Evaluating the Stability and Digestibility of Long-Chain Omega-3 Algal Oil Nanoemulsions Prepared with Lecithin and Tween 40 Emulsifiers Using an In Vitro Digestion Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Lecithin (LE), and Combined LE/Tween 40 (LTN) Algal Oil Nanoemulsions and Algal Oil Mix
2.2.2. In Vitro Digestion Model
2.2.3. Measurement of Droplet Size
2.2.4. Isolation of the Aqueous Phase from Digested Fluid
2.2.5. Extraction of Lipid of Aqueous Phase
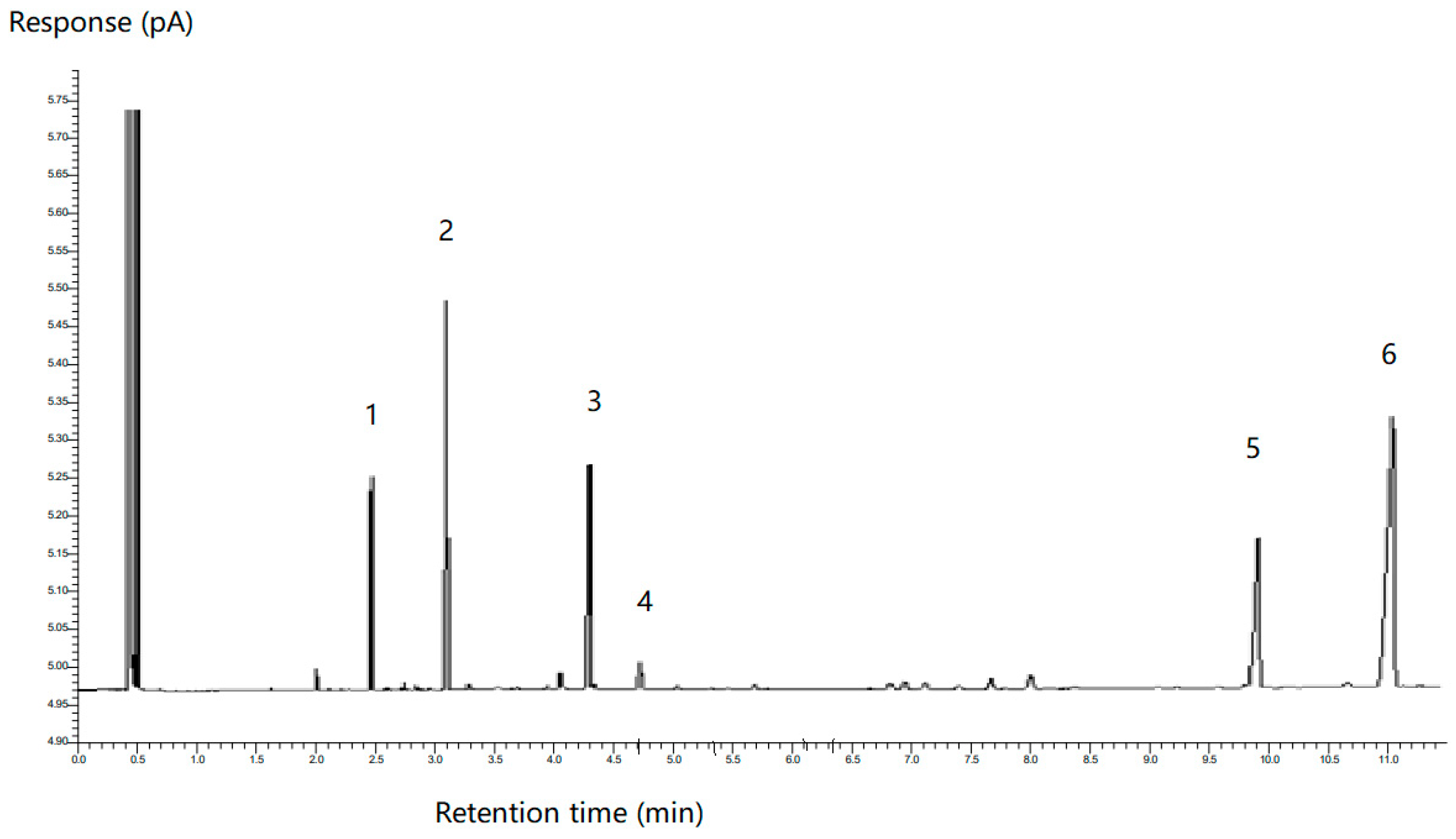
2.2.6. Determination of Fatty Acid Composition by GC
2.2.7. GC-FID Analysis
2.2.8. Statistical Analysis
3. Results
3.1. Droplet Size and Stability Measurements of Algal Oil Mix and Nanoemulsions
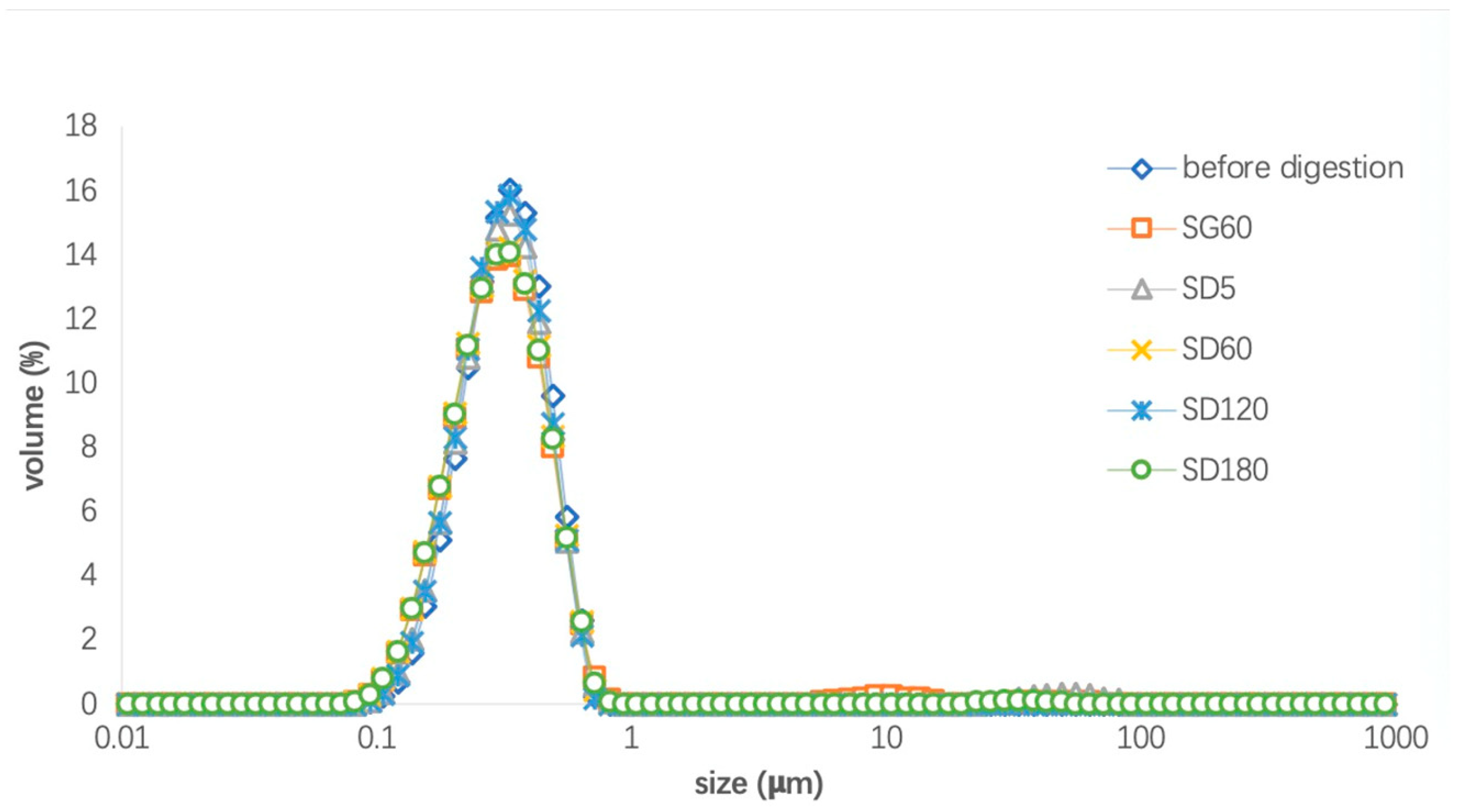
3.2. Droplet Size Distribution of the LTN Nanoemulsion and Algal Oil Mix during In Vitro Digestion
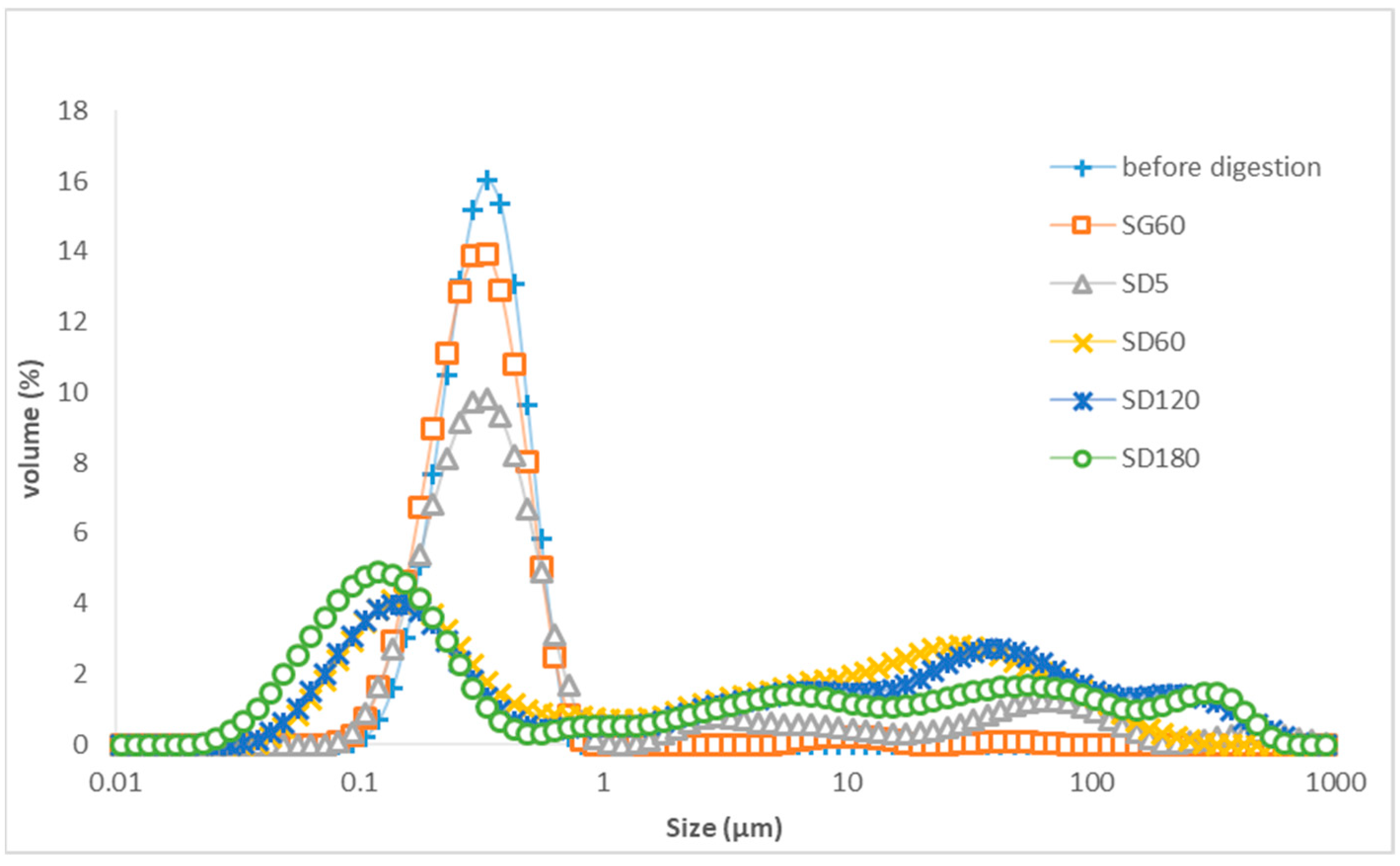
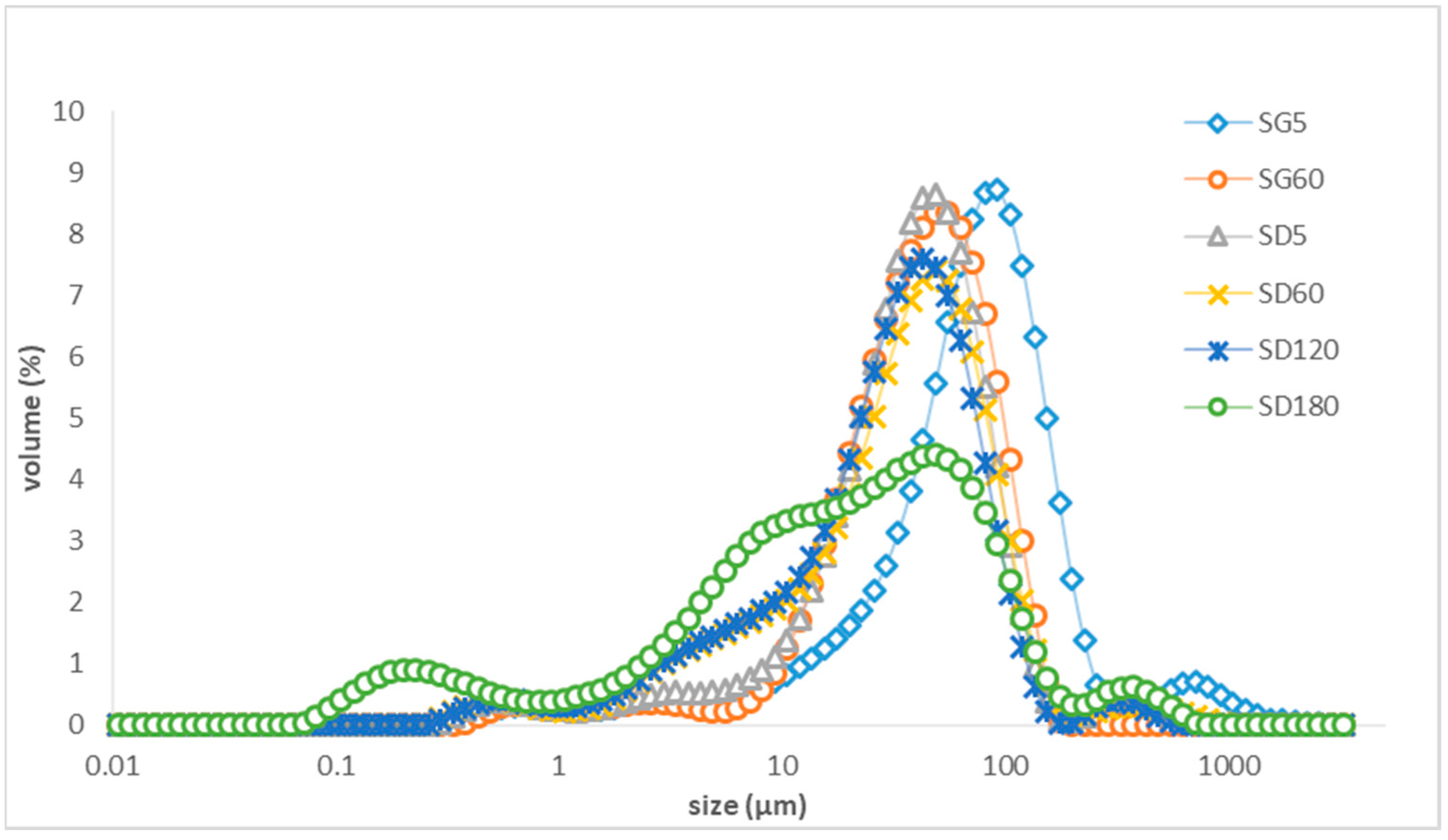
3.3. The Droplet Size Distribution of the Aqueous Phase of LTN Nanoemulsion and Algal Oil Mix Samples after Digestion
3.4. Digestibility of DHA of LTN Nanoemulsion and Algal Oil Mix during In Vitro Digestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, D.; Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.; Wei, L.; et al. associations of fish consumption with risk of cardiovascular disease and mortality among individuals with or without vascular disease from 58 countries. JAMA Intern. Med. 2021, 181, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Xiong, K.; Xu, L.; Zhang, B.; Ma, A. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutrients 2021, 13, 2342. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Meyer, B.J.; Mori, T.A.; Burke, V.; Mansour, J.; Patch, C.S.; Tapsell, L.C.; Noakes, M.; Clifton, P.A.; Barden, A.; et al. Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br. J. Nutr. 2007, 97, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 polyunsaturated fatty acids intake and blood pressure: A dose-response meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on omega-3 polyunsaturated fatty acids on cardiovascular health. Nutrients 2022, 14, 5146. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chiu, W.C.; Hsu, Y.P.; Lo, Y.L.; Wang, Y.H. Effects of omega-3 fatty acids on muscle mass, muscle strength and muscle performance among the elderly: A meta-analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef] [PubMed]
- Bates, B.; Collins, D.; Jones, K.; Page, P.; Roberts, C.; Steer, T.; Swan, G. National Diet and Nutrition Survey Rolling Programme Years 9 to 11 (2016/2017 to 2018/2019). Public Health England. 2020. Available online: https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019 (accessed on 15 June 2024).
- Derbyshire, E. Oily fish and omega-3s across the life stages: A focus on intakes and future directions. Front. Nutr. 2019, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Swetha, N.; Mathanghi, S.K. Towards sustainable omega-3 fatty acids production—A comprehensive review on extraction methods, oxidative stability and bio-availability enhancement. Food Chem. Adv. 2024, 4, 100603. [Google Scholar] [CrossRef]
- Lane, K.E.; Wilson, M.; Hellon, T.G.; Davies, I.G. Bioavailability and conversion of plant based sources of omega-3 fatty acids—A scoping review to update supplementation options for vegetarians and vegans. Crit. Rev. Food Sci. Nutr. 2022, 62, 4982–4997. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to docosahexaenoic acid (DHA) and maintenance of normal (fasting) blood concentrations of triglycerides (ID 533, 691, 3150), protection of blood lipids from oxidative damage (ID 630), contribution to the maintenance or achievement of a normal body weight (ID 629), brain, eye and nerve development (ID 627, 689, 704, 742, 3148, 3151), maintenance of normal brain function (ID 565, 626, 631, 689, 690, 704, 742, 3148, 3151), maintenance of normal vision (ID 627, 632, 743, 3149) and maintenance of normal spermatozoa motility (ID 628) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1734. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of a health claim related to DHA and contribution to normal brain development pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3840. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and brain, eye and nerve development (ID 501, 513, 540), maintenance of normal brain function (ID 497, 501, 510, 513, 519, 521, 534, 540, 688, 1323, 1360, 4294), maintenance of normal vision (ID 508, 510, 513, 519, 529, 540, 688, 2905, 4294), maintenance of normal cardiac function (ID 510, 688, 1360), “maternal health; pregnancy and nursing” (ID 514), “to fulfil increased omega-3 fatty acids need during pregnancy” (ID 539), “skin and digestive tract epithelial cells maintenance” (ID 525), enhancement of mood (ID 536), “membranes cell structure” (ID 4295), “anti-inflammatory action” (ID 4688) and maintenance of normal blood LDL-cholesterol concentrations (ID 4719) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2078. [Google Scholar] [CrossRef]
- Laidlaw, M.; Cockerline, C.A.; Rowe, W.J. A randomized clinical trial to determine the efficacy of manufacturers’ recommended doses of omega-3 fatty acids from different sources in facilitating cardiovascular disease risk reduction. Lipids Health Dis. 2014, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Hilleman, D.E.; Wiggins, B.S.; Bottorff, M.B. Critical differences between dietary supplement and prescription omega-3 fatty acids: A narrative review. Adv. Ther. 2020, 37, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Johnson, J.; Rooney, M.W.; Kyle, M.L.; Kling, D.F. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova® compared to Lovaza® in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 2012, 6, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Kruger, M.C.; Wynne, C.; Waaka, D.; Li, W.; Frampton, C.; Wolber, F.M.; Eason, C. Bioavailability of orally administered active lipid compounds from four different greenshell™ mussel formats. Mar. Drugs 2020, 18, 524. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ciftci, O.N. In vitro bioaccessibility of fish oil-loaded hollow solid lipid micro-and nanoparticles. Food Funct. 2020, 11, 8637–8647. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Q.; Li, W.; Wright, A.J. Emulsification of algal oil with soy lecithin improved DHA bioaccessibility but did not change overall in vitro digestibility. Food Funct. 2014, 5, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Barrow, C.J.; Nolan, C.; Holub, B.J. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J. Funct. Foods 2009, 1, 38–43. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; McClements, D.J.; Martín-Belloso, O. Chapter 15—Nanoemulsion design for the delivery of omega-3 fatty acids: Formation, oxidative stability, and digestibility. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Moltke Sørensen, A.-D., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 295–319. [Google Scholar]
- Leong, T.S.H.; Wooster, T.J.; Kentish, S.E.; Ashokkumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochemistry 2009, 16, 721–727. [Google Scholar] [CrossRef]
- Miao, M.; Chen, L.; McClements, D. Bioactive Delivery Systems for Lipophilic Nutraceuticals: Formulation, Fabrication, and Application; Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Li, W.; Smith, C.J.; Derbyshire, E.J.; Lane, K.E. Nano-Emulsions, Methods of Making the Same and Uses Thereof. 2014. Intellectual Properties Office. Available online: https://www.ipo.gov.uk/p-ipsum/Case/PublicationNumber/GB2511028 (accessed on 20 May 2024).
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-grade nanoemulsions: Preparation, stability and application in encapsulation of bioactive compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; Karsli, G.T.; Durmuş, M.; Yazgan, H.; Oztop, H.M.; McClements, D.J.; Ozogul, F. Recent developments in industrial applications of nanoemulsions. Adv. Colloid Interface Sci. 2022, 304, 102685. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Mendenhall, M. Chapter 35—Advances in lecithin-based nanoemulsions within the animal and human nutritional markets. In Microencapsulation in the Food Industry, 2nd ed.; Sobel, R., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 573–584. [Google Scholar]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.R.; Pulikkal, A.K. Nano emulsions stabilized by natural emulsifiers: A comprehensive review on feasibility, stability and bio-applicability. J. Drug Deliv. Sci. Technol. 2024, 92, 105303. [Google Scholar] [CrossRef]
- Lane, K.E.; Li, W.; Smith, C.; Derbyshire, E. The bioavailability of an omega-3-rich algal oil is improved by nanoemulsion technology using yogurt as a food vehicle. Int. J. Food Sci. Technol. 2014, 49, 1264–1271. [Google Scholar] [CrossRef]
- Lane, K.E.; Li, W.; Smith, C.J.; Derbyshire, E.J. The development of vegetarian omega-3 oil in water nanoemulsions suitable for integration into functional food products. J. Funct. Foods 2016, 23, 306–314. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Sui, Z.; Lu, W.; Corke, H. Soybean lecithin-stabilized oil-in-water (O/W) emulsions increase the stability and in vitro bioaccessibility of bioactive nutrients. Food Chem. 2021, 338, 128071. [Google Scholar] [CrossRef] [PubMed]
- Omer, E.; Chiodi, C. Fat digestion and absorption: Normal physiology and pathophysiology of malabsorption, including diagnostic testing. Nutr. Clin. Pract. 2024, 39, S6–S16. [Google Scholar] [CrossRef]
- Besseling, R.; Arribas-Bueno, R.; van Tuijn, R.; Gerich, A. Realtime droplet size monitoring of nano-emulsions during high pressure homogenization; InProcess-LSP: Oss, The Netherlands, 2021; pp. 1–12. [Google Scholar] [CrossRef]
- AOAC International. AOAC Fatty Acid in Oils and Fats Preparation of Methyl Ester Boron Trifluoride Method; AOAC International: Washington, DC, USA, 2000; pp. 19–20. [Google Scholar]
- Nobbmann, U. D90, D50, D10, and Span—For DLS? Malvern Panalytical. 2017. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/insights/d90-d50-d10-and-span-for-dls (accessed on 9 July 2024).
- Boisen, S.; Eggum, B.O. Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr. Res. Rev. 1991, 4, 141–162. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Kimura, H.; Futami, Y.; Tarui, S.-i.; Shinomiya, T. Activation of human pancreatic lipase activity by calcium and bile salts. J. Biochem. 1982, 92, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lundin, L.; Golding, M.; Wooster, T.J. Understanding food structure and function in developing food for appetite control. Nutr. Diet. 2008, 65, S79–S85. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhong, L.; Liu, L.; Xie, L.; Tang, H.; Zhang, L.; Li, X. Comparison of surfactants at solubilizing, forming and stabilizing nanoemulsion of hesperidin. J. Food Eng. 2020, 281, 110000. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Gastrointestinal fate of emulsion-based ω-3 oil delivery systems stabilized by plant proteins: Lentil, pea, and faba bean proteins. J. Food Eng. 2017, 207, 90–98. [Google Scholar] [CrossRef]
- Cheng, H.M.; Mah, K.K.; Seluakumaran, K. Fat digestion: Bile salt, emulsification, micelles, lipases, chylomicrons. In Defining Physiology: Principles, Themes, Concepts. Volume 2: Neurophysiology and Gastrointestinal Systems; Springer International Publishing: Cham, Switzerland, 2020; pp. 63–65. [Google Scholar]
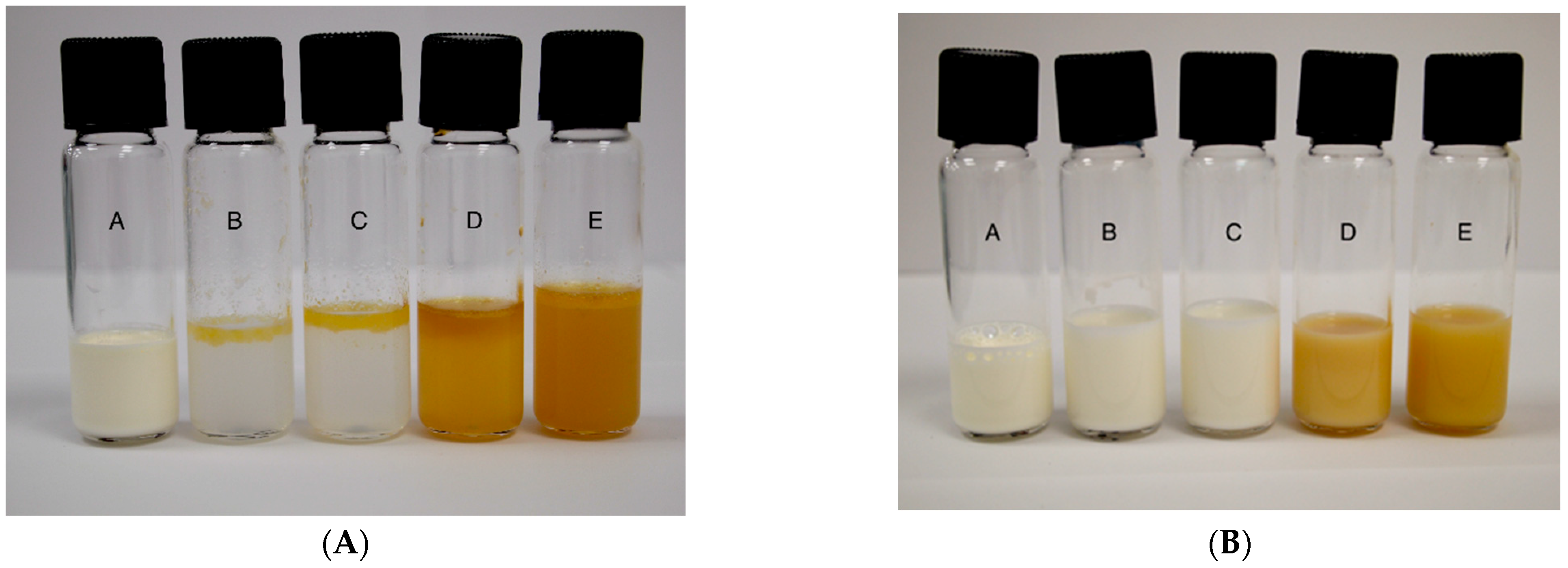



| Component | SGF | SDF | SBF | Final Mixture |
|---|---|---|---|---|
| (mM) | (mM) | (mM) | (mM) | |
| NaCl | 94.2 | 240 | 180 | 130.5 |
| NaH2PO4 | 4.4 | - | - | 1.4 |
| NaHCO3 | - | 80 | 137 | 45.7 |
| KCl | 22.1 | 15.1 | 10 | 13 |
| KH2PO4 | - | 1.2 | - | 0.4 |
| CaCl2·2H2O | 5.4 | 2.7 | 3 | 3 |
| NH4Cl | 11.4 | - | - | 3.5 |
| MgCl2 | - | 0.5 | - | 0.2 |
| Total | 137.6 | 340 | 330 | 197.5 |
| pH | 1.3 | 8.1 | 8.2 | 6.5 |
| Algal Oil Mix | LE Nanoemulsion | LTN Nanoemulsion | |
|---|---|---|---|
| Mean droplet size (d32 (µm)) | 73.6 ± 6.98 | 0.340 ± 0.00 a | 0.267 ± 0.00 a |
| Span | 1.24 | 2.91 | 1.78 |
| LTN Nanoemulsion | Algal Oil Mix | |
|---|---|---|
| Digestion Stage | d32 (µm) | d32 (µm) |
| Before digestion | 0.267 ± 0.00 | 73.6 ± 6.98 |
| Gastric 5 min | 0.245 ± 0.00 | 50.1 ± 1.81 c |
| Gastric 60 min | 0.238 ± 0.01 | 28.83 ± 0.06 cd |
| Duodenal 5 min | 0.326 ± 0.01 | 27.30 ± 0.30 cd |
| Duodenal 60 min | 14.20 ± 0.26 a | 21.67 ± 0.57 cd |
| Duodenal 120 min | 10.31 ± 0.52 ab | 19.82 ± 0.10 cd |
| Duodenal 180 min | 8.87 ± 2.56 ab | 18.97 ± 8.19 cd |
| Fatty Acid Sample | Myristic (%) | Palmitic (%) | Oleic (%) | Linoleic (%) | DPA (%) | DHA (%) |
|---|---|---|---|---|---|---|
| Before digestion | 5.39 ± 0.68 | 17.12 ± 2.33 | 16.37 ± 2.14 | 4.98 ± 0.71 | 16.74 ± 1.89 | 39.40 ± 4.15 |
| Aqueous phase of digested LTN | 3.53 ± 0.04 | 11.15 ± 0.48 | 28.17 ± 0.57 a | 12.53 ± 0.32 a | 15.74 ± 0.58 | 28.89 ± 0.74 a |
| Aqueous phase of digested algal oil mix | 5.83 ± 0.20 | 19.06 ± 0.53 | 29.38 ± 0.62 a | 15.34 ± 0.36 ab | 10.46 ± 0.39 ab | 19.95 ± 0.40 ab |
| Before Digestion DHA/LTN and Algal Oil Mix | Digested DHA/LTN | Digested DHA/ Algal Oil Mix | |
|---|---|---|---|
| DHA (mg/g) | 162.12 ± 17.07 | 47.34 ± 3.14 a | 16.53 ± 0.45 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Lane, K.E.; Li, W. Evaluating the Stability and Digestibility of Long-Chain Omega-3 Algal Oil Nanoemulsions Prepared with Lecithin and Tween 40 Emulsifiers Using an In Vitro Digestion Model. Foods 2024, 13, 2407. https://doi.org/10.3390/foods13152407
Zhou Q, Lane KE, Li W. Evaluating the Stability and Digestibility of Long-Chain Omega-3 Algal Oil Nanoemulsions Prepared with Lecithin and Tween 40 Emulsifiers Using an In Vitro Digestion Model. Foods. 2024; 13(15):2407. https://doi.org/10.3390/foods13152407
Chicago/Turabian StyleZhou, Qiqian, Katie E. Lane, and Weili Li. 2024. "Evaluating the Stability and Digestibility of Long-Chain Omega-3 Algal Oil Nanoemulsions Prepared with Lecithin and Tween 40 Emulsifiers Using an In Vitro Digestion Model" Foods 13, no. 15: 2407. https://doi.org/10.3390/foods13152407
APA StyleZhou, Q., Lane, K. E., & Li, W. (2024). Evaluating the Stability and Digestibility of Long-Chain Omega-3 Algal Oil Nanoemulsions Prepared with Lecithin and Tween 40 Emulsifiers Using an In Vitro Digestion Model. Foods, 13(15), 2407. https://doi.org/10.3390/foods13152407





