Fermentation Performances and Aroma Contributions of Selected Non-Saccharomyces Yeasts for Cherry Wine Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Must Preparation for Fermentation Assays
2.3. Fermentation Trials, Strain Inoculation and Microbial Analysis
2.4. Microbial Counts
2.5. Chemical Analysis of Must and Wine
2.6. Statistical Analysis
3. Results
3.1. Preliminary Screening on Fermentation Capacity
3.2. Fermentation of Cherry Must
3.3. Chemical Analysis of Wines
3.4. Statistical Analysis of Wines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dziadek, K.; Kopeć, A.; Tabaszewska, M. Potential of Sweet Cherry (Prunus avium L.) by-Products: Bioactive Compounds and Antioxidant Activity of Leaves and Petioles. Eur. Food Res. Technol. 2019, 245, 763–772. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Production of a Potentially Synbiotic Fermented Cornelian Cherry (Cornus mas L.) Beverage Using Lactobacillus paracasei K5 Immobilized on Wheat Bran. Biocatal. Agric. Biotechnol. 2019, 17, 347–351. [Google Scholar] [CrossRef]
- Merlino, V.M.; Fracassetti, D.; Di Canito, A.; Pizzi, S.; Borra, D.; Giuggioli, N.R.; Vigentini, I. Is the Consumer Ready for Innovative Fruit Wines? Perception and Acceptability of Young Consumers. Foods 2021, 10, 1545. [Google Scholar] [CrossRef]
- UNEP. United Nations Environment Programme: Food Waste Index Report 2021; UNEP: Nairobi, Kenya, 2021. [Google Scholar]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could Non-Saccharomyces Yeasts Contribute on Innovative Brewing Fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Bellut, K.; Krogerus, K.; Arendt, E.K. Lachancea fermentati Strains Isolated From Kombucha: Fundamental Insights, and Practical Application in Low Alcohol Beer Brewing. Front. Microbiol. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Capece, A.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Garrigós, V.; Matallana, E.; Aranda, A. Impact of Starmerella bacillaris and Zygosaccharomyces bailii on Ethanol Reduction 1 and Saccharomyces cerevisiae Metabolism during Mixed Wine Fermentations. bioRxiv 2021. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Loira, I.; Escott, C.; Carrau, F.; González, C.; Cuerda, R.; Morata, A. Application of Hanseniaspora vineae Yeast in the Production of Rosé Wines from a Blend of Tempranillo and Albillo Grapes. Fermentation 2021, 7, 141. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Li, P.; Gu, Q.; Yang, B. Use of Non-Saccharomyces Yeasts in Berry Wine Production: Inspiration from Their Applications in Winemaking. J. Agric. Food Chem. 2022, 70, 736–750. [Google Scholar] [CrossRef]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Evaluation of Different Saccharomyces cerevisiae Strains on the Profile of Volatile Compounds and Polyphenols in Cherry Wines. Food Chem. 2011, 127, 547–555. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae on Alcoholic Fermentation Behaviour and Wine Aroma of Cherry Wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Gong, H.S.; Zhao, Y.P.; Liu, W.L.; Jin, C.W. Sequential Culture with Torulaspora delbrueckii and Saccharomyces cerevisiae and Management of Fermentation Temperature to Improve Cherry Wine Quality. J. Sci. Food Agric. 2016, 96, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Zapparoli, G. Yeast-like Fungi and Yeasts in Withered Grape Carposphere: Characterization of Aureobasidium pullulans Population and Species Diversity. Int. J. Food Microbiol. 2019, 289, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of Yeasts for Apple Juice Fermentation and Production of Cider Volatile Compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Delfini, C.; Formica, J.V. Wine Microbiology; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9781482294644. [Google Scholar]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Granchi, L.; Ganucci, D.; Messini, A.; Vincenzini, M. Oenological Properties of Hanseniaspora osmophila and Kloeckera corticis from Wines Produced by Spontaneous Fermentations of Normal and Dried Grapes. FEMS Yeast Res. 2002, 2, 403–407. [Google Scholar] [PubMed]
- Guerrini, S.; Galli, V.; Barbato, D.; Facchini, G.; Mangani, S.; Pierguidi, L.; Granchi, L. Effects of Saccharomyces cerevisiae and Starmerella bacillaris on the Physicochemical and Sensory Characteristics of Sparkling Pear Cider (Perry). Eur. Food Res. Technol. 2023, 249, 341–352. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Investigating the Biochemical and Fermentation Attributes of Lachancea Species and Strains: Deciphering the Potential Contribution to Wine Chemical Composition. Int. J. Food Microbiol. 2019, 290, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhou, X.; Xiao, Z.; Niu, Y. Characterization of the Differences in the Aroma of Cherry Wines from Different Price Segments Using Gas Chromatography–Mass Spectrometry, Odor Activity Values, Sensory Analysis, and Aroma Reconstitution. Food Sci. Biotechnol. 2017, 26, 331–338. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the Perceptual Interaction among Ester Aroma Compounds in Cherry Wines by GC–MS, GC–O, Odor Threshold and Sensory Analysis: An Insight at the Molecular Level. Food Chem. 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of Non-Saccharomyces Yeasts to Be Used in Grape Musts with High Alcoholic Potential: A Strategy to Obtain Wines with Reduced Ethanol Content. FEMS Yeast Res. 2017, 17, fox010. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Baran, Y.; Navascués, E.; Santos, A.; Calderón, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological Management of Acidity in Wine Industry: A Review. Int. J. Food Microbiol. 2022, 375, 109726. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Guo, C.; Yan, Y.; Sun, X.; Ma, T.; Zhang, J.; Li, C.; Gou, C.; Yue, T.; Yuan, Y. Contribution of Non-Saccharomyces Yeasts to Aroma-Active Compound Production, Phenolic Composition and Sensory Profile in Chinese Vidal Icewine. Food Biosci. 2022, 46, 101152. [Google Scholar] [CrossRef]
- Zhou, R.; Song, Q.; Xia, H.; Song, N.; Yang, Q.; Zhang, X.; Yao, L.; Yang, S.; Dai, J.; Chen, X. Isolation and Identification of Non-Saccharomyces Yeast Producing 2-Phenylethanol and Study of the Ehrlich Pathway and Shikimate Pathway. J. Fungi 2023, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.J.; Olivera, V.; Boido, E.; Dellacassa, E.; Carrau, F. Wine Aroma Characterization of the Two Main Fermentation Yeast Species of the Apiculate Genus Hanseniaspora. Fermentation 2021, 7, 162. [Google Scholar] [CrossRef]
- Giorello, F.; Valera, M.J.; Martin, V.; Parada, A.; Salzman, V.; Camesasca, L.; Fariña, L.; Boido, E.; Medina, K.; Dellacassa, E.; et al. Genomic and Transcriptomic Basis of Hanseniaspora vineae’s Impact on Flavor Diversity and Wine Quality. Appl. Environ. Microbiol. 2019, 85, e01959-18. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, J.; Martín, V.; Portillo, M.d.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Tondini, F.; Lang, T.; Chen, L.; Herderich, M.; Jiranek, V. Linking Gene Expression and Oenological Traits: Comparison between Torulaspora delbrueckii and Saccharomyces cerevisiae Strains. Int. J. Food Microbiol. 2019, 294, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy Sulphur Compounds, Higher Alcohols and Esters Production Profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii Grown as Pure and Mixed Cultures in Grape Must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef]
- Mukai, N.; Masaki, K.; Fujii, T.; Iefuji, H. Single Nucleotide Polymorphisms of PAD1 and FDC1 Show a Positive Relationship with Ferulic Acid Decarboxylation Ability among Industrial Yeasts Used in Alcoholic Beverage Production. J. Biosci. Bioeng. 2014, 118, 50–55. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Eric, K.; Jia, C.; Yu, H.; Zhu, J. Characterization of Odor-Active Compounds of Various Cherry Wines by Gas Chromatography-Mass Spectrometry, Gas Chromatography-Olfactometry and Their Correlation with Sensory Attributes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, C.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Marchante-Cuevas, L.; Heras, J.M.; Morata, A. Use of Lachancea thermotolerans for Biological vs. Chemical Acidification at Pilot-Scale in White Wines from Warm Areas. Fermentation 2021, 7, 193. [Google Scholar] [CrossRef]
- Russo, P.; Tufariello, M.; Renna, R.; Tristezza, M.; Taurino, M.; Palombi, L.; Capozzi, V.; Rizzello, C.G.; Grieco, F. New Insights into the Oenological Significance of Candida zemplinina: Impact of Selected Autochthonous Strains on the Volatile Profile of Apulian Wines. Microorganisms 2020, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Pati, S.; Palombi, L.; Grieco, F.; Losito, I. Use of Multivariate Statistics in the Processing of Data on Wine Volatile Compounds Obtained by HS-SPME-GC-MS. Foods 2022, 11, 910. [Google Scholar] [CrossRef] [PubMed]
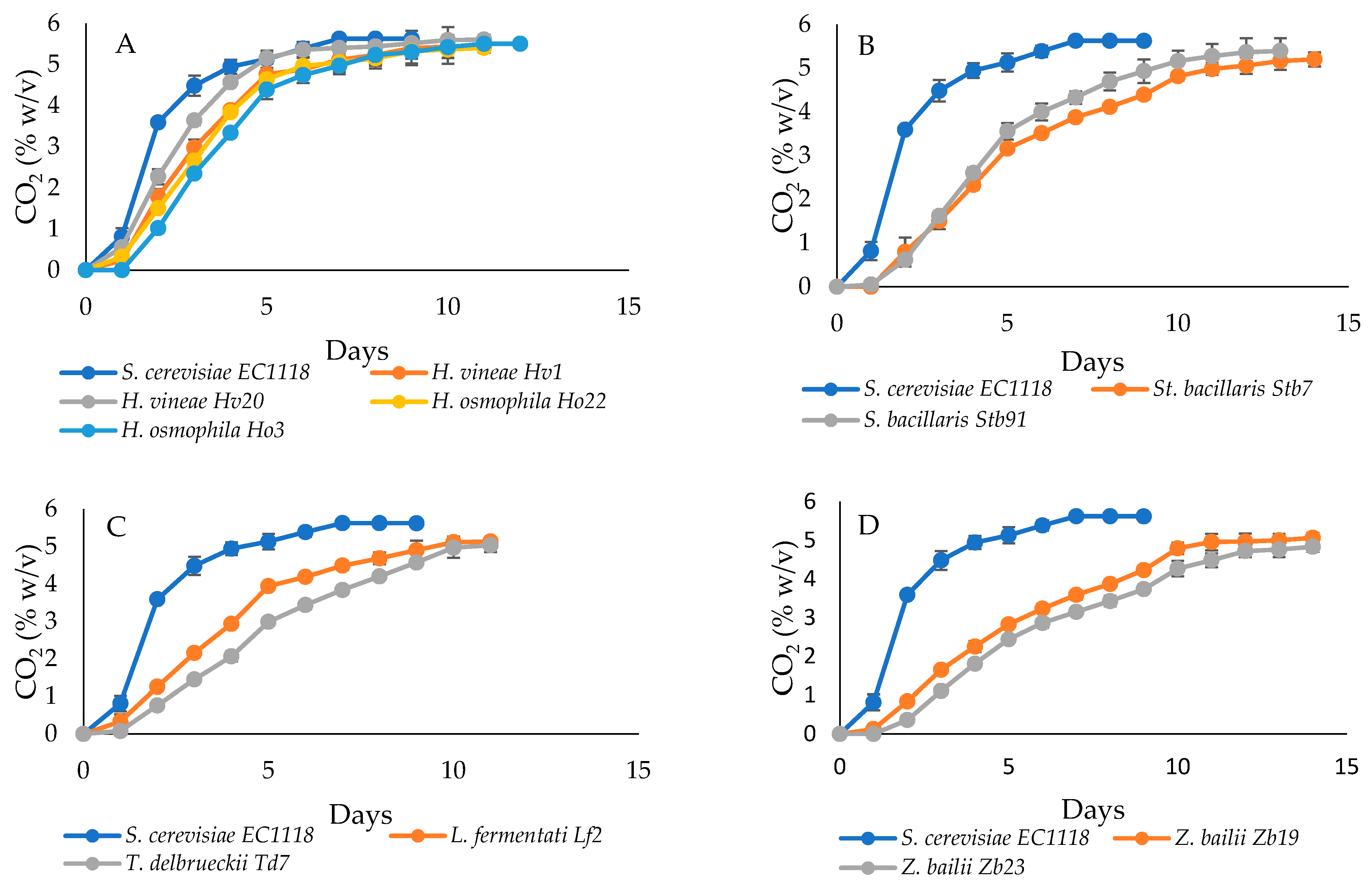

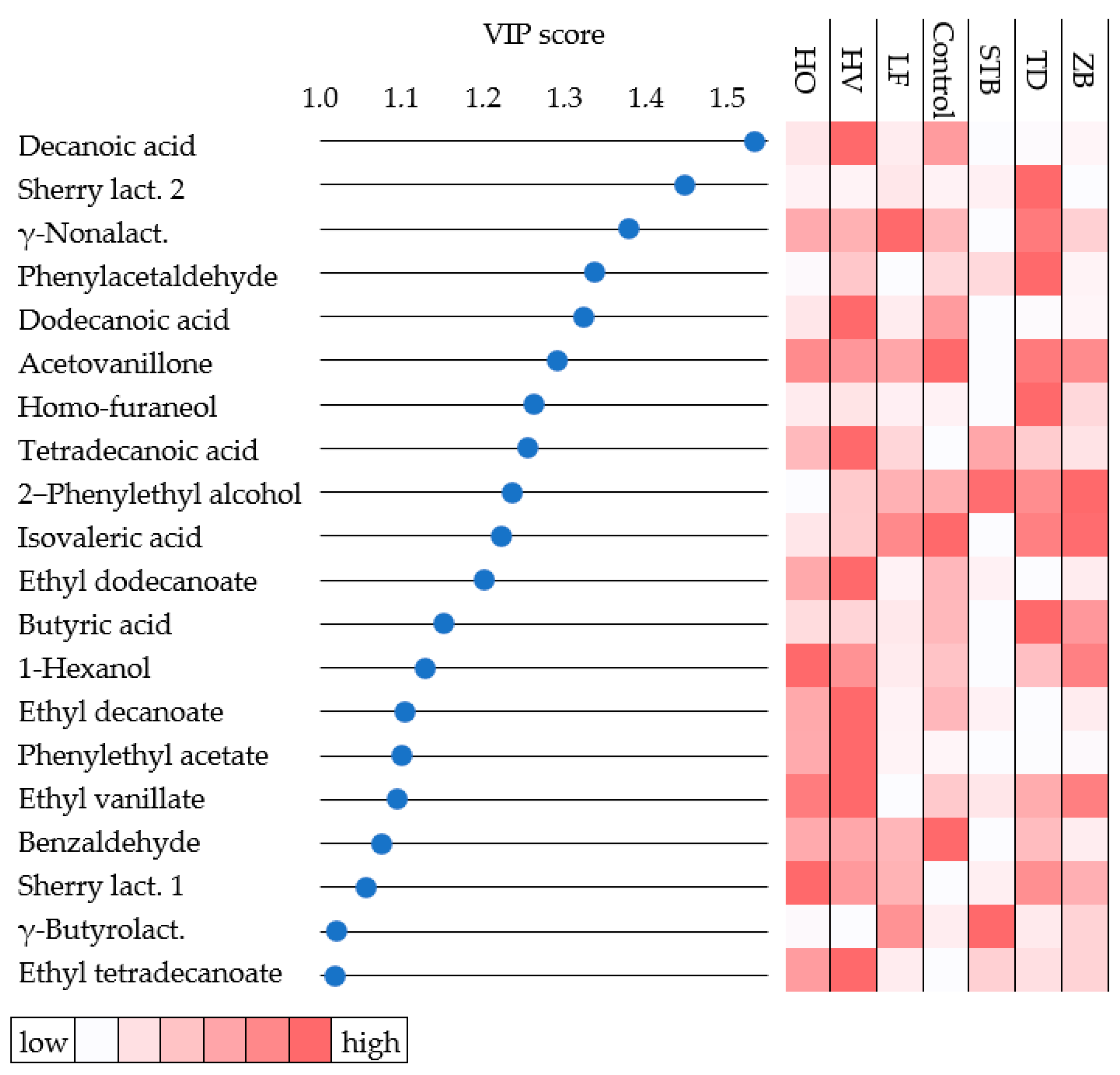
| Strains | Total Amount | Amount after 3 d | Average Daily Rate | Maximum Daily Rate |
|---|---|---|---|---|
| S. cerevisiae EC1118® | 7.6 ± 0.2 | 6.8 ± 0 | 0.4 ± 0 | 3.3 ± 0.1 |
| St. bacillaris Stb7 | 7.6 ± 0.1 | 0.9 ± 0.1 ** | 0.4 ± 0.0 | 1.2 ± 0.1 ** |
| H. osmophila Ho22 | 7.5 ± 0.2 | 0 ± 0 ** | 0.4 ± 0.0 | 1.3 ± 0.1 ** |
| St. bacillaris Stb142 | 7.5 ± 0.2 | 0 ± 0 ** | 0.3 ± 0 | 1.2 ± 0.1 ** |
| St. bacillaris Stb3 | 7.5 ± 0 | 0 ± 0 ** | 0.3 ± 0 | 1.3 ± 0 ** |
| H. vineae Hv1 | 7.4 ± 0 | 3.7 ± 0.2 ** | 0.4 ± 0 | 2.3 ± 0.1 ** |
| H. vineae Hv20 | 7.3 ± 0.2 | 1.7 ± 0 ** | 0.4 ± 0.2 | 1.6 ± 0.1 ** |
| St. bacillaris Stb91 | 7.3 ± 0.1 | 2.1 ± 0 ** | 0.4 ± 0 | 1.5 ± 0.1 ** |
| T. delbrueckii Td7 | 7.3 ± 0.1 | 0.0 ± 0 ** | 0.3 ± 0 | 1.2 ± 0 ** |
| Z. bailii Zb19 | 7.3 ± 0.1 | 1.8 ± 0 ** | 0.3 ± 0 | 1.2 ±0.1 ** |
| Z. bailii Zb23 | 7.3 ± 0 | 0.9 ± 0.1 ** | 0.3 ± 0 | 0.8 ± 0.1 ** |
| H. osmophila Ho3 | 7.2 ± 0.1 * | 1.7 ± 0.1 ** | 0.4 ± 0.1 | 1.3 ± 0 ** |
| H. vineae Hv45 | 7.1 ± 0 * | 0.1 ± 0 ** | 0.4 ± 0 | 1.9 ± 0.1 ** |
| St. bacillaris Stb34 | 7.1 ± 0.1 * | 0 ± 0 ** | 0.3 ± 0 | 1.5 ± 0.1 ** |
| Z. bailii Zb17 | 7.1 ± 0.1 * | 1.2 ± 0.2 ** | 0.3 ± 0 | 1.0 ± 0.1 ** |
| L. fermentati Lf2 | 6.7 ± 0.1 ** | 2.6 ± 0.1 ** | 0.3 ± 0 | 1.5 ± 0.1 ** |
| L. lanzarotensis Ll5 | 6.0 ± 0.2** | 0.1 ± 0.1 ** | 0.3 ± 0.1 | 1.2 ± 0.1 ** |
| H. pseudoguilliermondii YR6 | 5.8 ± 0.2 ** | 0 ± 0 ** | 0.3 ± 0 * | 0.9 ± 0.1 ** |
| H. valbiensis Y2D | 5.6 ± 0.1 ** | 0.1 ± 0 ** | 0.3 ± 0 | 1.1 ± 0 ** |
| L. lanzarotensis Ll7 | 5.4 ± 0.1 ** | 2.6 ± 0.1 ** | 0.4 ± 0 | 1.5 ± 0.2 ** |
| W. anomalus Wa847 | 5.0 ± 0.2 ** | 0.1 ± 0.1 ** | 0.2 ± 0.1 | 0.4 ± 0 ** |
| H. meyeri Y1A | 3.9 ± 0.1 ** | 1.4 ± 0 ** | 0.2 ± 0 * | 1.0 ± 0.2 ** |
| RI 1 | ID 2 | Control | TD | HV | HO | LF | STB | ZB | |
|---|---|---|---|---|---|---|---|---|---|
| Primary compounds | |||||||||
| pH | 3.7 ± 0.0 cd | 3.6 ± 0.0 d | 3.8 ± 0.0 a | 3.8 ± 0.0 b | 3.3 ± 0.0 e | 3.7 ± 0.0 c | 3.9 ± 0.1 a | ||
| Glucose and fructose (g/L) | 2.7 ± 0.1 e | 10.5 ± 1.0 c | 4.7 ± 0.5 de | 6.8 ± 1.7 cd | 6.0 ± 0.4 de | 19.1 ± 2.7 b | 35.9 ± 1.8 a | ||
| Ethanol (% v/v) | 8.0 ± 0.0 a | 7.6 ± 0.1 b | 8.1 ± 0.0 a | 8.0 ± 0.1 a | 7.4 ± 0.0 bc | 7.1 ± 0.1 c | 6.1 ± 0.4 d | ||
| Acetic acid (g/L) | 0.2 ± 0.0 c | 0.3 ± 0.0 c | 0.4 ± 0.0 ab | 0.3 ± 0.0 bc | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.5 ± 0.1 a | ||
| Malic acid (g/L) | 8.4 ± 0.0 a | 8.3 ± 0.2 a | 6.7 ± 0.2 c | 7.2 ± 0.0 bc | 7.6 ± 0.0 b | 7.0 ± 0.2 c | 8.2 ± 0.3 a | ||
| Lactic acid (g/L) | 0.3 ± 0.2 d | 0.2 ± 0.2 d | 0.2 ± 0.0 d | 0.2 ± 0.1 d | 4.4 ± 0.1 a | 0.7 ± 0.1 c | 2.7 ± 0.2 b | ||
| Glycerol (g/L) | 2.9 ± 0.1 b | 2.8 ± 0.1 b | 1.8 ± 0.2 c | 1.9 ± 0.1 c | 4.9 ± 0.1 a | 4.7 ± 0.1 a | 4.9 ± 0.1 a | ||
| Gluconic acid (g/L) | 4.3 ± 0.1 c | 4.5 ± 0.1 ab | 4.4 ± 0.1 bc | 4.4 ± 0.1 abc | 4.0 ± 0.0 d | 4.6 ± 0.1 a | 4.3 ± 0.1 bc | ||
| Total polyphenols (mg/L) | 2427.3 ± 42.5 d | 2579.7 ± 18.0 c | 2495.3 ± 2.1 cd | 2548.3 ± 19.2 cd | 2263.7 ± 13.2 e | 2937.7 ± 93.0 b | 3966.7 ± 72.0 a | ||
| Colour intensity | 3.6 ± 0.0 d | 4.1 ± 0.0 ab | 3.7 ± 0.0 cd | 3.9 ± 0.0 bc | 3.7 ± 0.0 cd | 4.3 ± 0.1 a | 4.2 ± 0.1 a | ||
| Esters | |||||||||
| Hexyl acetate (µg/L) | 1284 | MS | 7.0 ± 0.7 a | 0.8 ± 0.0 b | 0.8 ± 0.0 b | 0.8 ± 0.0 b | 1.6 ± 0.8 b | 0.8 ± 0.0 b | 0.9 ± 0.1 b |
| Isoamyl acetate (µg/L) | 1120 | S, MS | 944.7 ± 1.8 a | 12.9 ± 0.1 e | 62.5 ± 10.6 c | 44.2 ± 0.0 d | 155.9 ± 10.0 b | 4.5 ± 0.9 e | 46.6 ± 7.6 cd |
| Phenylethyl acetate (µg/L) | 1827 | MS | 269.8 ± 3.0 cd | 45.0 ± 1.3 cd | 4808.2 ± 289.1 a | 2764.3 ± 20.6 b | 334.0 ± 0.9 c | 12.4 ± 3.6 d | 120.5 ± 17.3 cd |
| Ethyl butyrate (µg/L) | 1040 | S, MS | 62.6 ± 3.4 a | 25.5 ± 2.0 c | 21.5 ± 1.2 c | 24.6 ± 0.9 c | 26.3 ± 2.6 bc | 31.8 ± 0.5 b | 24.7 ± 1.9 c |
| Ethyl hexanoate (µg/L) | 1242 | S, MS | 284.2 ± 4.0 a | 15.6 ± 1.0 b | 10.2 ± 1.7 c | 4.4 ± 0.1 de | 7.5 ± 0.9 cd | 1.6 ± 0.2 e | 5.3 ± 0.4 de |
| Ethyl octanoate (µg/L) | 1445 | S, MS | 472.0 ± 37.9 a | 32.5 ± 3.2 b | 63.8 ± 5.0 b | 70.3 ± 12.3 b | 26.7 ± 9.5 b | 34.2 ± 3.6 b | 53.8 ± 3.7 b |
| Ethyl decanoate (µg/L) | 1644 | MS | 153.8 ± 39.5 b | 40.2 ± 3.3 c | 277.3 ± 3.1 a | 178.2 ± 5.0 b | 56.5 ± 11.6 c | 59.1 ± 8.6 c | 67.9 ± 8.8 c |
| Ethyl dodecanoate (µg/L) | 1840 | MS | 11.1 ± 0.6 cd | 5.8 ± 0.1 d | 20.6 ± 3.9 b | 47.1 ± 1.7 a | 9.7 ± 1.6 cd | 13.2 ± 2.6 c | 12.8 ± 2.3 d |
| Ethyl tetradecanoate (µg/L) | 1998 | MS | 1.7 ± 0.6 d | 5.2 ± 0.2 cd | 18.9 ± 2.2 a | 13.1 ± 0.6 b | 3.7 ± 1.5 cd | 6.9 ± 2.3 c | 6.6 ± 1.2 c |
| Ethyl 4-hydroxybutyrate (µg/L) | 1800 | MS | 7903.7 ± 449.7 a | 3242.7 ± 113.8 b | 1019.3 ± 13.9 cd | 751.8 ± 64.5 d | 3631.0 ± 170.1 b | 1347.3 ± 135.0 c | 1524.1 ± 4.6 c |
| Diethyl succinate (µg/L) | 1682 | MS | 27.8 ± 3.2 a | 7.5 ± 0.1 b | 5.9 ± 0.2 b | 6.0 ± 0.8 b | 9.1 ± 0.2 b | 7.2 ± 0.6 b | 9.0 ± 0.2 b |
| Ethyl lactate (µg/L) | 1372 | MS | 478.0 ± 81.6 b | 243.1 ± 17.9 b | 502.8 ± 17.3 b | 244.1 ± 7.0 b | 5852.3 ± 661.5 a | 200.3 ± 13.3 b | 260.6 ± 21.0 b |
| Diethyl malate (µg/L) | 2047 | MS | 19.2 ± 0.8 b | 12.7 ± 0.6 c | 5.6 ± 0.0 e | 8.4 ± 0.0 de | 44.9 ± 3.1 a | 9.4 ± 0.5 cd | 7.9 ± 0.9 de |
| Ethyl vanillate (µg/L) | 2653 | MS | 4.8 ± 0.1 bc | 5.4 ± 0.3 abc | 6.5 ± 0.0 a | 6.2 ± 0.2 ab | 3.9 ± 0.8 c | 4.3 ±0.2 c | 6.1 ± 1.1 ab |
| Ethyl cinnamate (µg/L) | 2164 | MS | 2.5 ± 0.3 bc | 2.3 ± 0.1 bc | 3.4 ± 0.3 ab | 4.0 ± 1.0 a | 1.4 ± 0.2 c | 2.7 ± 0.5 abc | 2.5 ± 0.5 bc |
| Acids | |||||||||
| Butyric acid (µg/L) | 1626 | S, MS | 162.8 ± 34.4 abc | 215.2 ± 31.9 a | 143.4 ± 5.1 bc | 138.5 ± 12.5 bc | 130.0 ± 32.7 bc | 115.4 ± 1.0 c | 184.9 ± 13.6 a |
| Isovaleric acid (µg/L) | 1671 | MS | 176.3 ± 38.0 a | 166.9 ± 15.5 ab | 134.1 ± 6.6 abc | 122.2 ± 10.5 bc | 163.1 ± 12.0 ab | 111.8 ± 1.7 c | 175.2 ± 0.2 a |
| Hexanoic acid (µg/L) | 1864 | S, MS | 1217.7 ± 87.0 a | 177.2 ± 14.8 b | 140.5 ± 3.8 bc | 85.9 ± 0.6 bcd | 114.1 ± 4.5 bcd | 44.4 ± 8.6 d | 81.3 ± 10.5 cd |
| Octanoic acid (µg/L) | 2058 | S, MS | 3470.1 ± 58.3 a | 254.7 ± 11.3 c | 526.5 ± 1.8 b | 131.3 ± 1.4 d | 178.8 ± 9.1 d | 41.1 ± 0.2 e | 170.2 ± 19.2 d |
| Decanoic acid (µg/L) | 2265 | MS | 1874.5 ± 4.9 b | 121.0 ± 2.3 d | 2753.3 ± 105.3 a | 505.5 ± 46.6 c | 400.5 ± 72.6 c | 82.1 ± 15.5 d | 211.5 ± 12.7 d |
| Dodecanoic acid (µg/L) | 2482 | MS | 50.1 ± 5.9 bc | 21.6 ± 1.1 b | 86.4 ± 5.4 b | 272.7 ± 45.2 a | 49.5 ± 12.0 bc | 33.9 ± 0.1 c | 48.4 ± 6.5 bc |
| Tetradecanoic acid (µg/L) | 2688 | MS | 20.9 ± 8.0 b | 32.2 ± 4.0 ab | 54.6 ± 11.1 a | 36.6 ± 0.9 ab | 29.9 ± 7.9 b | 41.0 ± 15.0 ab | 26.9 ± 0.9 b |
| Homovanillic acid (µg/L) | 3099 | MS | 64.8 ± 6.6 a | 36.7 ± 2.9 cd | 46.6 ± 1.7 b | 41.1 ± 1.7 bc | 29.7 ± 3.7 d | 30.7 ± 0.7 d | 48.2 ± 0.3 b |
| Hydroxycinnamic acid (µg/L) | 1630 | MS | 54.6 ± 0.8 ab | 51.2 ± 0.3 cd | 56.2 ± 0.3 a | 53.1 ± 0.4 bc | 19.0 ± 0.3 e | 49.5 ± 0.5 d | 53.8 ± 2.4 abc |
| Alcohols | |||||||||
| 1-Hexanol (µg/L) | 1375 | S, MS | 92.3 ± 0.8 c | 93.5 ± 0.3 c | 106.9 ± 1.5 b | 118.7 ± 4.9 a | 80.5 ± 0.3 d | 74.9 ± 1.3 d | 112.2 ± 1.2 b |
| cis-3-Hexen-1-ol (µg/L) | 1405 | MS | 13.5 ± 1.2 a | 11.0 ± 0.1 b | 10.3 ± 0.2 b | 10.7 ± 1.0 b | 7.4 ± 0.4 c | 6.9 ± 0.1 c | 8.1 ± 0.2 c |
| Benzyl alcohol (µg/L) | 1893 | MS | 10,791.6 ± 1176.7 a | 11,506.5 ± 345.6 a | 11,643.3 ± 79.5 a | 11,741.0 ± 482.7 a | 8889.2 ± 397.2 b | 9011.4 ± 249.7 b | 10,761.7 ± 281.6 a |
| 3-Methylthio-1-propanol (µg/L) | 1700 | MS | 227.0 ± 29.1 a | 15.2 ± 2.5 d | 36.3 ± 4.0 bcd | 25.7 ± 4.6 cd | 54.3 ± 2.7 bc | 57.3 ± 2.4 bc | 65.8 ± 4.0 b |
| Anisyl alcohol (µg/L) | 2694 | MS | 70.5 ± 0.7 c | 103.1 ± 2.8 ab | 109.3 ± 0.9 a | 109.9 ± 1.3 a | 73.3 ± 5.1 c | 100.3 ± 0.7 b | 106.1 ± 3.6 ab |
| Vanillic alcohol (µg/L) | 2782 | MS | 49.2 ± 8.1 b | 69.5 ± 2.0 a | 72.5 ± 0.2 a | 73.3 ± 1.1 a | 32.1 ± 1.9 c | 56.4 ± 0.1 b | 54.9 ± 7.1 b |
| 2-Phenylethanol (mg/L) | 1910 | MS | 12.0 ± 0.3 c | 14.0 ± 0.2 b | 10.2 ± 0.9 d | 6.9 ± 0.3 e | 11.7 ± 0.1 c | 15.9 ± 0.6 a | 16.2 ± 0.6 a |
| Phenols | |||||||||
| 4-Vinylguaiacol (µg/L) | 2260 | S, MS | 279.7 ± 4.3 a | 55.5 ± 5.6 b | 16.8 ± 2.0 c | 8.8 ± 0.8 c | 16.6 ± 2.6 c | 11.3 ± 1.9 c | 13.1 ± 4.9 c |
| 4-Vinylphenol (µg/L) | 2406 | MS | 1571.0 ± 106.0 a | 17.0 ± 1.2 b | 10.9 ± 0.6 b | 5.3 ± 0.5 b | 11.2 ± 2.1 b | 5.2 ± 0.9 b | 9.1 ± 0.5 b |
| Eugenol (µg/L) | 1835 | MS | 103.2 ± 1.3 c | 130.1 ± 3.9 a | 131.0 ± 4.6 a | 129.0 ± 1.8 a | 64.5 ± 2.7 d | 71.6 ± 0.5 d | 115.4 ± 0.6 b |
| Vanillin (µg/L) | 2574 | MS | 5.5 ± 2.2 | 4.9 ± 0.1 | 5.8 ± 0.1 | 7.1 ± 2.0 | 5.6 ± 2.0 | 6.1 ± 0.9 | 7.0 ± 0.7 |
| Acetovanillone (µg/L) | 2664 | MS | 26.9 ± 1.8 | 26.3 ± 3.3 | 25.1 ± 0.3 | 25.6 ± 0.8 | 24.5 ± 3.7 | 20.9 ± 0.2 | 25.6 ± 2.3 |
| Phenol (µg/L) | 2004 | MS | 2.0 ± 0.3 b | 2.6 ± 0.1 a | 2.5 ± 0.4 ab | 2.9 ± 0.1 a | 2.4 ± 0.1 ab | 2.6 ± 0.1 a | 2.6 ± 0.1 a |
| Lactones | |||||||||
| γ-Nonalactone (µg/L) | 2068 | S, MS | 12.2 ± 0.6 b | 18.4 ± 0.9 a | 13.0 ± 0.0 b | 13.5 ± 0.2 b | 19.9 ± 0.7 a | 5.3 ± 0.5 d | 9.8 ± 1.4 c |
| γ-Butyrolactone (µg/L) | 1635 | MS | 298.9 ± 12.9 de | 310.9 ± 3.8 d | 234.5 ± 0.4 e | 249.0 ± 1.4 de | 659.8 ± 35.3 b | 816.3 ± 49.1 a | 401.0 ± 8.7 c |
| Ethyl pyroglutamate (µg/L) | 2630 | MS | 55.4 ± 8.0 bc | 59.7 ± 5.4 bc | 41.5 ± 2.5 c | 40.1 ± 2.4 c | 241.5 ± 23.1 a | 71.4 ± 2.3 b | 60.0 ± 0.4 bc |
| Sherry lactone 1 (µg/L) | 1805 | MS | 16.7 ± 5.7 c | 34.3 ± 1.5 ab | 32.8 ± 0.1 ab | 40.1 ± 3.2 a | 28.8 ± 3.7 b | 18.9 ± 1.7 c | 29.1 ± 2.9 b |
| Sherry lactone 2 (µg/L) | 1972 | MS | 79.2 ± 18.1 bc | 265.7 ± 4.1 a | 77.8 ± 1.0 bc | 78.9 ± 4.8 bc | 95.1 ± 13.6 b | 82.1 ± 2.8 bc | 64.8 ± 6.5 c |
| Aldehydes | |||||||||
| Phenylacetaldehyde (µg/L) | 1658 | MS | 9.1 ± 1.6 b | 19.3 ± 2.3 a | 10.5 ± 2.4 b | 5.7 ± 0.1 b | 5.4 ± 3.7 b | 8.9 ± 1.4 b | 6.3 ± 1.1 b |
| Benzaldehyde (µg/L) | 1520 | S, MS | 874.1 ± 17.9 a | 634.5 ± 16.7 b | 696.4 ± 54.6 b | 686.8 ± 46.7 b | 652.2 ± 35.5 b | 440.3 ± 51.3 c | 489.1 ± 62.5 c |
| Furaneol (µg/L) | 1993 | MS | 1.8 ± 0.7 c | 4.5 ± 0.9 a | 3.0 ± 0.0 b | 3.0 ± 0.6 b | 3.6 ± 0.6 ab | 2.7 ± 0.1 bc | 3.6 ± 0.9 ab |
| Homo-furaneol (µg/L) | 2067 | MS | 10.8 ± 2.5 b | 29.4 ± 2.9 a | 12.9 ± 1.3 b | 11.8 ± 5.1 b | 11.2 ± 0.3 b | 9.3 ± 0.2 b | 14.4 ± 0.4 b |
| Terpenes | |||||||||
| Linalool (µg/L) | 1550 | MS | 8.3 ± 0.1 e | 10.4 ± 0.0 c | 6.7 ± 0.3 f | 7.4 ± 0.1 ef | 15.4 ± 0.3 a | 13.2 ± 0.2 b | 9.6 ± 0.6 d |
| α−Terpineol (µg/L) | 1705 | MS | 2.9 ± 0.1 cd | 3.9 ± 0.8 bc | 2.2 ± 0.1 d | 3.1 ± 0.2 cd | 6.9 ± 0.4 a | 4.6 ± 0.6 b | 4.5 ±0.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, F.; Avesani, M.; Lorenzini, M.; Zapparoli, G.; Simonato, B. Fermentation Performances and Aroma Contributions of Selected Non-Saccharomyces Yeasts for Cherry Wine Production. Foods 2024, 13, 2455. https://doi.org/10.3390/foods13152455
Bianchi F, Avesani M, Lorenzini M, Zapparoli G, Simonato B. Fermentation Performances and Aroma Contributions of Selected Non-Saccharomyces Yeasts for Cherry Wine Production. Foods. 2024; 13(15):2455. https://doi.org/10.3390/foods13152455
Chicago/Turabian StyleBianchi, Federico, Michele Avesani, Marilinda Lorenzini, Giacomo Zapparoli, and Barbara Simonato. 2024. "Fermentation Performances and Aroma Contributions of Selected Non-Saccharomyces Yeasts for Cherry Wine Production" Foods 13, no. 15: 2455. https://doi.org/10.3390/foods13152455
APA StyleBianchi, F., Avesani, M., Lorenzini, M., Zapparoli, G., & Simonato, B. (2024). Fermentation Performances and Aroma Contributions of Selected Non-Saccharomyces Yeasts for Cherry Wine Production. Foods, 13(15), 2455. https://doi.org/10.3390/foods13152455







