Suppressive Effect of Coffee Leaves on Lipid Digestion and Absorption In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Plant Material
2.3. Determination of Proximate Composition
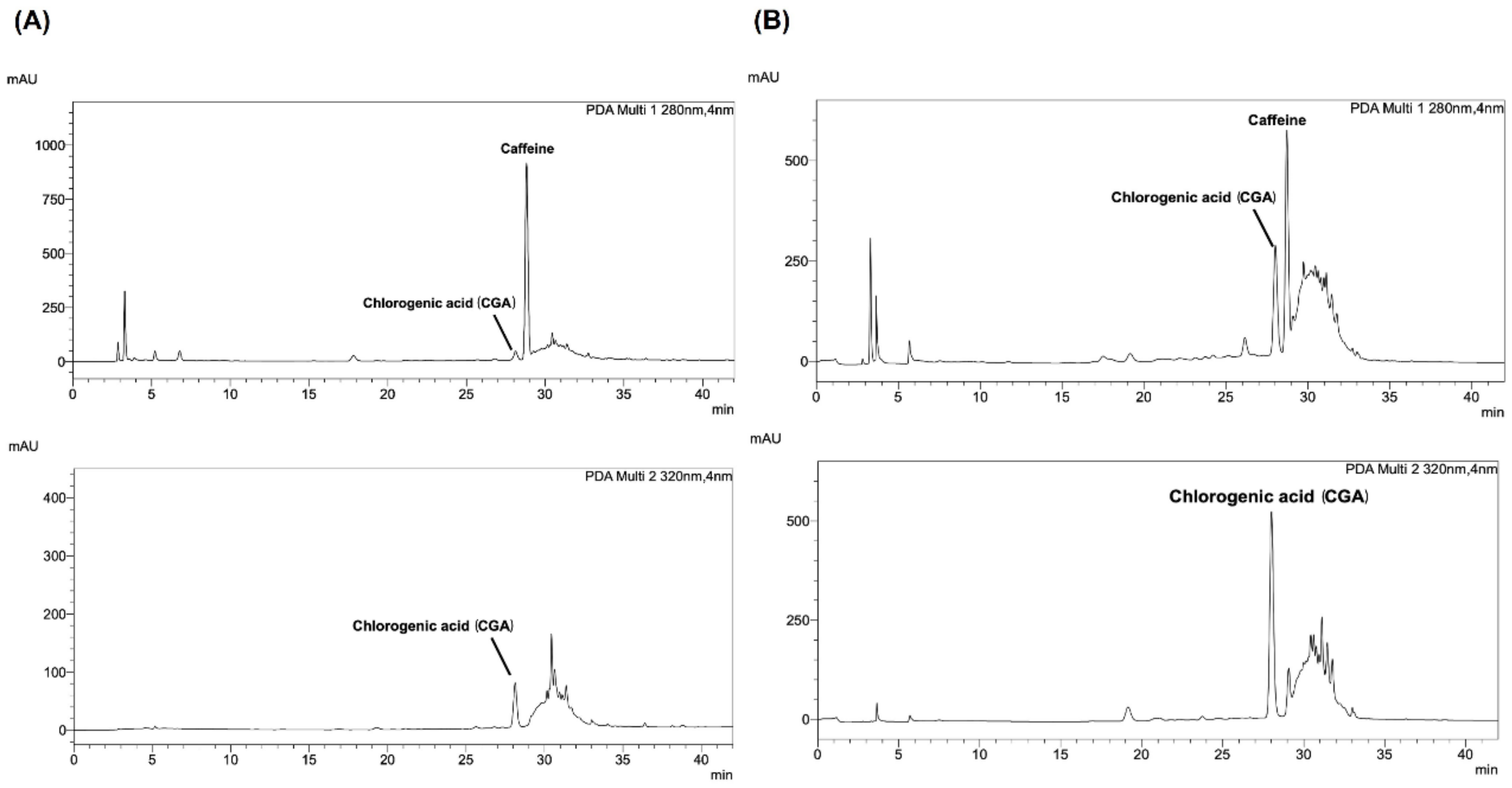
2.4. Determination of High-Performance Liquid Chromatography (HPLC) Analysis
2.5. Determination of Lipid Digestion and Absorption In Vitro
2.5.1. Determination of Pancreatic Lipase Inhibition
2.5.2. Determination of Cholesterol Micelles’ Solubility
2.5.3. Determination of Bile Acid Binding
2.6. Cholesterol Uptake in Caco-2 Cells
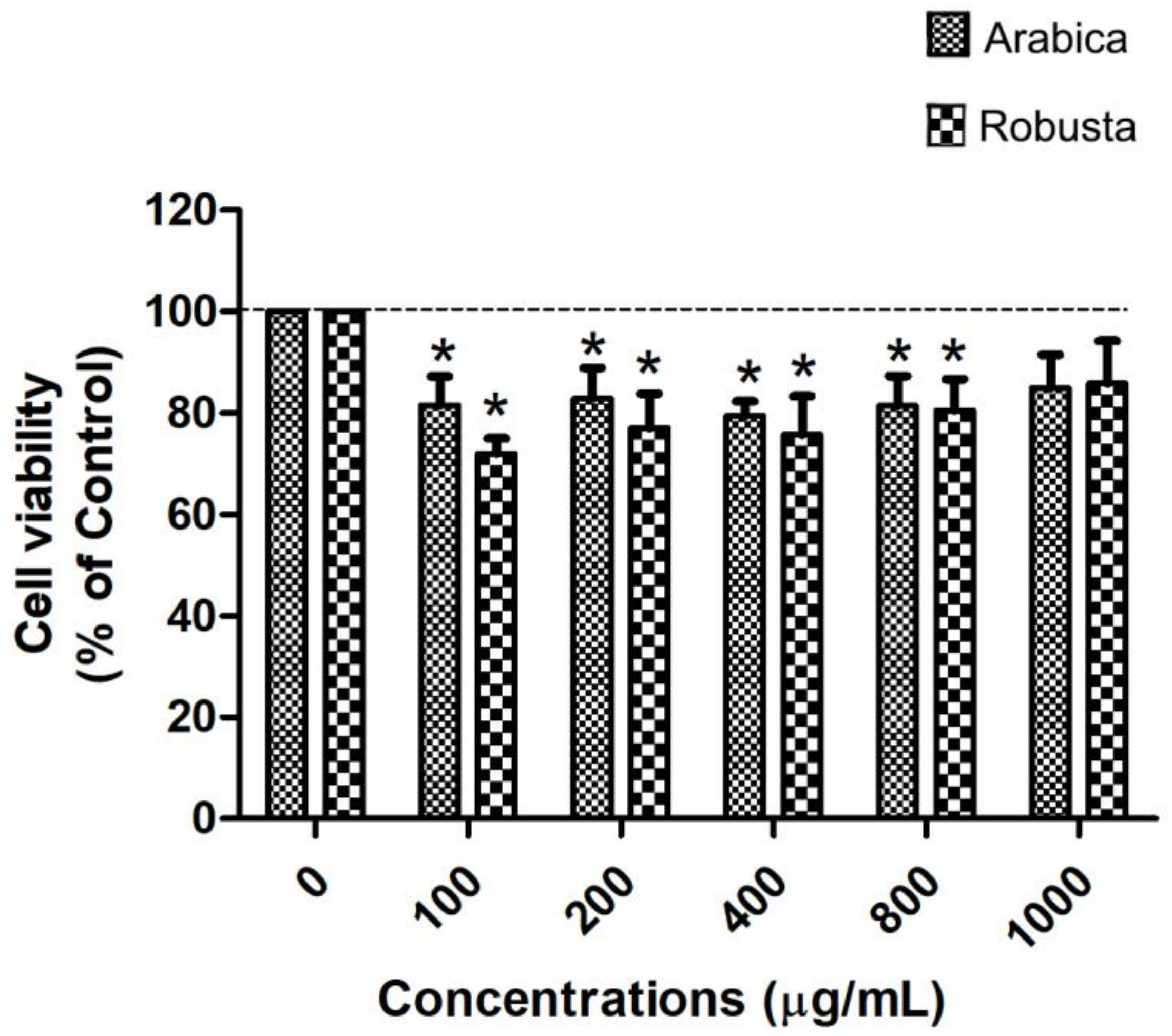
2.6.1. Determination of Cell Viability
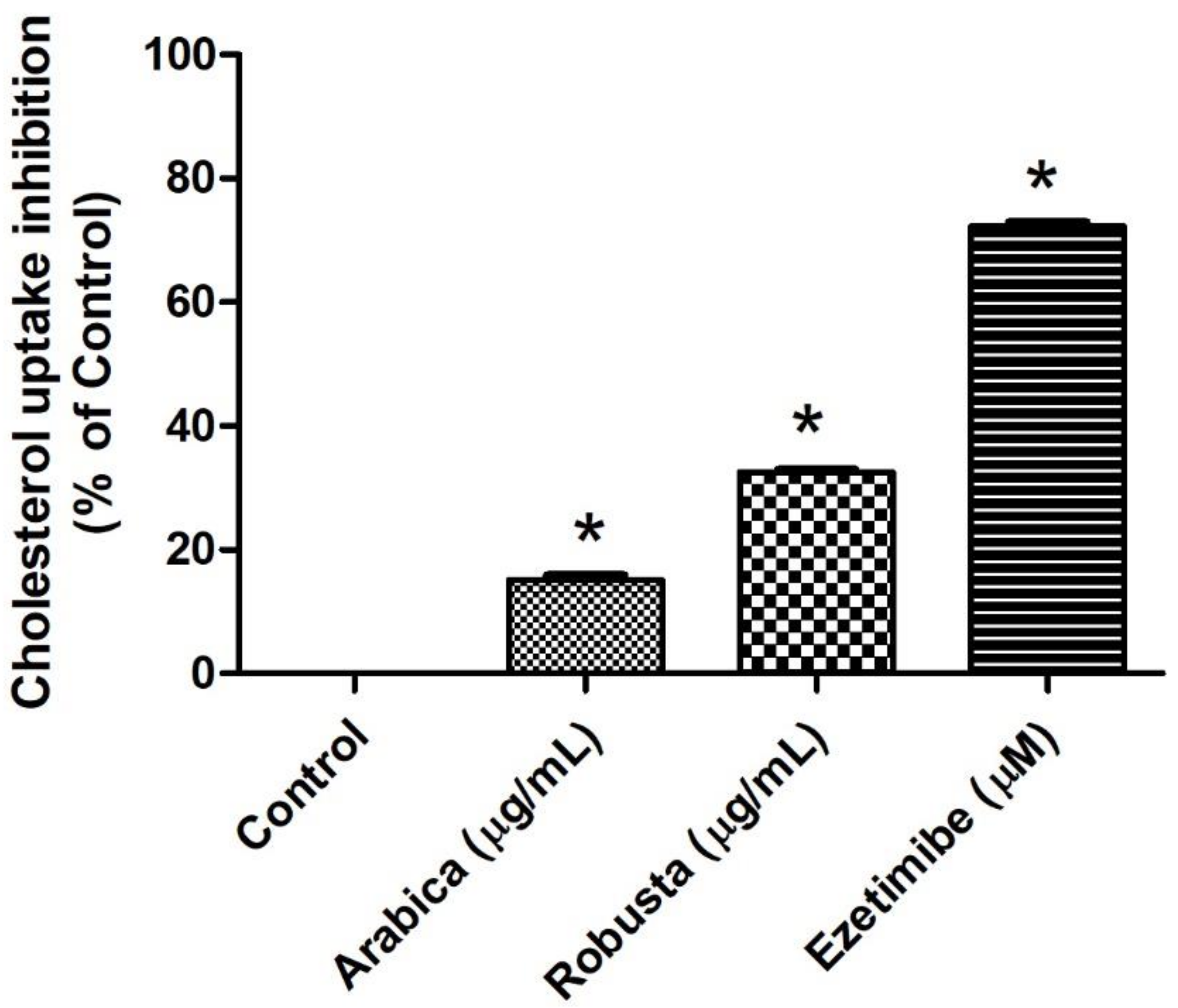
2.6.2. Determination of Cholesterol Uptake in Caco-2 Cells
2.7. Molecular Docking
2.8. Statistical Analyses
3. Results and Discussion
3.1. Nutrition Components and Bioactive Compounds in Coffee Leaf Extract
3.2. Arabica and Robusta Coffee Leaf Extracts Exhibited Inhibitory Effects on Lipase Activity
3.3. The Effect of Arabica and Robusta Coffee Leaf Extracts on the Micellar Solubility of Cholesterol and Bile Acid Binding
3.4. Arabica and Robusta Coffee Leaf Extracts Inhibited Cholesterol Uptake in Caco-2 Cells
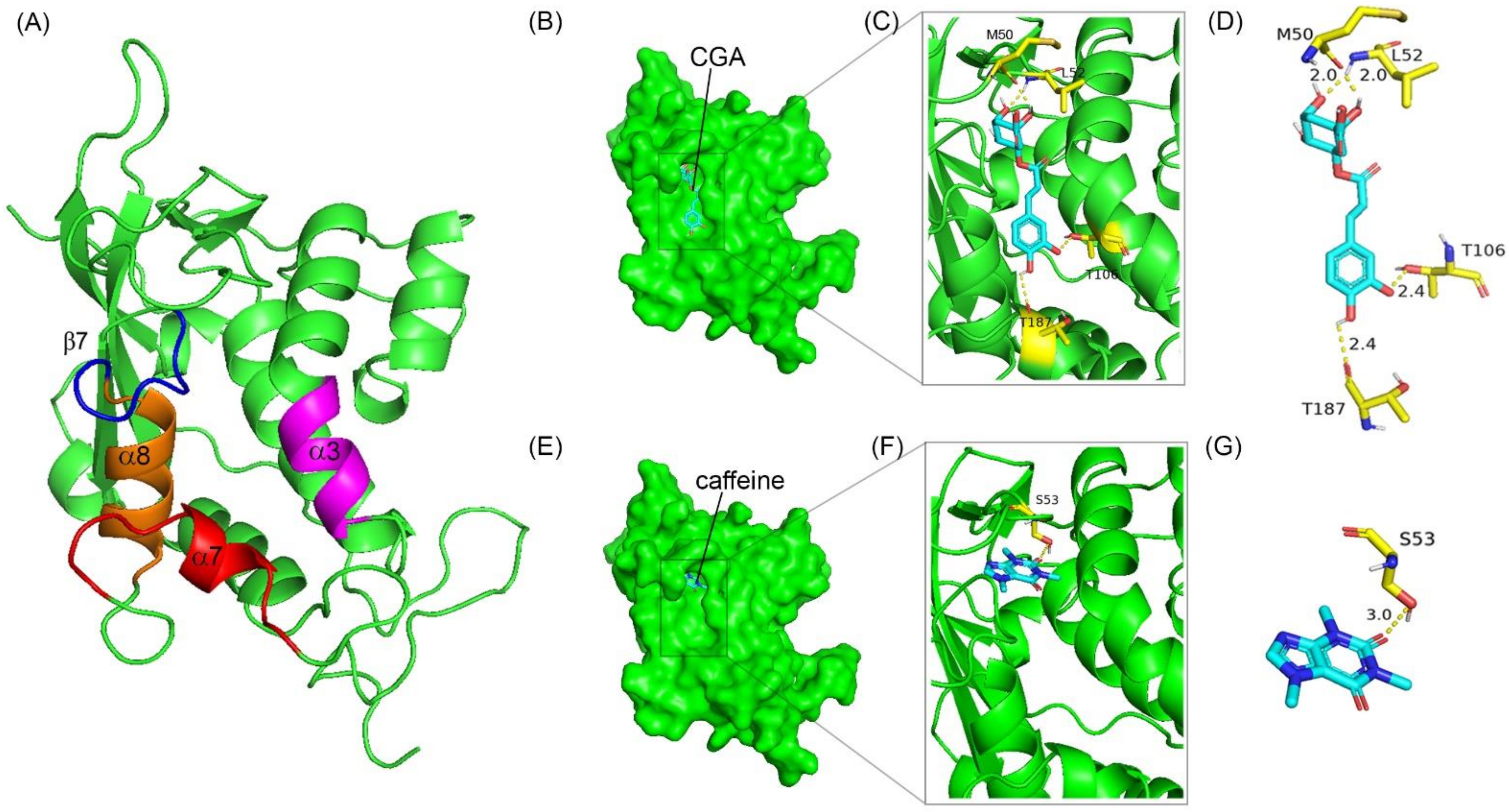
3.5. CGA and Caffeine Can Bind to Niemann–Pick C1-Like 1 (NPC1L1) at the Cholesterol Binding Pocket
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kruit, J.K.; Groen, A.K.; van Berkel, T.J.; Kuipers, F. Emerging roles of the intestine in control of cholesterol metabolism. World J. Gastroenterol. 2006, 12, 6429–6439. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Miettinen, T.A. The effect of cholesterol absorption inhibition on low density lipoprotein cholesterol level. Atherosclerosis 1995, 117, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.A. Medicinal Plants of the World, Volume 3: Chemical Constituents, Traditional and Modern Medicinal Uses; Springer Science & Business Media: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Campa, C.; Mondolot, L.; Rakotondravao, A.; Bidel, L.P.; Gargadennec, A.; Couturon, E.; LaFisca, P.; Rakotomalala, J.J.; Jay-Allemand, C.; Davis, A.P. A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: Biological implications and uses. Ann. Bot. 2012, 110, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.P.D.; Shimizu, M.M.; Moura, J.C.; Catharino, R.R.; Ramos, R.A.; Ribeiro, R.V.; Mazzafera, P. Looking for the physiological role of anthocyanins in the leaves of Coffea arabica. Photochem. Photobiol. 2012, 88, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Patay, E.; Nemeth, T.; Nemeth, T.S.; Filep, R.; Vlase, L.; Papp, N. Histological and phytochemical studies of Coffea benghalensis B. Heyne Ex Schult. compared with Coffea arabica L. Farmacia 2016, 64, 125–130. [Google Scholar]
- Rusinek, R.; Dobrzański, B., Jr.; Gawrysiak-Witulska, M.; Siger, A.; Żytek, A.; Karami, H.; Umar, A.; Lipa, T.; Gancarz, M. Effect of the roasting level on the content of bioactive and aromatic compounds in Arabica coffee beans. Int. Agrophys. 2024, 38, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Das, M.; Kumar, G.S.; Murthy, P.S. Coffee leaf extract exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced C57BL6 obese mice. 3 Biotech 2023, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.H.S.; Salles, B.C.C.; Duarte, S.M.d.S.; da Silva, M.A.; Viana, A.L.M.; Moraes, G.d.O.I.d.; Figueiredo, S.A.; Ferreira, E.B.; Rodrigues, M.R.; Paula, F.B.d.A. Leaf extract of Coffea arabica L. reduces lipid peroxidation and has anti-platelet effect in a rat dyslipidemia model. Braz. J. Pharm. Sci. 2022, 58, e19562. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Muanprasat, C.; Pasachan, T.; Pongchaidecha, A.; Amornlerdpison, D.; Srimaroeng, C. Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine 2019, 52, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Park, C.S.; Park, Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci. Biotechnol. 2019, 28, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.X.; Lyu, J.L.; Wu, P.Y.; Wen, K.C.; Chang, C.C.; Chiang, H.M. Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions. Int. J. Mol. Sci. 2023, 24, 12367. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.L.; Nascimento, G.O.; Lopes, G.S.; Matos, W.O.; Cunha, R.L.; Malta, M.R.; Liska, G.R.; Owen, R.W.; Trevisan, M.T.S. The concentration of polyphenolic compounds and trace elements in the Coffea arabica leaves: Potential chemometric pattern recognition of coffee leaf rust resistance. Food Res. Int. 2020, 134, 109221. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International; AOAC International: Rockville, MA, USA, 1990. [Google Scholar]
- Aubry, S.; Aubert, G.; Cresteil, T.; Crich, D. Synthesis and biological investigation of the β-thiolactone and β-lactam analogs of tetrahydrolipstatin. Org. Biomol. Chem. 2012, 10, 2629–2632. [Google Scholar] [CrossRef] [PubMed]
- Hadvary, P.; Sidler, W.; Meister, W.; Vetter, W.; Wolfer, H. The lipase inhibitor tetrahydrolipstatin binds covalently to the putative active site serine of pancreatic lipase. J. Biol. Chem. 1991, 266, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Kirana, C.; Rogers, P.F.; Bennett, L.E.; Abeywardena, M.Y.; Patten, G.S. Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactives. J. Agric. Food Chem. 2005, 53, 4623–4627. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Wäsche, A. In vitro binding of bile acids by lupin protein isolates and their hydrolysates. Food Chem. 2004, 88, 179–184. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Montis, A.; Souard, F.; Delporte, C.; Stoffelen, P.; Stevigny, C.; Van Antwerpen, P. Coffee Leaves: An Upcoming Novel Food? Planta Med. 2021, 87, 949–963. [Google Scholar] [CrossRef]
- Monteiro, A.; Colomban, S.; Azinheira, H.G.; Guerra-Guimaraes, L.; Do Ceu Silva, M.; Navarini, L.; Resmini, M. Dietary Antioxidants in Coffee Leaves: Impact of Botanical Origin and Maturity on Chlorogenic Acids and Xanthones. Antioxidants 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cangeloni, L.; Bonechi, C.; Leone, G.; Consumi, M.; Andreassi, M.; Magnani, A.; Rossi, C.; Tamasi, G. Characterization of Extracts of Coffee Leaves (Coffea arabica L.) by Spectroscopic and Chromatographic/Spectrometric Techniques. Foods 2022, 11, 2495. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.H.; Kim, D.W.; Shin, M.J.; Kwon, S.W.; Kim, Y.N.; Kim, D.S.; Lim, S.S.; Kim, J.; Park, J.; Eum, W.S.; et al. Chlorogenic Acid Improves Neuroprotective Effect of PEP-1-Ribosomal Protein S3 Against Ischemic Insult. Exp. Neurobiol. 2011, 20, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Chan, S.Y.; Puddey, I.B.; Devine, A.; Wattanapenpaiboon, N.; Wahlqvist, M.L.; Lukito, W.; Burke, V.; Ward, N.C.; Prince, R.L.; et al. Phenolic acid metabolites as biomarkers for tea- and coffee-derived polyphenol exposure in human subjects. Br. J. Nutr. 2004, 91, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.S.; Donovan, J.L.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Fruit hydroxycinnamic acids inhibit human low-density lipoprotein oxidation in vitro. J. Agric. Food Chem. 1998, 46, 1783–1787. [Google Scholar] [CrossRef]
- Rodriguez de Sotillo, D.V.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef] [PubMed]
- McClendon, K.S.; Riche, D.M.; Uwaifo, G.I. Orlistat: Current status in clinical therapeutics. Expert Opin. Drug Saf. 2009, 8, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Yuniarti, E.; Saputri, F.C.; Mun‘Im, A. Natural Deep Eutectic Solvent Extraction and Evaluation of Caffeine and Chlorogenic Acid from Green Coffee Beans of Coffea canephora. Indian J. Pharm. Sci. 2019, 81, 1062–1069. [Google Scholar] [CrossRef]
- Hu, B.; Cui, F.; Yin, F.; Zeng, X.; Sun, Y.; Li, Y. Caffeoylquinic acids competitively inhibit pancreatic lipase through binding to the catalytic triad. Int. J. Biol. Macromol. 2015, 80, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Wikiera, A.; Mika, M.; Zyla, K. Methylxanthine drugs are human pancreatic lipase inhibitors. Pol. J. Food Nutr. Sci. 2012, 62, 109–113. [Google Scholar] [CrossRef][Green Version]
- Insull, W., Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: A scientific review. South. Med. J. 2006, 99, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Nutescu, E.A.; Shapiro, N.L. Ezetimibe: A selective cholesterol absorption inhibitor. Pharmacotherapy 2003, 23, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Ge, L.; Qi, W.; Zhang, L.; Miao, H.H.; Li, B.L.; Yang, M.; Song, B.L. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. J. Biol. Chem. 2011, 286, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Palnitkar, M.; Deisenhofer, J. The structure of the NPC1L1 N-terminal domain in a closed conformation. PLoS ONE 2011, 6, e18722. [Google Scholar] [CrossRef] [PubMed]
- Nihei, W.; Nagafuku, M.; Hayamizu, H.; Odagiri, Y.; Tamura, Y.; Kikuchi, Y.; Veillon, L.; Kanoh, H.; Inamori, K.; Arai, K.; et al. NPC1L1-dependent intestinal cholesterol absorption requires ganglioside GM3 in membrane microdomains. J. Lipid Res. 2018, 59, 2181–2187. [Google Scholar] [CrossRef]
- Feng, D.; Ohlsson, L.; Duan, R.D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis. 2010, 9, 40. [Google Scholar] [CrossRef]
- Betters, J.L.; Yu, L. NPC1L1 and cholesterol transport. FEBS Lett. 2010, 584, 2740–2747. [Google Scholar] [CrossRef]
- Davies, J.P.; Scott, C.; Oishi, K.; Liapis, A.; Ioannou, Y.A. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 2005, 280, 12710–12720. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.J.; Gupta, E.K.; Ito, M.K. Acute coffee ingestion does not affect LDL cholesterol level. Ann. Pharmacother. 2005, 39, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Romańczyk, M.; Zembala, M.O.; Filipiak, K.J. Coffee and lipid profile: From theory to everyday practice. Folia Cardiol. 2023, 18, 24–30. [Google Scholar] [CrossRef]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L.M.; Van Gent, T.; Buytenhek, R.; Princen, H.M.G.; Katan, M.B. Consumption of French-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J. Intern. Med. 2000, 248, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Meyboom, S.; Kuilman, M.; Rexwinkel, H.; Vissers, M.N.; Klerk, M.; Katan, M.B. Comparison of effect of cafetiere and filtered coffee on serum concentrations of liver aminotransferases and lipids: Six month randomised controlled trial. BMJ 1996, 313, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Weusten-Van der Wouw, M.P.; Katan, M.B.; Viani, R.; Huggett, A.C.; Liardon, R.; Liardon, R.; Thelle, D.S.; Ahola, I.; Aro, A. Identity of the cholesterol-raising factor from boiled coffee and its effects on liver function enzymes. J. Lipid Res. 1994, 35, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.; Ranheim, T.; Nenseter, M.S.; Huggett, A.C.; Drevon, C.A. Effect of a coffee lipid (cafestol) on cholesterol metabolism in human skin fibroblasts. J. Lipid Res. 1998, 39, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Rustan, A.C.; Halvorsen, B.; Huggett, A.C.; Ranheim, T.; Drevon, C.A. Effect of coffee lipids (cafestol and kahweol) on regulation of cholesterol metabolism in HepG2 cells. Arter. Thromb. Vasc. Biol. 1997, 17, 2140–2149. [Google Scholar] [CrossRef]
- Ranheim, T.; Halvorsen, B.; Huggett, A.C.; Blomhoff, R.; Drevon, C.A. Effect of a coffee lipid (cafestol) on regulation of lipid metabolism in CaCo-2 cells. J. Lipid Res. 1995, 36, 2079–2089. [Google Scholar] [CrossRef] [PubMed]

| Leaf | %Moisture | %Dry Matter | %Fiber | %Ash | %Fat | %Protein | %NFE | Energy/100 g (Kcal) |
|---|---|---|---|---|---|---|---|---|
| Arabica | 7.20 ± 0.85 | 99.28 ± 0.01 | 85.63 ± 0.25 | 5.59 ± 0.03 | 1.53 ± 0.04 | 17.73 ± 0.23 * | 18.03 ± 0.74 * | 425.70 ± 2.26 |
| Robusta | 7.38 ± 0.05 | 99.10 ± 0.06 | 85.64 ± 0.03 | 6.99 ± 0.17 * | 1.22 ± 0.04 | 14.33 ± 0.15 | 16.15 ± 0.40 | 425.80 ± 4.38 |
| Leaf | Chlorogenic Acid (mg/g Extract) | RT (min) | Caffeine (mg/g Extract) | RT (min) |
|---|---|---|---|---|
| Arabica | 2.91 ± 0.22 | 27.997 | 19.82 ± 0.75 | 27.989 |
| Robusta | 18.15 ± 0.91 | 27.970 | 14.90 ± 0.76 | 28.679 |
| Substance | Bile Acid Binding (IC50) (µg/mL) | Micellar Solubility Inhibition (IC50) (µg/mL) | ||
|---|---|---|---|---|
| TC | TD | GC | ||
| Arabica coffee leaf extract | 1804.00 ± 1.26 | 4905.00 ± 3.10 | 998.00 ± 1.07 | 2527.00 ± 1.09 |
| Robusta coffee leaf extract | 2215.00 ± 1.20 | 4760.00 ± 2.71 | 828.70 ± 1.08 | 2542.00 ± 1.18 |
| Cholestyramine | 1050.00 ± 1.08 | 642.40 ± 1.12 | 1039.00 ± 1.09 | 602.90 ± 1.07 |
| No. | Protein | Ligand | Binding Energy (kcal/mol) | No of H-Bonds | Amino Acid Involved in Interaction | Intermolecular H-Bonding Distance (Å) |
|---|---|---|---|---|---|---|
| 1 | NPC1L1-NTD | Chlorogenic acid (CGA) | −6.1 | 4 | MET50 LEU52 THR106 THR187 | 2.0 2.0 2.4 2.4 |
| 2 | NPC1L1-NTD | Caffeine | −5.1 | 1 | SER 53 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansri, V.; Sroyraya, M.; Phisalprapa, P.; Yosboonruang, A.; Ontawong, A.; Saokaew, S.; Goh, B.-H.; Trisat, K.; Phewchan, P.; Rawangkan, A.; et al. Suppressive Effect of Coffee Leaves on Lipid Digestion and Absorption In Vitro. Foods 2024, 13, 2445. https://doi.org/10.3390/foods13152445
Sansri V, Sroyraya M, Phisalprapa P, Yosboonruang A, Ontawong A, Saokaew S, Goh B-H, Trisat K, Phewchan P, Rawangkan A, et al. Suppressive Effect of Coffee Leaves on Lipid Digestion and Absorption In Vitro. Foods. 2024; 13(15):2445. https://doi.org/10.3390/foods13152445
Chicago/Turabian StyleSansri, Veerawat, Morakot Sroyraya, Pochamana Phisalprapa, Atchariya Yosboonruang, Atcharaporn Ontawong, Surasak Saokaew, Bey-Hing Goh, Kanittaporn Trisat, Premchirakorn Phewchan, Anchalee Rawangkan, and et al. 2024. "Suppressive Effect of Coffee Leaves on Lipid Digestion and Absorption In Vitro" Foods 13, no. 15: 2445. https://doi.org/10.3390/foods13152445
APA StyleSansri, V., Sroyraya, M., Phisalprapa, P., Yosboonruang, A., Ontawong, A., Saokaew, S., Goh, B.-H., Trisat, K., Phewchan, P., Rawangkan, A., Limpeanchob, N., & Duangjai, A. (2024). Suppressive Effect of Coffee Leaves on Lipid Digestion and Absorption In Vitro. Foods, 13(15), 2445. https://doi.org/10.3390/foods13152445









