Immunochromatographic Strip Based on Tetrahedral DNA Immunoprobe for the Detection of Aflatoxin B1 in Rice Bran Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Equipment
2.3. Self-Assembly of TDNs
2.4. Construction and Characterization of the Immunoprobes
2.5. Estimation of the Labeling Density of Effective Antibody
2.6. Investigation of the Immunoaffinity of the Immunoprobes
2.7. Sample Pretreatment
2.8. Fabrication of the ICS
2.9. Qualitative and Quantitative Detection of AFB1 by ICSs
3. Results
3.1. Characterization of the TDNs
3.2. Characterization of Immunoprobes
3.3. Determination of the Labeling Density of the Effective Antibody
3.4. Investigation of the Immunoaffinity
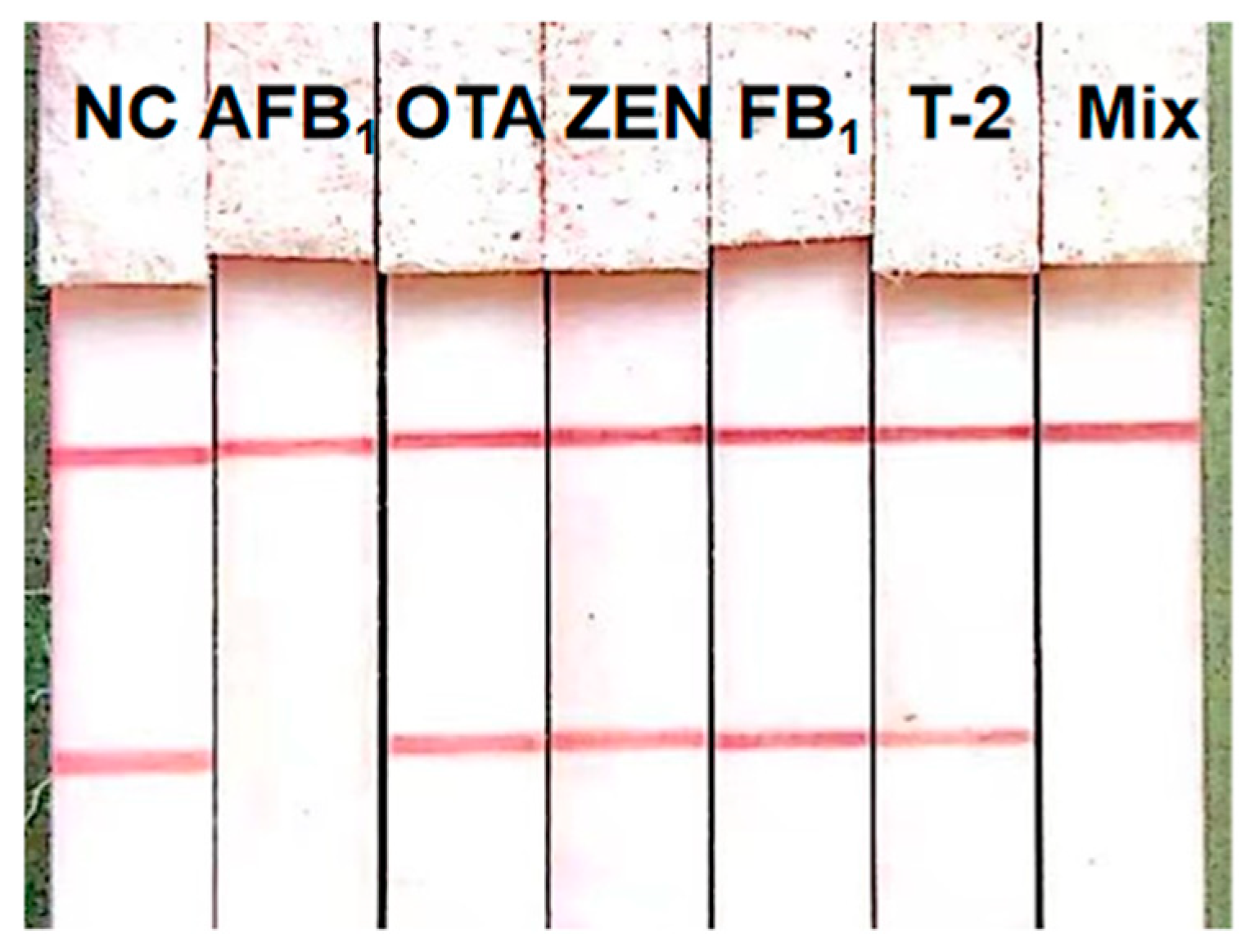
3.5. AFB1 Analysis of the Proposed ICS
3.6. Assessment of the ICS
3.7. Method Verification Using UPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pok, P.S.; Londoño, V.A.G.; Vicente, S.; Romero, S.M.; Pacín, A.; Tolaba, M.; Alzamora, S.M.; Resnik, S.L. Evaluation of citrus flavonoids against Aspergillus parasiticus in maize: Aflatoxins reduction and ultrastructure alterations. Food Chem. 2020, 318, 126414. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Biocontrol of aflatoxins using non-aflatoxigenic Aspergillus flavus: A literature review. J. Fungi. 2021, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ren, C.; Wang, C.; An, K.; Cui, H.; Hao, N.; Wang, K. Gold nanoparticles mediated designing of versatile aptasensor for colorimetric/electrochemical dual-channel detection of aflatoxin B1. Biosens. Bioelectron. 2020, 166, 112443. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pu, H.; Sun, D.W. Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: Principles and recent applications. Trends Food Sci. Tech. 2021, 110, 393–404. [Google Scholar] [CrossRef]
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Ferre, F.S. Worldwide occurrence of mycotoxins in rice. Food Control 2016, 62, 291–298. [Google Scholar] [CrossRef]
- Junsai, T.; Poapolathep, S.; Sutjarit, S.; Giorgi, M.; Zhang, Z.; Logrieco, A.F.; Li, P.; Poapolathep, A. Determination of multiple mycotoxins and their natural occurrence in edible vegetable oils using liquid chromatography-tandem mass spectrometry. Foods 2021, 10, 2795. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, Y.; Chang, M.; Tang, L.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Antioxidant interaction of α-tocopherol, γ-oryzanol and phytosterol in rice bran oil. Food Chem. 2021, 343, 128431. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Reddy, K.R.N. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, Y.; Song, J.; Liu, Q.; Song, L.; Fei, X.; Li, X. A novel multimode biosensor for sensitive detection of AFB1 in food based on Mxenes nano enzymes. Food Chem. 2023, 426, 136645. [Google Scholar] [CrossRef]
- Zuo, J.; Yan, T.; Tang, X.; Zhang, Q.; Li, P. Dual-modal immunosensor made with the multifunction nanobody for fluorescent/colorimetric sensitive detection of aflatoxin B1 in maize. ACS Appl. Mater. Interfaces 2023, 15, 2771–2780. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Pan, A.; Zhang, C.; Guo, M.; Lou, C.; Zhang, J.; Wu, H.; Wang, X. Fast extraction of aflatoxins, ochratoxins and enniatins from maize with magnetic covalent organic framework prior to HPLC-MS/MS detection. Food Chem. 2023, 404, 134464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, N.; Yan, Z.; Yu, D.; Wang, L.; Wang, K.; Wei, X.; Wu, A. Development and validation of the one-step purification method coupled to LC-MS/MS for simultaneous determination of four aflatoxins in fermented tea. Food Chem. 2021, 354, 129497. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Singh, R. Determining aflatoxins in raw peanuts using immunoaffinity column as sample clean-up method followed by normal-phase HPLC-FLD analysis. Food Control 2022, 139, 109065. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.; Li, Y.; He, T.; Yang, Y.; Liu, M. Development of a biotinylated nanobody for sensitive detection of aflatoxin B1 in cereal via ELISA. Talanta 2022, 239, 123125. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, Y.; Chen, T.; Yang, B.; Ding, M.; Wen, H.; Xiao, J.; Cheng, G.; Tao, H.; Peng, D. Quantitative Determination of Aflatoxin B1 in Maize and Feed by ELISA and Time-Resolved Fluorescent Immunoassay Based on Monoclonal Antibodies. Foods 2024, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Zhang, X.; Hu, X.; Huang, X.; Liang, N.; Shen, T.; Zou, X.; Shi, J. A DNA tetrahedral scaffolds-based electrochemical biosensor for simultaneous detection of AFB1 and OTA. Food Chem. 2024, 442, 138312. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein. Microchim. Acta 2021, 188, 105. [Google Scholar] [CrossRef]
- Zhuang, Q.Q.; Chen, R.T.; Zheng, Y.J.; Huang, K.Y.; Peng, H.P.; Lin, Z.; Xia, X.H.; Chen, W.; Deng, H.H. Detection of tetanus toxoid with fluorescent tetanus human IgG-AuNC-based immunochromatography test strip. Biosens. Bioelectron. 2021, 177, 112977. [Google Scholar] [CrossRef]
- Bian, L.; Li, Z.; He, A.; Wu, B.; Yang, H.; Wu, Y.; Hu, F.; Lin, G.; Zhang, D. Ultrabright nanoparticle-labeled lateral flow immunoassay for detection of anti-SARS-CoV-2 neutralizing antibodies in human serum. Biomaterials 2022, 288, 121694. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, F.; Chen, X.; Fang, H.; Zha, C.; Huang, J.; Sun, X.; Ahmed, M.B.M.; Guo, Y.; Liu, Y. Dual-ratiometric aptasensor for simultaneous detection of malathion and profenofos based on hairpin tetrahedral DNA nanostructures. Biosens. Bioelectron. 2023, 227, 114853. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, J.; Liu, Z.; Mi, Q.; Niu, L.; Zhang, J. Design nanoprobe based on DNA tetrahedron supported hybridization chain reaction and its application to in situ analysis of bacteria. Chem. Eng. J. 2023, 466, 143099. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, T.; Lin, Y. Functionalizing framework nucleic-acid-based nanostructures for biomedical application. Adv. Mater. 2022, 34, 2107820. [Google Scholar] [CrossRef]
- Lin, M.; Wang, J.; Zhou, G.; Wang, J.; Wu, N.; Lu, J.; Gao, J.; Chen, X.; Shi, D.J.; Zou, X.; et al. Programmable engineering of a biosensing interface with tetrahedral DNA nanostructures for ultrasensitive DNA detection. Angew. Chem. Int. Edit. 2015, 54, 2151–2155. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J.; Zhang, S.; Mo, F.; Su, S.; Li, Y.; Fang, L.; Deng, J.; Huang, H.; Luo, Z.; et al. Electrochemical biosensor for DNA methylation detection through hybridization chain-amplified reaction coupled with a tetrahedral DNA nanostructure. ACS Appl. Mater. Interfaces 2019, 11, 3745–3752. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jia, J.; Yang, R.; Chen, D.; Wang, J.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z. Ratiometric immunosensor for GP73 detection based on the ratios of electrochemiluminescence and electrochemical signal using DNA tetrahedral nanostructure as the carrier of stable reference signal. Anal. Chem. 2019, 91, 3717–3724. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Hu, X.; Gyimah, E.; Yakubu, S.; Wang, K.; Wu, X.; Zhang, Z. Electrochemical biosensor based on tetrahedral DNA nanostructures and G-quadruplex–hemin conformation for the ultrasensitive detection of microRNA-21 in serum. Anal. Chem. 2019, 91, 7353–7359. [Google Scholar] [CrossRef]
- Xiong, X.; Yuan, W.; Li, Y.; Lu, Y.; Xiong, X.; Li, Y.; Liu, Y.; Lu, L. Sensitive electrochemical detection of aflatoxin B1 using DNA tetrahedron-nanostructure as substrate of antibody ordered assembly and template of aniline polymerization. Food Chem. 2020, 331, 127368. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Hu, Y.; Chen, R.; Dou, R.; Liu, K.; Cui, C. Translocation of tetrahedral DNA nanostructures through a solid-state nanopore. Nanoscale 2019, 11, 6263–6269. [Google Scholar] [CrossRef]
- Gao, L.; Liu, L.; Tian, Y.; Yang, Q.; Wu, P.; Fan, C.; Zhao, Q.; Li, F. Probing the formation kinetics and thermodynamics with rationally designed analytical tools enables one-pot synthesis and purification of a tetrahedral DNA nanostructure. Anal. Chem. 2021, 93, 7045–7053. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.M.; Zhou, Z.; Li, M.X.; Zhao, W.; Xu, J.J.; Chen, H.Y. DNA tetrahedral scaffolds-based platform for the construction of electrochemiluminescence biosensor. Biosens. Bioelectron. 2017, 90, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Zhang, H.; Zhu, D.; Li, J.; San, L.; Wang, Z.; Wang, C.; Wang, Y.; Wang, L.; Zuo, X.; et al. A novel ultrasensitive electrochemical DNA sensor based on double tetrahedral nanostructures. Biosens. Bioelectron. 2015, 71, 434–438. [Google Scholar] [CrossRef]
- Fan, Z.; Yao, B.; Ding, Y.; Zhao, J.; Xie, M.; Zhang, K. Entropy-driven amplified electrochemiluminescence biosensor for RdRp gene of SARS-CoV-2 detection with self-assembled DNA tetrahedron scaffolds. Biosens. Bioelectron. 2021, 178, 113015. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xue, L.; Zhang, M.; Li, C.; Xiang, Y.; Liu, P.; Li, G. An electrochemical sensor for Oct4 detection in human tissue based on target-induced steric hindrance effect on a tetrahedral DNA nanostructure. Biosens. Bioelectron. 2019, 127, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical detection of nucleic acids, proteins, small molecules and cells using a DNA-nanostructure-based universal biosensing platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, M.; Liu, C.; Tian, Y.; Fang, S.; Yang, H.; Li, B.; Liu, Q. Colloidal gold immunochromatographic test strips for broad-pectrum detection of Salmonella. Food Control 2021, 126, 108052. [Google Scholar] [CrossRef]
- Shi, X.; Zhan, W.; Chen, G.; Yu, Q.; Liu, Q.; Du, H.; Cao, L.; Liu, X.; Yuan, L.; Chen, H. Regulation of protein binding capability of surfaces via host-guest interactions: Effects of localized and average ligand density. Langmuir 2015, 31, 6172–6178. [Google Scholar] [CrossRef]
- Damphathik, C.; Songsiriritthigul, C.; Lerdsri, J.; Jakmunee, J.; Wongnongwa, Y.; Jungsuttiwong, S.; Ortner, A.; Kalcher, K.; Samphao, A. A novel immunosensor based on cobalt oxide nanocomposite modified single walled carbon nanohorns for the selective detection of aflatoxin B1. Talanta 2023, 258, 124472. [Google Scholar] [CrossRef]
- Liu, B.H.; Hsu, Y.T.; Lu, C.C.; Yu, F.Y. Detecting aflatoxin B1 in foods and feeds by using sensitive rapid enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Food Control 2013, 30, 184–189. [Google Scholar] [CrossRef]
- Zhang, D.; Li, P.; Yang, Y.; Zhang, Q.; Zhang, W.; Xiao, Z.; Ding, X. A high selective immunochromatographic assay for rapid detection of aflatoxin B1. Talanta 2011, 85, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.B.; Yang, Z.Y.; Kim, J.S.; Kim, J.Y.; Kang, S.J.; Woo, G.J.; Chung, Y.C.; Eremin, S.A.; Chung, D.H. Development of immunochromatography strip-test using nanocolloidal gold-antibody probe for the rapid detection of aflatoxin B1 in grain and feed samples. J. Microbiol. Biotechn. 2007, 17, 1629–1637. [Google Scholar]
- Yan, X.; Persaud, K.C. The Optimization of a Lateral Flow Immunoassay for Detection of Aflatoxin B 1 in Potable Water Samples. IEEE Sens. J. 2018, 19, 404–412. [Google Scholar] [CrossRef]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Xie, J.; Huang, Z. Rapid and simultaneous detection of aflatoxin B1, zearalenone, and T-2 toxin in medicinal and edible food using gold immunochromatographic test strip. Foods 2023, 12, 633. [Google Scholar] [CrossRef]


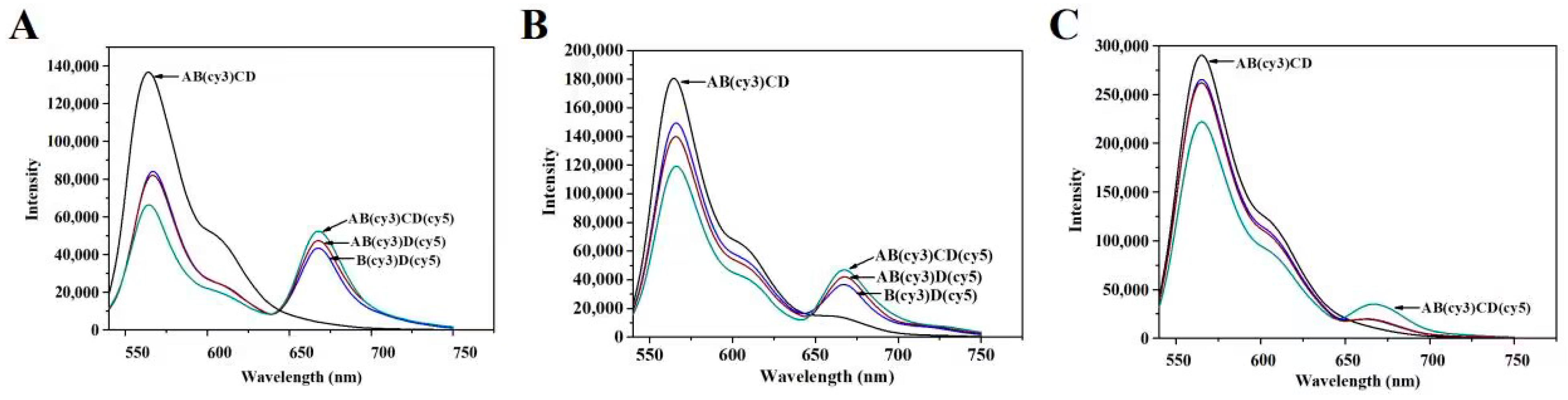

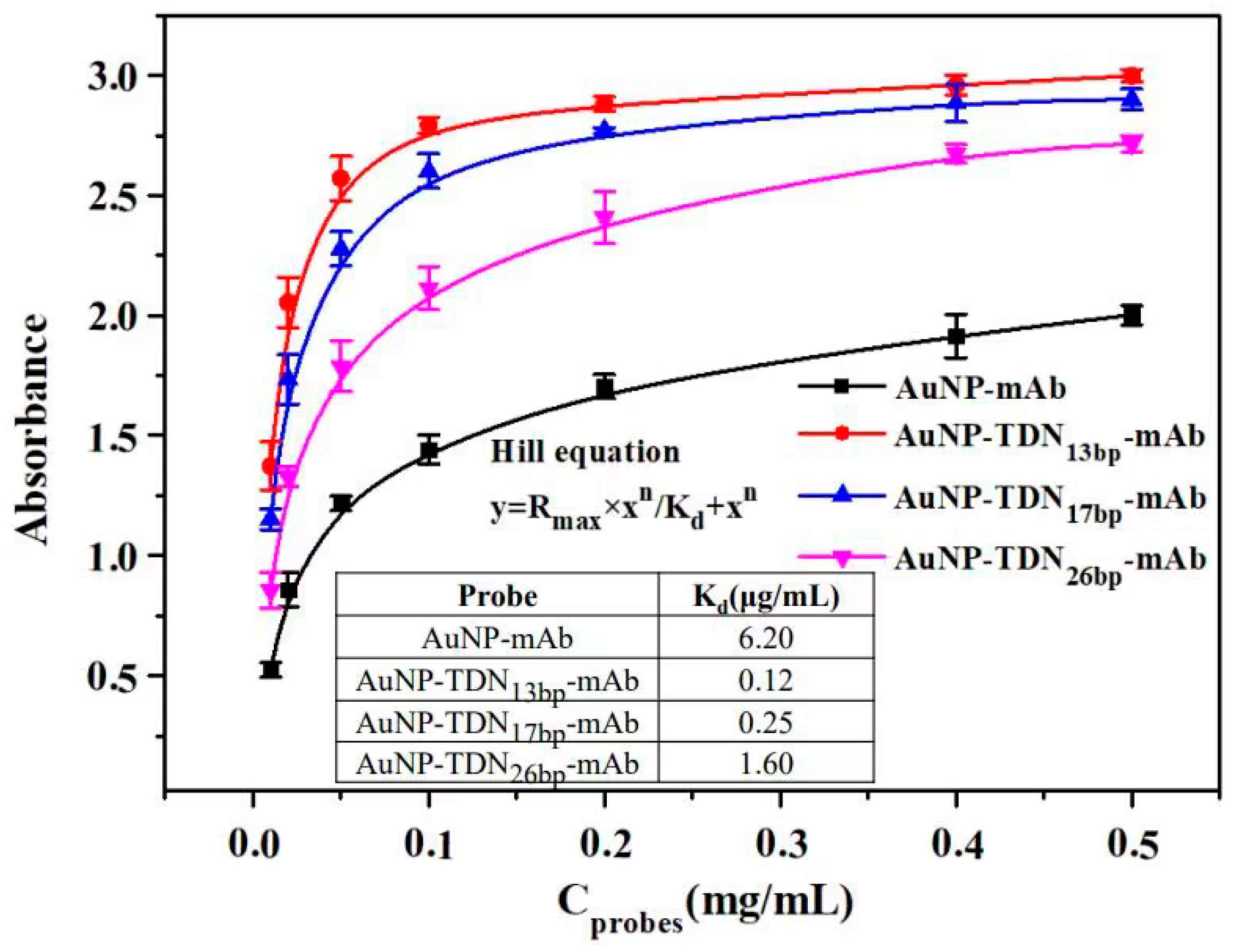

| Immune Probe | Antibody Coupling Density (mAb/AuNP) | Effective Antibody Density (Effective mAb/AuNP) | Proportion of Effective Antibodies (%) |
|---|---|---|---|
| AuNP-TDN13bp-mAb | 19 | 18 | 94.7 |
| AuNP-TDN17bp-mAb | 16 | 16 | 100 |
| AuNP-TDN26bp-mAb | 8 | 8 | 100 |
| AuNP-mAb | 31 | 10 | 32.3 |
| Spiked (ng/mL) | ICS Visual Observation Results (n = 20) | |
|---|---|---|
| Detection Results | Abnormality Rate% | |
| 0 | + a | 0 |
| 0.05 | + | 0 |
| 0.1 | + | 5 |
| 0.3 | ± b | 0 |
| 1 | − c | 0 |
| 2 | − | 0 |
| AFB1 (ng/mL) | Intra-Assay (n = 6) | Inter-Assay (n = 6) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | Recovery (%) | CV (%) | Mean ± SD (ng/mL) | Recovery (%) | CV (%) | |
| 0.1 | 0.095 ± 0.03 | 95.0 | 6.17 | 0.093 ± 0.03 | 93.0 | 7.47 |
| 0.3 | 0.267 ± 0.06 | 89.0 | 8.03 | 0.255 ± 0.05 | 85.0 | 7.12 |
| 0.5 | 0.491 ± 0.02 | 98.2 | 5.23 | 0.480 ± 0.05 | 96.0 | 6.78 |
| Probe | VLOD (ng/mL) | Specificity (Interfering Mycotoxins) | Accuracy | Repeatability (Abnormality Rate) | Ref. |
|---|---|---|---|---|---|
| AuNP-mAb | 2 | AFB2/AFG1/AFG2 | cd-ELISA | / | [40] |
| AuNP-mAb | 1 | AFB2/AFG1/AFG2 | ic-ELISA HPLC | 10% (ic-ELISA) | [41] |
| AuNP-mAb | 0.5 | AFB2/AFG1/AFG2/ OTA/Citrinin/ Patulin/ZEN/T-2 | HPLC | / | [42] |
| AuNP-mAb | 0.5 | / | ELISA | / | [43] |
| GNPs-mAb | 0.5 | AFB2/AFG1/AFG2 | / | 0% | [44] |
| GNP-mAb | 0.5 | ZEN/T-2/FB1/ DON/OTA | / | / | [45] |
| AuNP-TDN-mAb | 0.2 | OTA/ZEN/FB1/T-2 | UPLC | 5% | This study |
| Sample | Spiked (ng/mL) | Mean (ng/mL) ± SD (ng/mL) | |
|---|---|---|---|
| ICS | UPLC | ||
| Rice bran oil | 0.05 | 0.053 ± 0.036 | 0.055 ± 0.018 |
| 0.1 | 0.108 ± 0.022 | 0.094 ± 0.019 | |
| 0.3 | 0.307 ± 0.051 | 0.325 ± 0.034 | |
| 0.5 | 0.511 ± 0.016 | 0.469 ± 0.051 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Qu, W.; Hao, X.; Fang, M.; Yang, Q.; Li, Y.; Gong, Z.; Li, P. Immunochromatographic Strip Based on Tetrahedral DNA Immunoprobe for the Detection of Aflatoxin B1 in Rice Bran Oil. Foods 2024, 13, 2410. https://doi.org/10.3390/foods13152410
Xu L, Qu W, Hao X, Fang M, Yang Q, Li Y, Gong Z, Li P. Immunochromatographic Strip Based on Tetrahedral DNA Immunoprobe for the Detection of Aflatoxin B1 in Rice Bran Oil. Foods. 2024; 13(15):2410. https://doi.org/10.3390/foods13152410
Chicago/Turabian StyleXu, Lin, Wenli Qu, Xiaotong Hao, Min Fang, Qing Yang, Yuzhi Li, Zhiyong Gong, and Peiwu Li. 2024. "Immunochromatographic Strip Based on Tetrahedral DNA Immunoprobe for the Detection of Aflatoxin B1 in Rice Bran Oil" Foods 13, no. 15: 2410. https://doi.org/10.3390/foods13152410






