Influence of Foliar Zinc Application on Cadmium and Zinc Bioaccessibility in Brassica chinensis L.: In Vitro Digestion and Chemical Sequential Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Zn Fertilizers, and Soil
2.2. Pot Experiment
2.3. Measurement of Total Concentrations of Cd and Zn
2.4. Extraction of Chemical Forms of Cd and Zn
2.5. In Vitro Gastrointestinal Digestion
2.6. The Combined Test of In Vitro Digestion and Sequential Extraction
2.7. Data and Statistical Analyses
3. Results and Discussion
3.1. Cd and Zn Concentrations in Pakchoi Shoots
3.2. Chemical Forms of Cd and Zn in Pakchoi Shoots
3.3. Cd Bioaccessibility in Pakchoi Shoots and Health Risk Assessment of Cd
3.4. Zn Bioaccessibility in Pakchoi Shoots
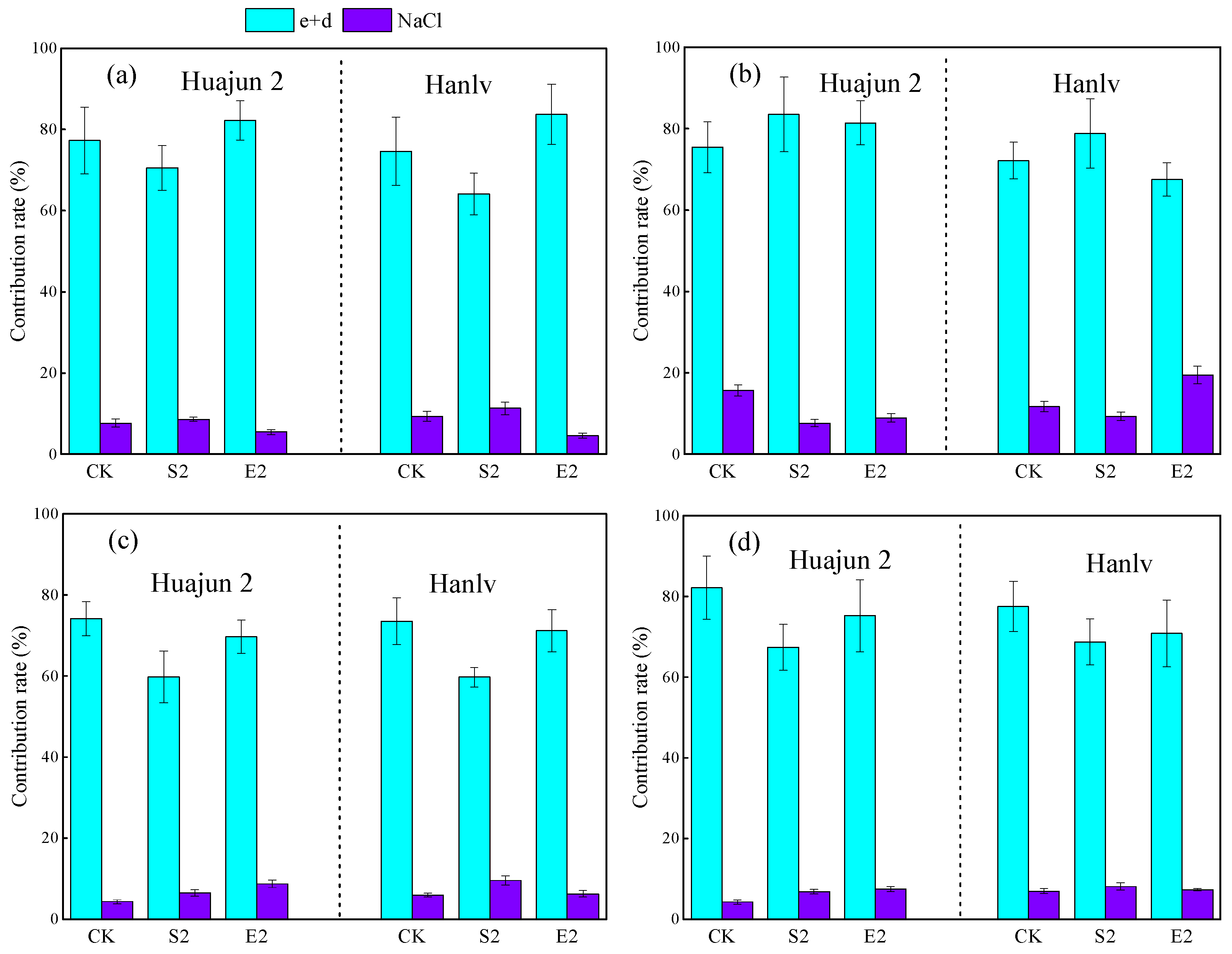
3.5. Relationships between Chemical Forms and Bioaccessibility of Cd and Zn
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 58, 1–453. [Google Scholar]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Mubeen, S.; Yuan, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, X.; Zhao, D.; Wang, P.; Zhao, F. Relative bioavailability of cadmium in rice: Assessment, modeling, and application for risk assessment. Foods 2023, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, 0177978. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, A.; Widell, A.; Olsson, I.M.; Grawe, K.P. Cadmium in food chain and health effects in sensitive population groups. Biometals 2004, 17, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Technical solutions for the safe utilization of heavy metal-contaminated farmland in China: A critical review. Land Degrad. Dev. 2019, 30, 1773–1784. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Du, B.; Cui, H.; Fan, X.; Zhou, D.; Zhou, J. Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant system and translocation of Cd in low- and high-Cd wheat cultivars. Environ. Pollut. 2020, 265, 115045. [Google Scholar] [CrossRef] [PubMed]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Cui, Y. In vitro digestion/Caco-2 cell model to estimate cadmium and lead bioaccessibility/bioavailability in two vegetables: The influence of cooking and additives. Food Chem. Toxicol. 2013, 59, 215–221. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, X.; Zhang, Z.; Liang, H.; Gu, M.; Shen, F.; Shohag, M.J.I.; Li, X. In vivo–in vitro correlations for the assessment of cadmium bioavailability in vegetables. J. Agric. Food Chem. 2021, 69, 12295–12304. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Liu, D.; Shohag, M.J.I.; Zehra, A.; He, Z.; Feng, Y.; Yang, X. Foliar application of zinc and selenium alleviates cadmium and lead toxicity of water spinach–Bioavailability/cytotoxicity study with human cell lines. Environ. Int. 2020, 145, 106122. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Xu, Y.; Wang, L.; Huang, Q.; Yan, X.; Liu, C. Effects of foliar application of manganese sulfate and zinc sulfate on bioaccessibility of cadmium, manganese, and zinc in wheat grains. J. Agro-Environ. Sci. 2020, 39, 2181–2189. [Google Scholar]
- Lin, Q.; Hamid, Y.; Wang, H.; Lu, M.; Gao, X.; Zou, T.; Chen, Z.; Hussain, B.; Feng, Y.; Li, T.; et al. Co-foliar application of zinc and nano-silicon to rice helps in reducing cadmium exposure risk: Investigations through in-vitro digestion with human cell line bioavailability assay. J. Hazard. Mater. 2024, 468, 133822. [Google Scholar] [CrossRef]
- Liao, W.; Wang, G.; Li, K.; Zhao, W.; Wu, Y. Effect of cooking on speciation and in vitro bioaccessibility of Hg and As from rice, using ordinary and pressure cookers. Biol. Trace Elem. Res. 2019, 187, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, N.; Cai, X.; Du, H.; Fu, Y.; Geng, Z.; Sultana, S.; Sun, G.; Cui, Y. Assessment of arsenic distribution, bioaccessibility and speciation in rice utilizing continuous extraction and in vitro digestion. Food Chem. 2021, 346, 128969. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wang, P.; Li, Y.; Du, H.; Chen, X.; Sun, G.; Cui, Y. Arsenic in rice bran products: In vitro oral bioaccessibility, arsenic transformation by human gut microbiota, and human health risk assessment. J. Agric. Food Chem. 2019, 67, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Cui, X. Zinc chemical forms and organic acid exudation in non-heading Chinese cabbages under zinc stress. Agric. Sci. 2012, 3, 562–566. [Google Scholar] [CrossRef]
- Su, Y.; Liu, J.; Lu, Z.; Wang, X.; Zhang, Z.; Shi, G. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, C.; Wang, P.; Wang, C.; Huang, Y.; Zhang, X.; Liu, Z. Rice vegetative organs alleviate cadmium toxicity by altering the chemical forms of cadmium and increasing the ratio of calcium to manganese. Ecotoxicol. Environ. Saf. 2019, 184, 109640. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Sun, Y.; Liang, X.; Lin, D. Identification of pakchoi cultivars with low cadmium accumulation and soil factors that affect their cadmium uptake and translocation. Front. Environ. Sci. Eng. 2014, 8, 877–887. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 45–278. [Google Scholar]
- Xin, J.; Huang, B. Subcellular distribution and chemical forms of cadmium in two hot pepper cultivars differing in cadmium accumulation. J. Agric. Food Chem. 2014, 62, 508–515. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, X.; Li, Y.; Li, Y.; Xia, H.; Li, Z.; Zhuang, P. Use of dietary components to reduce the bioaccessibility and bioavailability of cadmium in rice. J. Agric. Food Chem. 2020, 68, 4166–4175. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Li, J.; Chen, L.; Zhou, C.; He, X. Residue behavior and dietary intake risk assessment of imidaclothiz in pakchoi (Brassica chinensis L.). Sci. Agric. Sin. 2020, 53, 3587–3596. [Google Scholar]
- Zhang, Y.; Liu, P.; Jin, Y.; Wang, C.; Min, J.; Wu, Y. Dietary exposure and risk assessment to cadmium of the adult population of Jiangsu province, China: Comparing between semi-probabilistic and fully probabilistic approaches. Hum. Ecol. Risk Assess. 2016, 22, 226–240. [Google Scholar] [CrossRef]
- Saifullah; Sarwar, N.; Bibi, S.; Ahmad, M.; Ok, Y.S. Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ. Earth Sci. 2013, 71, 1663–1672. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Yan, X.; Liang, X.; Sun, Y.; Xu, Y. Pivotal role for root cell wall polysaccharides in cultivar-dependent cadmium accumulation in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2020, 194, 110369. [Google Scholar] [CrossRef]
- Haslett, B.S.; Reid, R.J.; Rengel, Z. Zinc mobility in wheat: Uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Yang, X. Biofortification and bioavailability of rice grain zinc as affected by different forms of foliar zinc fertilization. PLoS ONE 2012, 7, e45428. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhou, Y.; Yang, Z.; Lin, B.; Yuan, J.; Wu, S. Comparisons in subcellular and biochemical behaviors of cadmium between low-Cd and high-Cd accumulation cultivars of pakchoi (Brassica chinensis L.). Front. Environ. Sci. Eng. 2013, 8, 226–238. [Google Scholar] [CrossRef]
- Yan, B.; Isaure, M.P.; Mounicou, S.; Castillo-Michel, H.; De Nolf, W.; Nguyen, C.; Cornu, J.Y. Cadmium distribution in mature durum wheat grains using dissection, laser ablation-ICP-MS and synchrotron techniques. Environ. Pollut. 2020, 260, 113987. [Google Scholar] [CrossRef]
- Mishra, B.; McDonald, L.M.; Roy, M.; Lanzirotti, A.; Myneni, S.C.B. Uptake and speciation of zinc in edible plants grown in smelter contaminated soils. PLoS ONE 2020, 15, e0226180. [Google Scholar] [CrossRef]
- Terzano, R.; Al Chami, Z.; Vekemans, B.; Janssens, K.; Miano, T.; Ruggiero, P. Zinc distribution and speciation within rocket plants (Eruca vesicaria L. Cavalieri) grown on a polluted soil amended with compost as determined by XRF microtomography and micro-XANES. J. Agric. Food Chem. 2008, 56, 3222–3231. [Google Scholar] [CrossRef]
- Lin, Y.F.; Aarts, M.G.M. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Sun, L.; Yang, J.; Fang, H.; Xu, C.; Peng, C.; Huang, H.; Lu, L.; Duan, D.; Zhang, X.; Shi, J. Mechanism study of sulfur fertilization mediating copper translocation and biotransformation in rice (Oryza sativa L.) plants. Environ. Pollut. 2017, 226, 426–434. [Google Scholar] [CrossRef]
- Lu, Y.; Nie, Z.; Liu, H.; Gao, W.; Qin, S.; Li, C.; Fu, H.; Zhao, P. Influence of zinc on the subcellular fractions and chemical forms of cadmium in winter wheat. J. Henan Agric. Univ. 2019, 53, 503–511. [Google Scholar]
- Doolette, C.L.; Read, T.L.; Li, C.; Scheckel, K.G.; Donner, E.; Kopittke, P.M.; Schjoerring, J.K.; Lombi, E. Foliar application of zinc sulphate and zinc EDTA to wheat leaves: Differences in mobility, distribution, and speciation. J. Exp. Bot. 2018, 69, 4469–4481. [Google Scholar] [CrossRef] [PubMed]
- Viipsi, K.; Sjöberg, S.; Tõnsuaadu, K.; Shchukarev, A. Hydroxy- and fluorapatite as sorbents in Cd(II)–Zn(II) multi-component solutions in the absence/presence of EDTA. J. Hazard. Mater. 2013, 252–253, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, P.; Li, R.; Xu, Y.; Sun, Y.; Liang, X.; Dai, J. Effect of foliar zinc application on bioaccessibility of cadmium and zinc in pakchoi. Environ. Sci. 2018, 39, 2944–2952. [Google Scholar]
- Exposure to Cadmium: A Major Public Health Concern. Available online: https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-19-4-3 (accessed on 1 May 2019).
- Mnisi, R.L.; Ndibewu, P.P.; Mafu, L.D.; Bwembya, G.C. Bioaccessibility and risk assessment of essential and non-essential elements in vegetables commonly consumed in Swaziland. Ecotoxicol. Environ. Saf. 2017, 144, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Heavy metal pollution and potential health risks of commercially available Chinese herbal medicines. Sci. Total Environ. 2019, 653, 748–757. [Google Scholar] [CrossRef]




| Chemical Forms | Treatments | Cd Concentrations (mg/kg FW) | |
|---|---|---|---|
| Huajun 2 | Hanlv | ||
| Fethanol | CK | 0.0338 ± 0.0047 ab | 0.0557 ± 0.0098 c |
| S1 | 0.0271 ± 0.0039 b | 0.0681 ± 0.0111 bc | |
| S2 | 0.0317 ± 0.0060 b | 0.0501 ± 0.0100 c | |
| E1 | 0.0269 ± 0.0024 b | 0.0858 ± 0.0154 b | |
| E2 | 0.0427 ± 0.0051 a | 0.159 ± 0.015 a | |
| Fd-H2O | CK | 0.107 ± 0.025 c | 0.397 ± 0.072 c |
| S1 | 0.063 ± 0.010 c | 0.115 ± 0.015 d | |
| S2 | 0.103 ± 0.011 c | 0.145 ± 0.021 d | |
| E1 | 0.336 ± 0.055 b | 0.772 ± 0.091 b | |
| E2 | 0.485 ± 0.099 a | 1.20 ± 0.17 a | |
| FNaCl | CK | 0.250 ± 0.030 b | 0.732 ± 0.045 a |
| S1 | 0.328 ± 0.054 ab | 0.425 ± 0.030 c | |
| S2 | 0.433 ± 0.083 a | 0.589 ± 0.029 b | |
| E1 | 0.114 ± 0.018 c | 0.335 ± 0.026 cd | |
| E2 | 0.108 ± 0.023 c | 0.235 ± 0.043 d | |
| FHAc | CK | 0.0327 ± 0.0031 ab | 0.0491 ± 0.0065 a |
| S1 | 0.0505 ± 0.0143 ab | 0.0444 ± 0.0058 a | |
| S2 | 0.0422 ± 0.0006 ab | 0.0420 ± 0.0092 a | |
| E1 | 0.0179 ± 0.0045 b | 0.0468 ± 0.0132 a | |
| E2 | 0.0531 ± 0.0120 a | 0.0137 ± 0.0038 a | |
| FHCl | CK | 0.00529 ± 0.00026 ab | 0.00929 ± 0.00188 ab |
| S1 | 0.00775 ± 0.00187 ab | 0.0101 ± 0.0021 ab | |
| S2 | 0.00582 ± 0.00124 ab | 0.00834 ± 0.00200 ab | |
| E1 | 0.00309 ± 0.00076 b | 0.0162 ± 0.0045 a | |
| E2 | 0.0142 ± 0.0038 a | 0.00364 ± 0.00047 b | |
| Chemical Forms | Treatments | Zn Concentrations (mg/kg FW) | |
|---|---|---|---|
| Huajun 2 | Hanlv | ||
| Fethanol | CK | 2.34 ± 0.28 e | 2.85 ± 0.33 e |
| S1 | 9.03 ± 1.19 c | 8.00 ± 0.54 c | |
| S2 | 15.3 ± 1.4 a | 13.8 ± 0.1 a | |
| E1 | 4.36 ± 0.66 d | 5.18 ± 0.44 d | |
| E2 | 12.8 ± 1.2 b | 10.4 ± 0.7 b | |
| Fd-H2O | CK | 1.59 ± 0.11 d | 1.79 ± 0.23 c |
| S1 | 3.11 ± 0.53 c | 4.17 ± 0.77 b | |
| S2 | 11.8 ± 1.1 a | 13.3 ± 1.3 a | |
| E1 | 3.25 ± 0.41 c | 3.24 ± 0.53 bc | |
| E2 | 6.65 ± 1.04 b | 11.6 ± 1.5 a | |
| FNaCl | CK | 2.12 ± 0.41 b | 2.79 ± 0.45 b |
| S1 | 3.56 ± 0.78 b | 7.51 ± 1.28 b | |
| S2 | 35.7 ± 3.5 a | 32.1 ± 6.7 a | |
| E1 | 4.93 ± 0.94 b | 3.83 ± 0.76 b | |
| E2 | 5.44 ± 1.02 b | 7.08 ± 1.86 b | |
| FHAc | CK | 1.18 ± 0.09 c | 1.52 ± 0.27 c |
| S1 | 7.15 ± 0.53 b | 7.17 ± 1.42 b | |
| S2 | 22.5 ± 4.0 a | 13.7 ± 0.4 a | |
| E1 | 1.38 ± 0.19 c | 1.90 ± 0.17 c | |
| E2 | 5.78 ± 1.47 b | 2.66 ± 0.56 c | |
| FHCl | CK | 0.68 ± 0.10 c | 0.93 ± 0.18 c |
| S1 | 2.47 ± 0.33 b | 3.02 ± 0.39 b | |
| S2 | 5.34 ± 0.67 a | 4.92 ± 0.74 a | |
| E1 | 0.86 ± 0.20 c | 1.52 ± 0.37 c | |
| E2 | 2.77 ± 0.28 b | 1.53 ± 0.35 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tao, X.; Liu, C.; Liang, X.; Xu, Y.; Sun, Y. Influence of Foliar Zinc Application on Cadmium and Zinc Bioaccessibility in Brassica chinensis L.: In Vitro Digestion and Chemical Sequential Extraction. Foods 2024, 13, 2430. https://doi.org/10.3390/foods13152430
Wang L, Tao X, Liu C, Liang X, Xu Y, Sun Y. Influence of Foliar Zinc Application on Cadmium and Zinc Bioaccessibility in Brassica chinensis L.: In Vitro Digestion and Chemical Sequential Extraction. Foods. 2024; 13(15):2430. https://doi.org/10.3390/foods13152430
Chicago/Turabian StyleWang, Lin, Xueying Tao, Chang Liu, Xuefeng Liang, Yingming Xu, and Yuebing Sun. 2024. "Influence of Foliar Zinc Application on Cadmium and Zinc Bioaccessibility in Brassica chinensis L.: In Vitro Digestion and Chemical Sequential Extraction" Foods 13, no. 15: 2430. https://doi.org/10.3390/foods13152430
APA StyleWang, L., Tao, X., Liu, C., Liang, X., Xu, Y., & Sun, Y. (2024). Influence of Foliar Zinc Application on Cadmium and Zinc Bioaccessibility in Brassica chinensis L.: In Vitro Digestion and Chemical Sequential Extraction. Foods, 13(15), 2430. https://doi.org/10.3390/foods13152430








