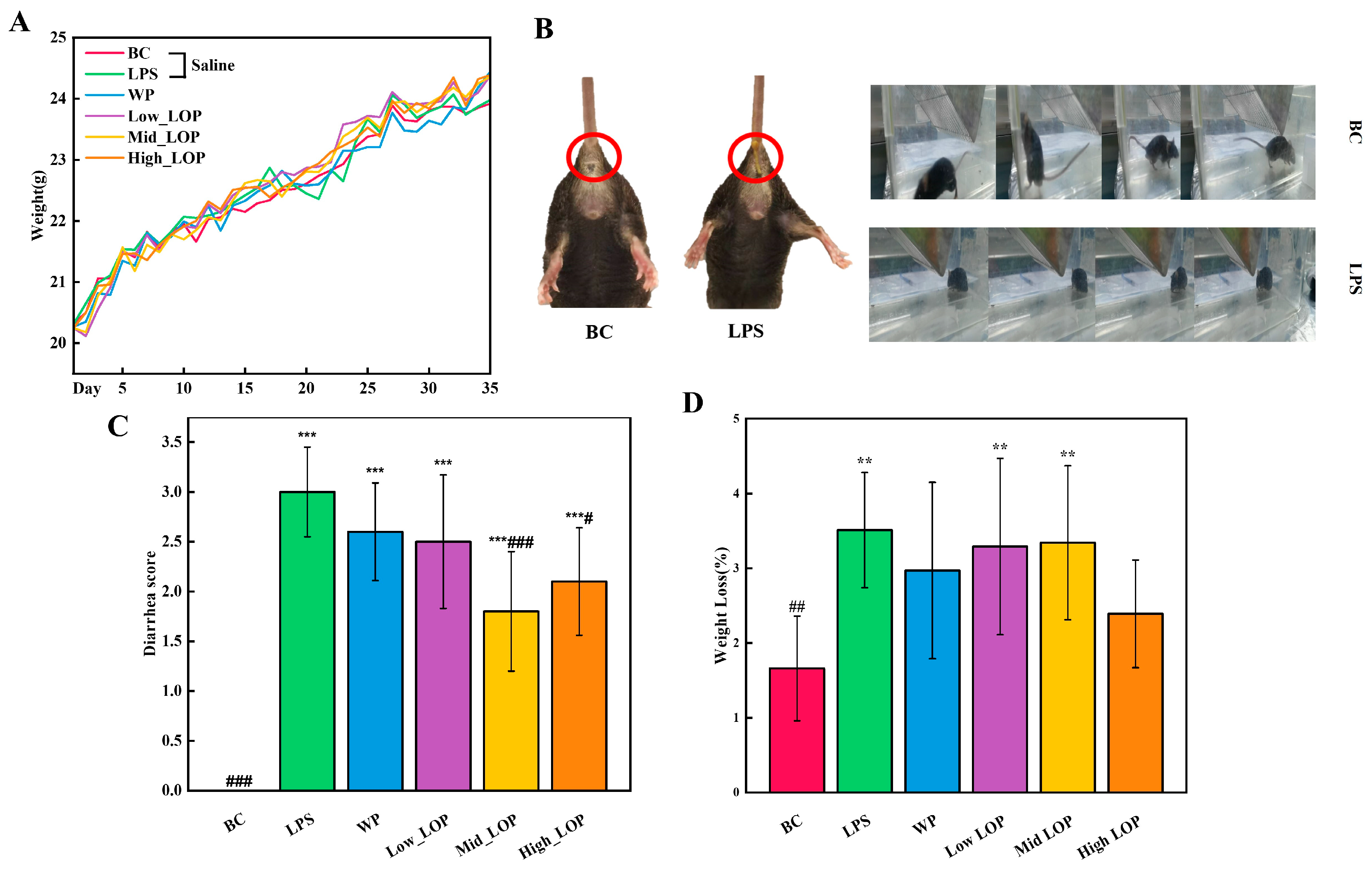
3.3. Effects of the LOPs on the Basic Body Condition of Mice
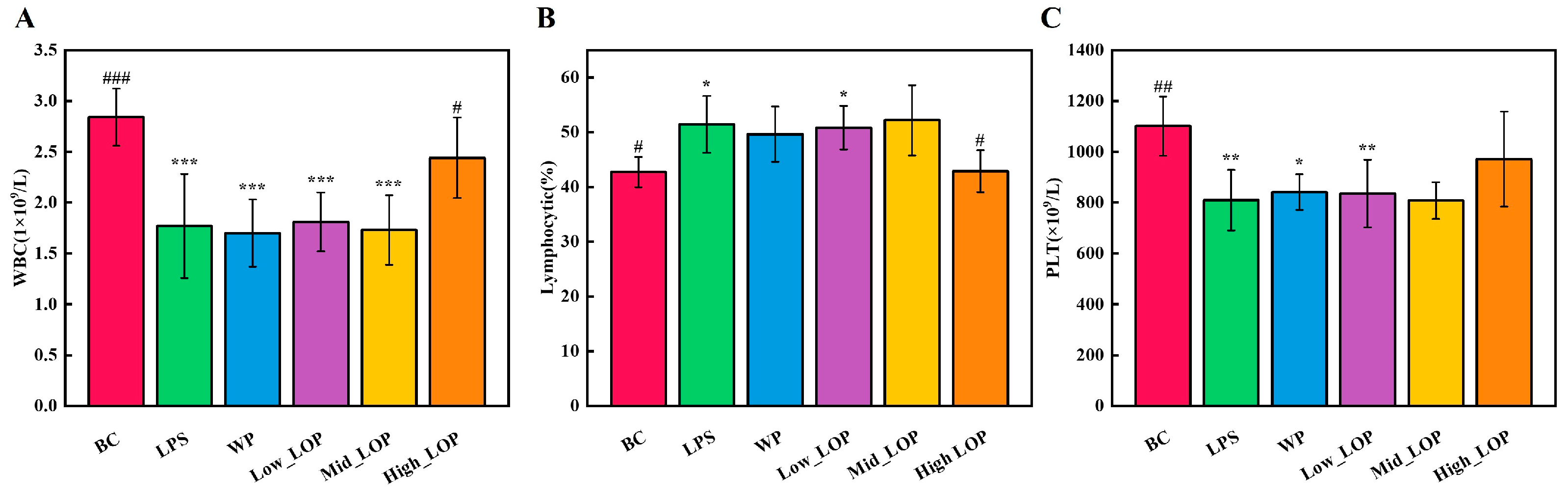
3.4. Blood Routine
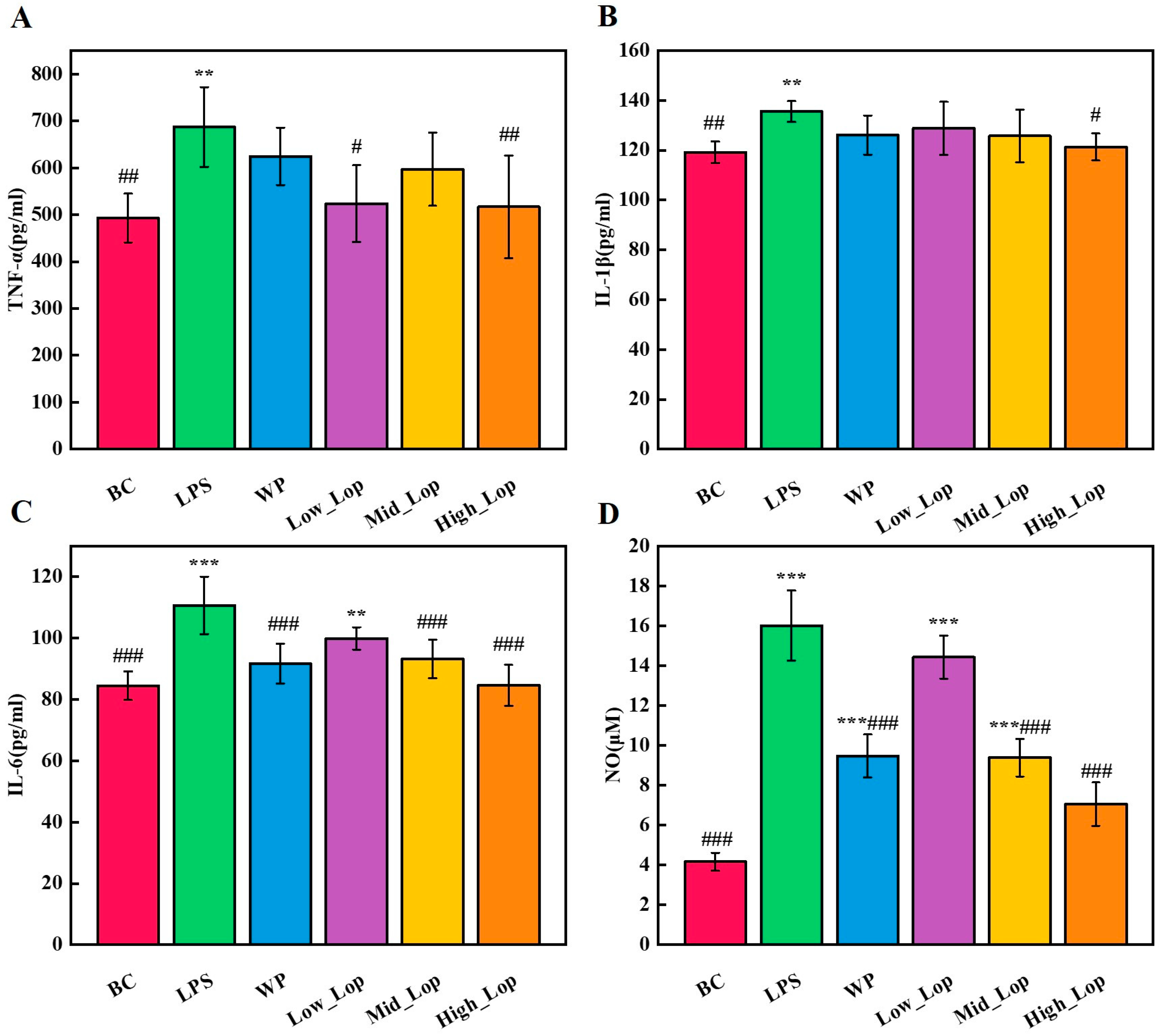
3.5. Anti-Inflammatory Effects of the LOPs
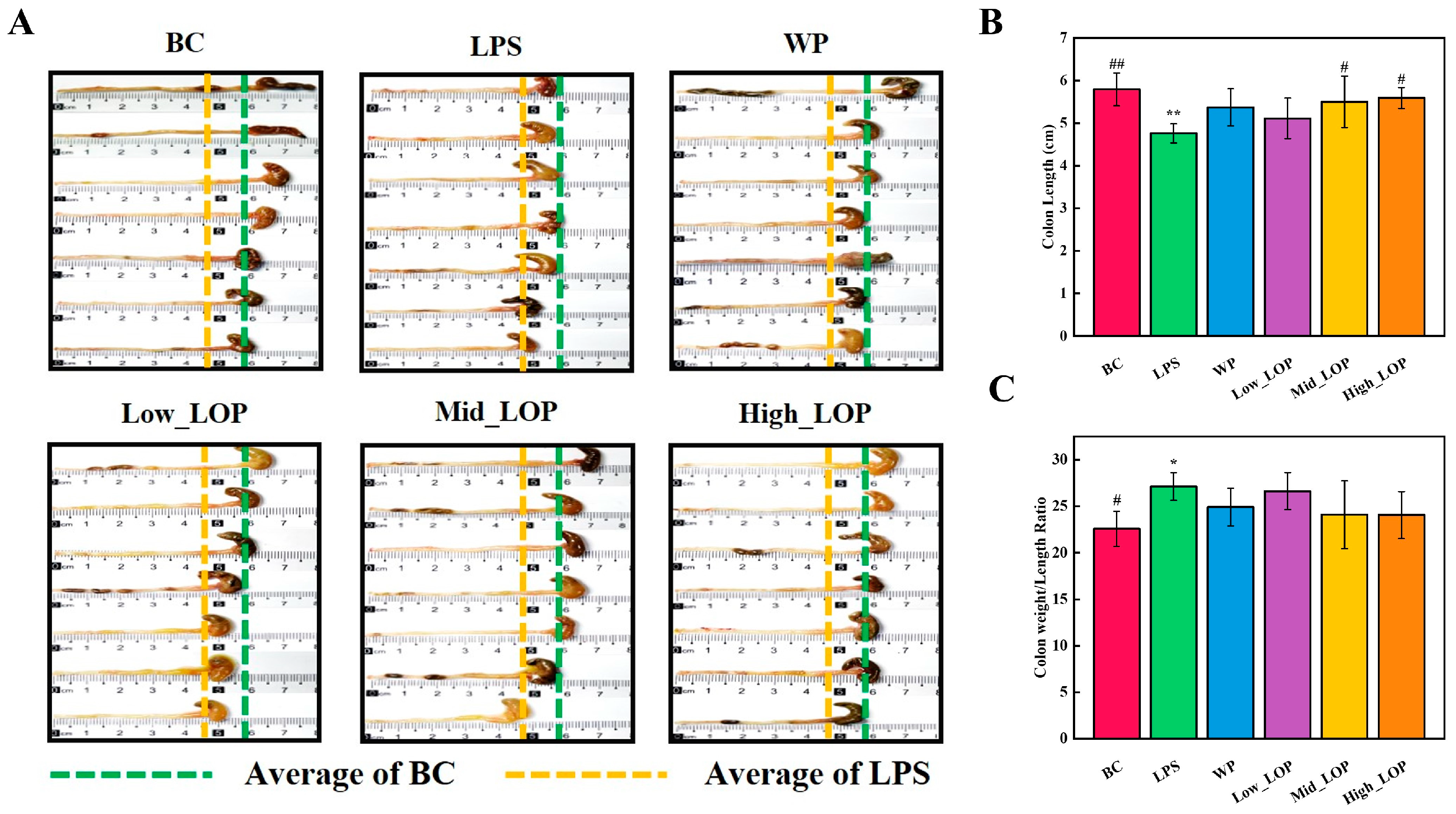
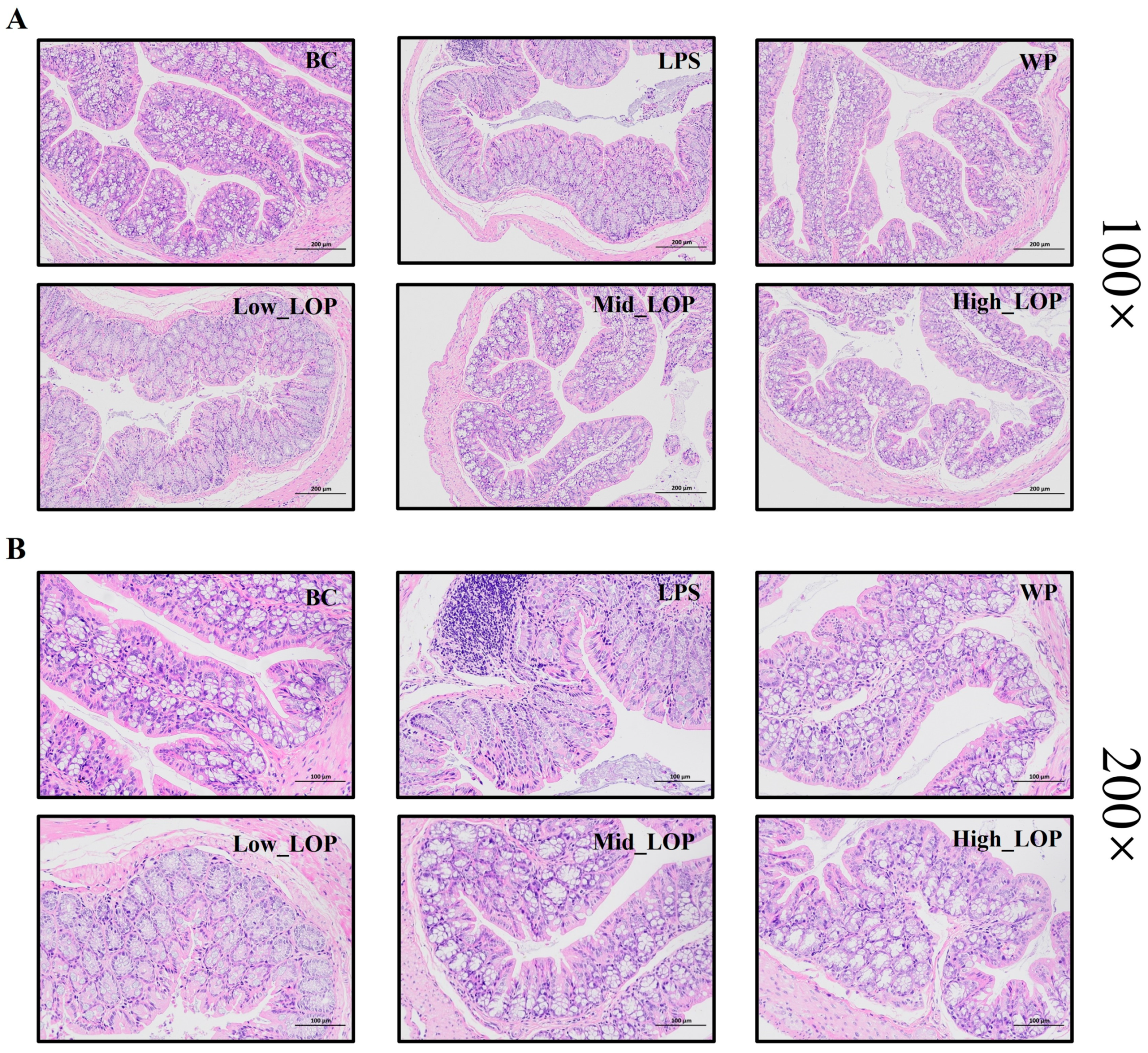
3.6. Histological Analysis
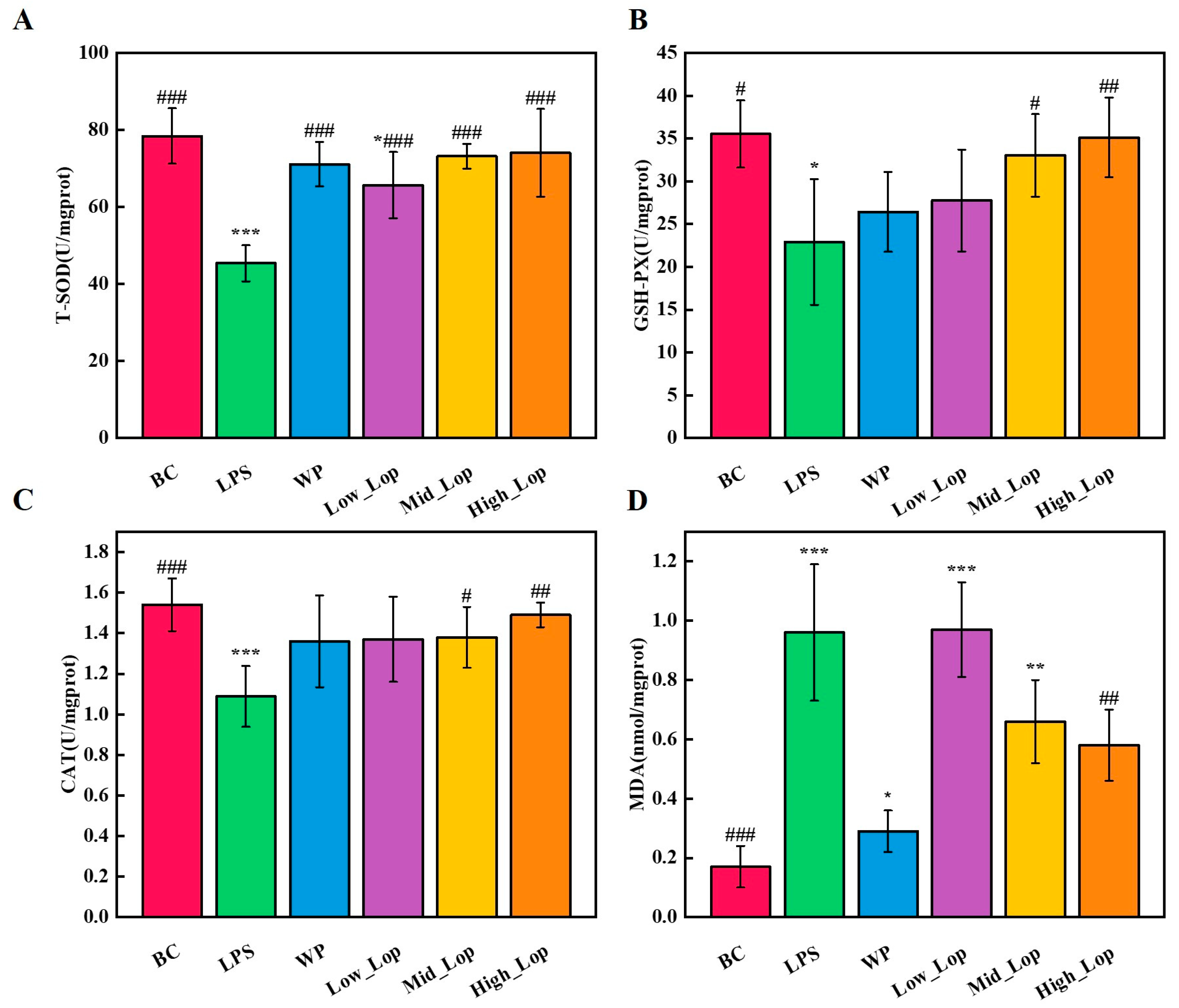
3.7. Colon Tissue Anti-Oxidant Analysis
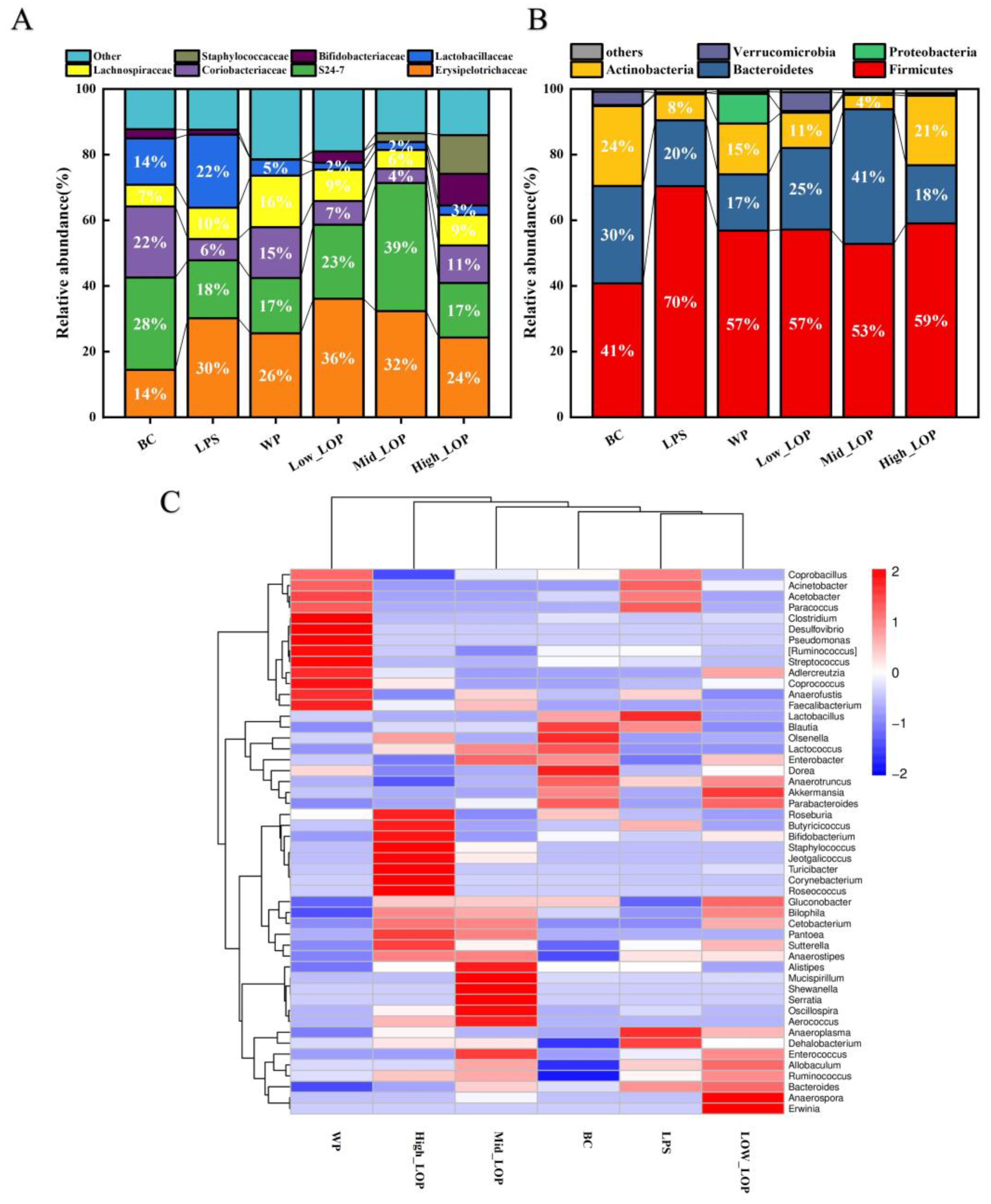
3.8. Effect of the LOPs on the Distribution of the Intestinal Flora
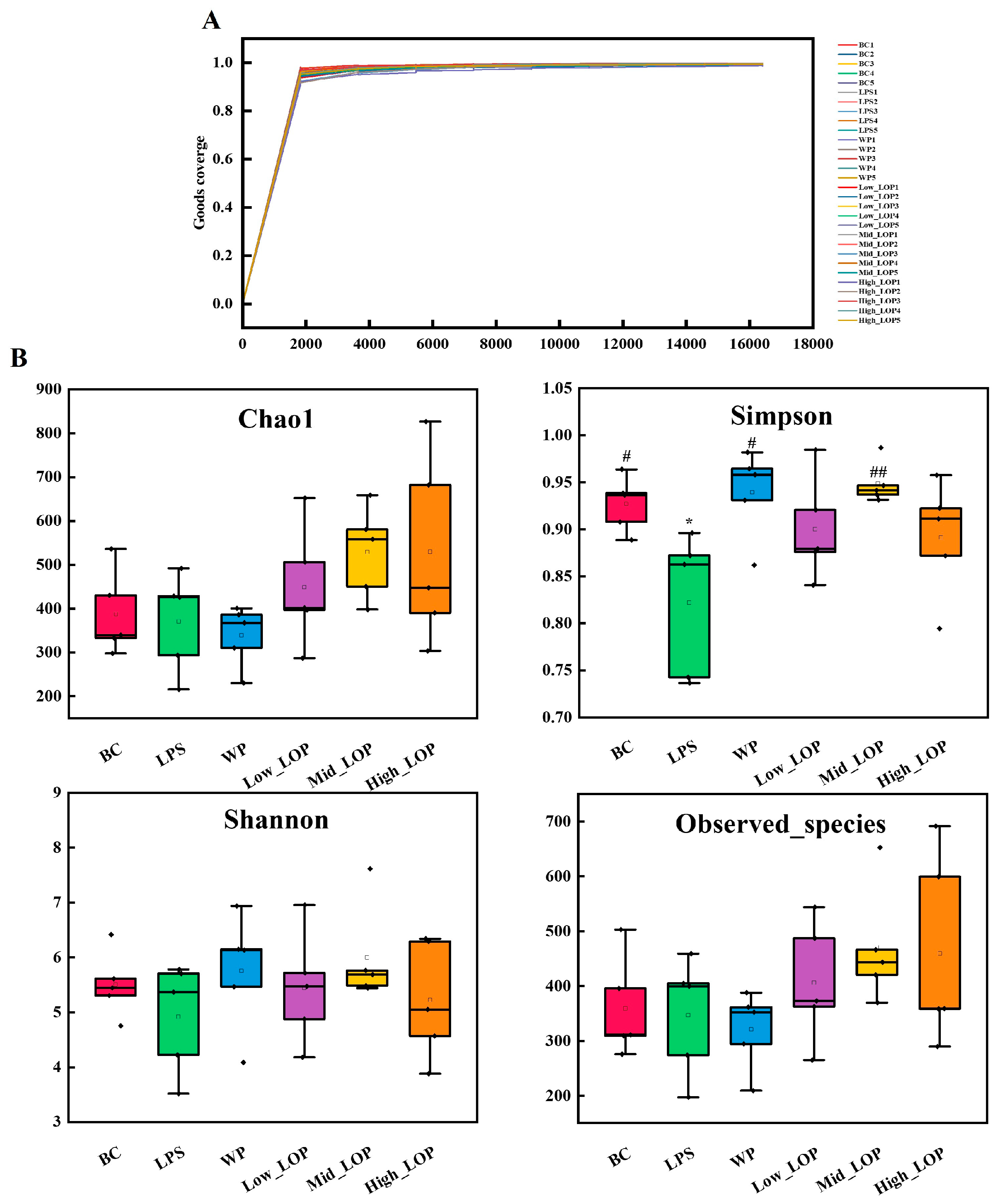
3.8.1. Quantity and Length of the Sequence
3.8.2. Microbial Diversity Analysis
3.8.3. Microbial Composition Analysis
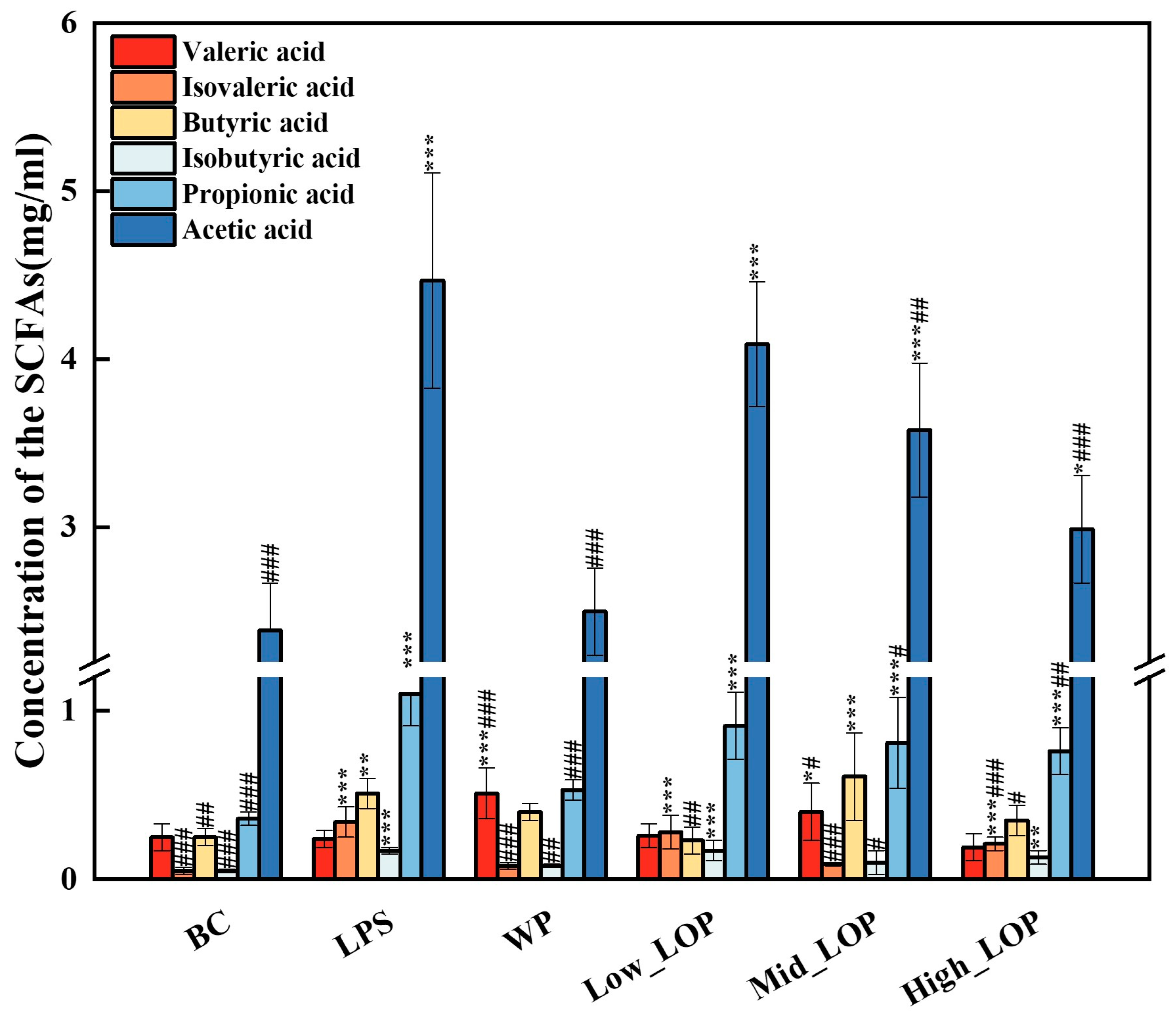
3.9. Faecal SCFAs Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Gusev, E.; Solomatina, L.; Zhuravleva, Y.; Sarapultsev, A. General Pathological Processes of Inflammation. Int. J. Mol. Sci. 2021, 22, 11453. [Google Scholar] [CrossRef]
- Huguet, J.M.; Barceló, L.F.; Suárez, P.; Sanchez, E.; Prieto, J.D.; García, V.; Sempere, J. Colorectal cancer screening and surveillance in patients with inflammatory bowel disease in 2021. World J. Gastroenterol. 2022, 28, 502–516. [Google Scholar] [CrossRef]
- Sutaria, R.; Mok, Z.H. Nanoparticle-enhanced mesalazine therapy for inflammatory bowel disease. Pharm. Sci. Adv. 2023, 1, 100014. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef]
- Li, Q.; Wu, W.j.; Fang, X.J.; Chen, H.J.; Han, Y.C.; Liu, R.L.; Niu, B.; Gao, H.Y. Structural characterization of a polysaccharide from bamboo (Phyllostachys edulis) shoot and its prevention effect on colitis mouse. Food Chem. 2022, 387, 132807. [Google Scholar] [CrossRef]
- Sun, Y.; Diao, F.R.; Niu, Y.B.; Li, X.Q.; Zhou, H.P.; Mei, Q.B.; Li, Y.H. Apple polysaccharide prevents from colitis-associated carcinogenesis through regulating macrophage polarization. Int. J. Biol. Macromol. 2020, 161, 704–711. [Google Scholar] [CrossRef]
- He, D.; Zeng, W.; Wang, Y.; Xing, Y.F.; Xiong, K.; Su, N.; Zhang, C.; Lu, Y.; Xing, X.H. Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment. Food Sci. Hum. Wellness 2022, 11, 1464–1474. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Deng, Z.; Cui, C.B.; Wang, Y.A.; Ni, J.J.; Zheng, L.F.; Wei, H.K.; Peng, J. FSGHF3 and peptides, prepared from fish skin gelatin, exert a protective effect on DSS-induced colitis via the Nrf2 pathway. Food Funct. 2020, 11, 414–423. [Google Scholar] [CrossRef]
- Xiang, X.W.; Zhou, X.L.; Wang, R.; Shu, C.H.; Zhou, Y.F.; Ying, X.G.; Zheng, B. Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Mar. Drugs 2021, 19, 127. [Google Scholar] [CrossRef]
- Xiang, X.W.; Jiang, Q.H.; Shao, W.; Li, J.H.; Zhou, Y.F.; Chen, L.; Deng, S.G.; Zheng, B.; Chen, Y.F. Protective Effects of Shrimp Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Nutr. 2021, 8, 773064. [Google Scholar] [CrossRef]
- Li, X.P.; Xu, Y.J.; Zhang, C.L.; Deng, L.; Chang, M.J.; YU, Z.Q.; Liu, D. Protective Effect of Calculus Bovis Sativus on Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 469506. [Google Scholar] [CrossRef]
- Wang, Q.K.; Li, W.; He, Y.H.; Ren, D.D.; Kow, f.; Song, L.L.; Yu, X.J. Novel antioxidative peptides from the protein hydrolysate of oysters (Crassostrea talienwhanensis). Food Chem. 2014, 145, 991–996. [Google Scholar] [CrossRef]
- Qian, B.j.; Zhao, X.; Yang, Y.; Tian, C.C. Antioxidant and anti-inflammatory peptide fraction from oyster soft tissue by enzymatic hydrolysis. Food Sci. Nutr. 2020, 8, 3947–3956. [Google Scholar] [CrossRef]
- Cheong, S.H.; Kim, E.K.; Hwang, J.W.; Kim, Y.S.; Lee, J.S.; Moon, S.H.; Jeon, B.T.; Park, J.S. Purification of a Novel Peptide Derived from a Shellfish, Crassostrea gigas, and Evaluation of Its Anticancer Property. J. Agric. Food Chem. 2013, 61, 11442–11446. [Google Scholar] [CrossRef]
- Li, J.Z.; Yang, L.; Li, G.Y.; Liu, S.Y.; Cao, W.H.; Lin, H.S.; Chen, Z.Q.; Qin, X.M.; Huang, J.Z.; Zheng, H.N. Low-molecular-weight oyster peptides ameliorate cyclophosphamide-chemotherapy side-effects in Lewis lung cancer mice by mitigating gut microbiota dysbiosis and immunosuppression. J. Funct. Foods 2022, 95, 105196. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Zhang, C.H.; Cao, W.H.; Qin, X.M.; Gao, J.L.; Zheng, H.N. The purification and identification of immunoregulatory peptides from oyster (Crassostrea hongkongensis) enzymatic hydrolysate. RSC Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef]
- Ahn, C.B.; Cho, Y.S.; Je, J.Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef]
- Xiang, X.W.; Zheng, H.Z.; Wang, R.; Chen, H.; Xiao, J.X.; Zheng, B.; Liu, S.L.; Ding, Y.T. Ameliorative Effects of Peptides Derived from Oyster (Crassostrea gigas) on Immunomodulatory Function and Gut Microbiota Structure in Cyclophosphamide-Treated Mice. Mar. Drugs 2021, 19, 456. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Jo, M.J.; Seo, Y.B.; Park, N.G.; Kim, G.D. Anti-Inflammatory Activity of β-thymosin Peptide Derived from Pacific Oyster (Crassostrea gigas) on NO and PGE2 Production by Down-Regulating NF-κB in LPS-Induced RAW264.7 Macrophage Cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef]
- Xie, C.L.; Kang, S.S.; Lu, C.; Choi, Y.J. Quantification of Multifunctional Dipeptide YA from Oyster Hydrolysate for Quality Control and Efficacy Evaluation. BioMed Res. Int. 2018, 2018, 8437379. [Google Scholar] [CrossRef]
- Sahu, K.K.; Minz, S.; Kaurav, M.; Pandey, R.S. Proteins and peptides: The need to improve them as promising therapeutics for ulcerative colitis. Artif. Cells Nanomed. Biotechnol. 2016, 44, 642–653. [Google Scholar] [CrossRef]
- Chorawala, M.R.; Chauhan, S.; Patel, R.; Shah, G. Cell Wall Contents of Probiotics (Lactobacillus species) Protect Against Lipopolysaccharide (LPS)-Induced Murine Colitis by Limiting Immuno-inflammation and Oxidative Stress. Probiotics Antimicrob. Proteins 2021, 13, 1005–1017. [Google Scholar] [CrossRef]
- Lv, H.Q.; Li, Q.Q.; Zhou, Z.N.; Fang, H.Y.; Chen, Q.X.; Shuai, Y.Y. The protective effect of 2′-Fucosyllactose on LPS-induced colitis suckling mice by ameliorating intestinal inflammation and modulating gut microbiota. Food Biosci. 2023, 51, 102317. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Liu, L.J.; Jin, Y.Y.; Pei, X.; Sun, W.J.; Wang, M.Q. Clostridium tyrobutyricum Protects against LPS-Induced Colonic Inflammation via IL-22 Signaling in Mice. Nutrients 2021, 13, 215. [Google Scholar] [CrossRef]
- Tan, H.Z.; Zhao, J.X.; Zhang, H.; Zhai, Q.X.; Chen, W. Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Appl. Microbiol. Biotechnol. 2019, 103, 2353–2365. [Google Scholar] [CrossRef]
- Han, H.S.; Kim, S.Y.; Shin, J.S.; Lee, H.H.; Chung, K.S.; Rhee, Y.K.; Cho, C.W.; Hong, H.D.; Lee, K.T. Polysaccharide fraction isolated from the leaves of Hordeum vulgare L. protects against colonic inflammation of systemic immune responses. J. Funct. Foods 2021, 87, 104765. [Google Scholar] [CrossRef]
- Inui, T.; Kawamura, N.; Nakama, R.; Inui, A.; Kastsuura, G. Degalactosylated Whey Protein Suppresses Inflammatory Responses Induced by Lipopolysaccharide in Mice. Front. Nutr. 2022, 9, 852355. [Google Scholar] [CrossRef]
- Shao, W.F.; Chen, R.; Lin, G.L.; Ran, K.J.; Zhang, Y.Y.; Yang, H.X.; Shangguan, J.X.; Zhao, Y.Z.; Xu, H.L. In situ mucoadhesive hydrogel capturing tripeptide KPV: The anti-inflammatory, antibacterial and repairing effect on chemotherapy-induced oral mucositis. Biomater. Sci. 2022, 10, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wu, N.; Yao, Y.; Chen, S.P.; Xu, M.S.; Yin, Z.P.; Zhao, Y.; Tu, Y.G. Anti-inflammatory effects of tripeptide WLS on TNF-α-induced HT-29 cells and DSS-induced colitis in mice. Food Funct. 2022, 13, 9496–9512. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Kierkegaard-Brøchner, S.; Murphy, M.C. Injury prevention and management—Healthy joints and strong muscles. Br. J. Sports Med. 2023, 57, 1471. [Google Scholar] [CrossRef]
- Wei, X.X.; Li, N.; Wu, X.Y.; Cao, G.D.; Qiao, H.P.; Wang, J.; Hao, R.R. The preventive effect of Glycyrrhiza polysaccharide on lipopolysaccharide-induced acute colitis in mice by modulating gut microbial communities. Int. J. Biol. Macromol. 2023, 239, 124199. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lin, X.D.; Pan, X.Y.; Xu, H.Y.; Zhang, X.M.; Liang, G.Y.; Qiu, J.W.; Zhang, X.Y.; Gao, Y.; Tan, X.; et al. Development and validation of a blood routine-based extent and severity clinical decision support tool for ulcerative colitis. Sci. Rep. 2023, 13, 21368. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.Z.; Sun, N.; Mao, C.W.; Zheng, Z.H.; Lin, S.Y. Prevention and potential repair of colitis: Beneficial effects and regulatory mechanisms of food-derived anti-inflammatory peptides. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Luo, R.F.; Ye, N.J.; Hu, Y.C.; Fu, C.M.; Gao, R.; Fu, S.; Gao, F. Research progress on natural β-glucan in intestinal diseases. Int. J. Biol. Macromol. 2022, 219, 1244–1260. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Luo, J.; Liu, Y.; Yu, S.Y.; Liu, J. Rheum tanguticum polysaccharide alleviates DSS-induced ulcerative colitis and regulates intestinal microbiota in mice. Food Biosci. 2023, 53, 102788. [Google Scholar] [CrossRef]
- Zeng, W.; He, D.; Xing, Y.F.; Liu, J.Y.; Su, N.; Zhang, C.; Wang, Y.; Xing, X.H. Internal connections between dietary intake and gut microbiota homeostasis in disease progression of ulcerative colitis: A review. Food Sci. Hum. Wellness 2021, 10, 119–130. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, Z.; Wu, M.Q.; She, Y.Q.; Li, L.Z.; Xu, Y.J.; Qin, K.H.; Hu, Z.P.; Yang, M.Y.; Lu, F.T.; et al. The Communication Between Intestinal Microbiota and Ulcerative Colitis: An Exploration of Pathogenesis, Animal Models, and Potential Therapeutic Strategies. Front. Med. 2021, 8, 766126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Chen, H.; Li, W.T.; He, Q.; Liang, J.Y.M.; Yan, X.H.; Yuan, Y.H.; Yue, T.L. Selenium-containing tea polysaccharides ameliorate DSS-induced ulcerative colitis via enhancing the intestinal barrier and regulating the gut microbiota. Int. J. Biol. Macromol. 2022, 209, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Zhang, Y.M.; Song, J.P.; Li, Y.Q.; Zhou, L.Y.; Xu, H.T.; Wu, K.Z.; Gao, J.; Zhao, M.M.; Zheng, Y. Bergamot polysaccharides relieve DSS-induced ulcerative colitis via regulating the gut microbiota and metabolites. Int. J. Biol. Macromol. 2023, 253, 127335. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.R.; Liu, J.; Zhang, X.; Wu, X.N.; Tang, S.X.; Sun, R.; Qian, C.L.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Chen, W.J.; Li, H.; Wright, J.; Lamendella, R.; Lukin, D.; Szymczak, W.; Sun, K.; Kelly, L.; Ghosh, S.; et al. Bacterial Swarmers Enriched During Intestinal Stress Ameliorate Damage. Gastroenterology 2021, 161, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Kinsella, R.; Harding, C.; Feldman, M. The Secrets of Acinetobacter Secretion. Trends Microbiol. 2017, 25, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Pawlak, E.; Rembacz, K.; Kotas, M.; Różańska, P.Ż.; Kujawa, D.; Łaczmański, Ł.; Piotrowski, P.; Bielawski, T.; Samochowiec, J.; et al. Associations of gut microbiota alterations with clinical, metabolic, and immune-inflammatory characteristics of chronic schizophrenia. J. Psychiatr. Res. 2024, 171, 152–160. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.J.; Luo, W.W.; Shen, Z.H.; Yang, Z.Y.; Xiao, M.W.; Tong, T.; Yang, Y.Y.; Wang, X.Y. Roseburia intestinalis: A Beneficial Gut Organism from the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Alessandri, G.; Sinderen, D.V.; Ventura, M. The genus Bifidobacterium: From genomics to functionality of an important component of the mammalian gut microbiota. Comput. Struct. Biotechnol. J. 2021, 19, 1472–1487. [Google Scholar] [CrossRef]
- Qu, D.W.; Gu, Z.N.; Feng, Z.N.; Feng, S.S.; Yu, L.L.; Tian, F.W.; Zhang, H.; Chen, W.; Zhai, Q.X. Differences in the effects and action modes of gut commensals against dextran sulfate sodium-induced intestinal inflammation. Food Sci. Hum. Wellness 2023, 13, 1201–1211. [Google Scholar] [CrossRef]
- Herp, S.; Brugiroux, S.; Garzetti, D.; Bleich, A.; Berry, D.; Stecher, B.; Eberl, C.; Hussain, S.; Walter, S.; Gerlach, R.; et al. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe 2019, 25, 681–694.e8. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.; Mackay, C.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Han, R.; Ma, Y.X.; Xiao, J.B.; You, L.J.; Pedisić, S.; Liao, L. The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria Lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food Chem. Toxicol. 2021, 149, 112001. [Google Scholar] [CrossRef] [PubMed]

| Low Molecular Weight Oyster Peptides g/100 g (or KJ) | |
|---|---|
| Carbohydrate | 22.10 |
| Peptides | 67.20 |
| Crude fat | 2.00 |
| Ash | 4.90 |
| Moisture | 5.83 |
| Energy | 1518.00 KJ |
| Amino Acid | Quantity in the LOPs (g/100 g) |
|---|---|
| Glutamate | 10.2 |
| Threonine | 3.06 |
| Serine | 2.97 |
| Proline | 3.43 |
| Glycine | 3.81 |
| Alanine | 3.44 |
| Valine | 3.00 |
| Methionine | 1.30 |
| Isoleucine | 3.00 |
| Leucine | 4.04 |
| Tyrosine | 2.03 |
| Phenylalanine | 2.05 |
| Histidine | 1.13 |
| Lysine | 5.64 |
| Arginine | 4.97 |
| Aspartate | 7.11 |
| The percentage of hydrophobic amino acids (%) | 39.50% |
| Ratio of essential amino acids to non-essential amino acids (%) | 70.28% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Lin, H.; Shen, W.; Cao, W.; Qin, X.; Gao, J.; Chen, Z.; Zheng, H.; Zhong, S.; Huang, H. The Preventive Effect of Low-Molecular Weight Oyster Peptides on Lipopolysaccharide-Induced Acute Colitis in Mice by Modulating Intestinal Microbiota Communities. Foods 2024, 13, 2391. https://doi.org/10.3390/foods13152391
Wu Q, Lin H, Shen W, Cao W, Qin X, Gao J, Chen Z, Zheng H, Zhong S, Huang H. The Preventive Effect of Low-Molecular Weight Oyster Peptides on Lipopolysaccharide-Induced Acute Colitis in Mice by Modulating Intestinal Microbiota Communities. Foods. 2024; 13(15):2391. https://doi.org/10.3390/foods13152391
Chicago/Turabian StyleWu, Qihang, Haisheng Lin, Weiqiang Shen, Wenhong Cao, Xiaoming Qin, Jialong Gao, Zhongqin Chen, Huina Zheng, Saiyi Zhong, and Haoyang Huang. 2024. "The Preventive Effect of Low-Molecular Weight Oyster Peptides on Lipopolysaccharide-Induced Acute Colitis in Mice by Modulating Intestinal Microbiota Communities" Foods 13, no. 15: 2391. https://doi.org/10.3390/foods13152391







