Impact of Mechanical and Manual Peeling on the Volatile Profile of White Pepper (Piper nigrum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Processes of Piper nigrum L.
2.3. Determination of Volatiles
2.4. Calculation of Relative Odor Activity Values (ROAVs)
2.5. Statistical Analysis
3. Results
3.1. The Contents of Volatile Components
3.2. The ROAVs of Volatile Components
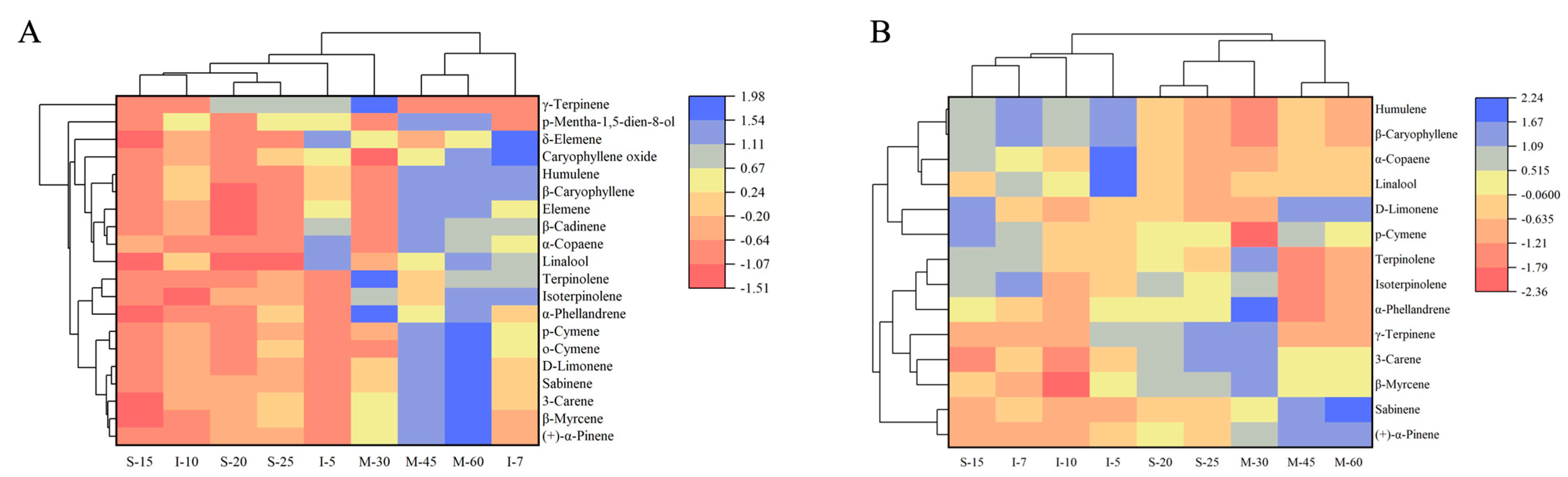
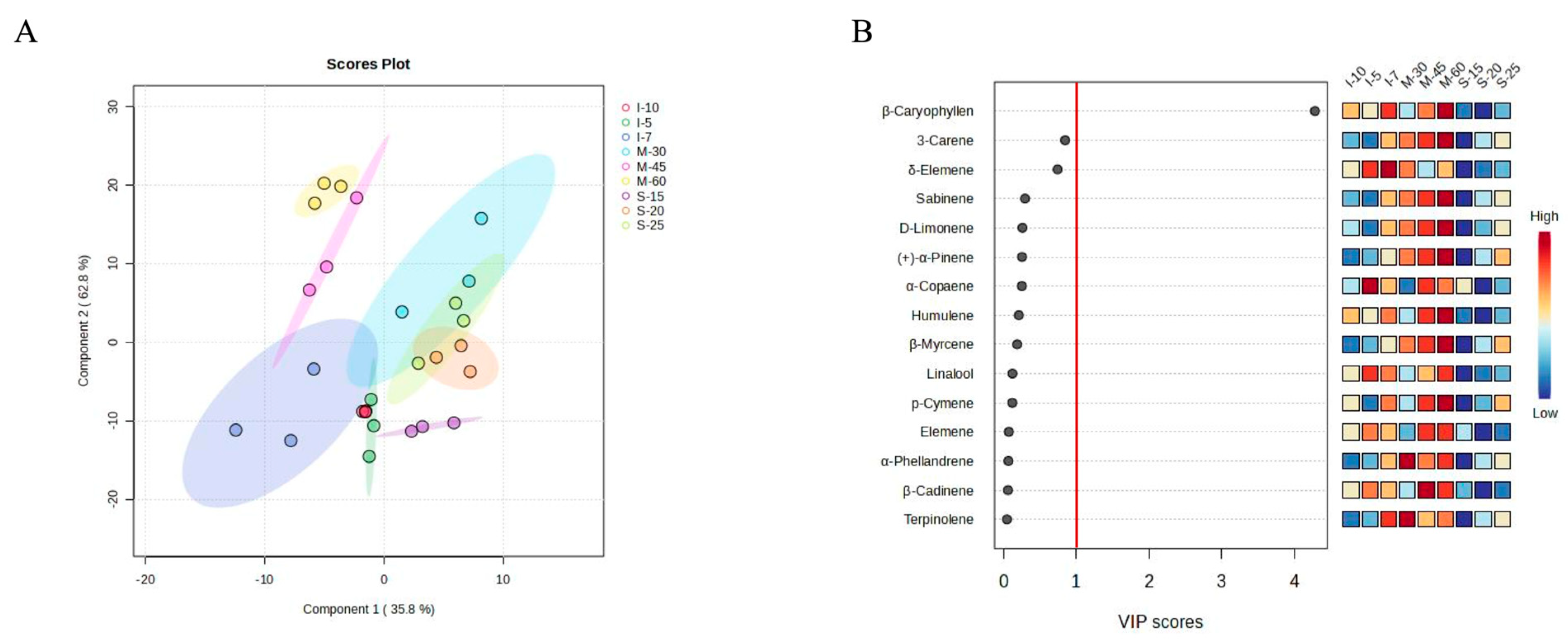
3.3. Correlation Analysis of Volatile Compounds
4. Discussion
4.1. Effect of Immersion on Volatile Compounds of Pepper
4.2. Effect of Mechanical Peeling on Volatile Compounds of Pepper
4.3. Effect of Steaming on Volatile Compounds of Pepper
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dludla, P.V.; Cirilli, I.; Marcheggiani, F.; Silvestri, S.; Orlando, P.; Muvhulawa, N.; Moetlediwa, M.T.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hlengwa, N.; et al. Bioactive Properties, Bioavailability Profiles, and Clinical Evidence of the Potential Benefits of Black Pepper (Piper nigrum) and Red Pepper (Capsicum annum) against Diverse Metabolic Complications. Molecules 2023, 28, 6569. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Norouzkhani, N.; Karimi, A.G.; Badami, N.; Jalalifar, E.; Mahmoudvand, B.; Ansari, A.; Sariyarighan, N.P.; Alijanzadeh, D.; Aghakhani, S.; Shayestehmehr, R.; et al. From kitchen to clinic: Pharmacotherapeutic potential of common spices in Indian cooking in age-related neurological disorders. Front. Pharmacol. 2022, 13, 960037. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; da Silva, J.K.R.; Setzer, W.N. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules 2019, 24, 4244. [Google Scholar] [CrossRef]
- Nair, K.P.P. The agronomy and economy of black pepper (Piper nigrum L.) the “King of Spices”. In Agronomy and Economy of Black Pepper and Cardamom; Sparks, D.L., Ed.; Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2004; Volume 82, pp. 271–389. [Google Scholar]
- Jelen, H.H.; Gracka, A. Analysis of black pepper volatiles by solid phase microextraction-gas chromatography: A comparison of terpenes profiles with hydrodistillation. J. Chromatogr. A 2015, 1418, 200–209. [Google Scholar] [CrossRef]
- Aziz, N.S.; Sofian-Seng, N.-S.; Razali, N.S.M.; Lim, S.J.; Mustapha, W.A.W. A review on conventional and biotechnological approaches in white pepper production. J. Sci. Food Agric. 2019, 99, 2665–2676. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, D.W.; Shin, Y.; Kim, Y.J. Comparative study of the bioactive compounds, flavours and minerals present in black pepper before and after removing the outer skin. LWT Food Sci. Technol. 2020, 125, 109356. [Google Scholar] [CrossRef]
- Arief, R.W.; Mustikawati, D.R.; Asnawi, R. Alteration of The Content of Piperine and Essential Oil from Black Pepper and White Pepper After a Year Storage. IOP Conf. Ser. Earth Environ. Sci. 2023, 1172, 012047. [Google Scholar] [CrossRef]
- Rosa, F.; Rodiawan; Prayitnoadi, R.P. Stress Analysis and Safety Factor of Shaft on Pepper Peeler Machine to Reduce Environmental Pollution. IOP Conf. Ser. Earth Environ. Sci. 2019, 353, 012031. [Google Scholar] [CrossRef]
- Hu, Q.S.; Zhang, J.C.; Xu, C.B.; Li, C.F.; Liu, S.X. The Dynamic Microbiota Profile During Pepper (Piper nigrum L.) Peeling by Solid-State Fermentation. Curr. Microbiol. 2017, 74, 739–746. [Google Scholar] [CrossRef]
- Milenkovic, A.N.; Stanojevic, J.S.; Troter, D.Z.; Pejcic, M.G.; Stojanovic-Radic, Z.Z.; Cvetkovic, D.J.; Stanojevic, L.P. Chemical composition, antimicrobial and antioxidant activities of essential oils isolated from black (Piper nigrum L.) and cubeb pepper (Piper cubeba L.) fruits from the Serbian market. J. Essent. Oil Res. 2023, 35, 262–273. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Vellaikumar, S.; Murugan, M.; Dhanya, M.K.; Karthikeyan, A.; Akilan, M.; Ariharasutharsan, G.; Nimisha, M.; Aiswarya, S. Assessment of phytochemical diversity in essential oil composition of eighteen Piper nigrum (L.) accessions from southern India. J. Essent. Oil Res. 2021, 33, 549–558. [Google Scholar] [CrossRef]
- Tran, T.H.; Ngo, T.C.Q.; Dao, T.P.; Nguyen, P.T.N.; Pham, T.N.; Le, X.T.; Vo, D.M.H.; Minh, P.T.H.; Linh, H.T.K. Effect of microwaves energy on volatile compounds in pepper (Piper nigrum L.) leaves essential oil. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 032013. [Google Scholar] [CrossRef]
- Fan, R.; Qin, X.W.; Hu, R.S.; Hu, L.S.; Wu, B.D.; Hao, C.Y. Studies on the chemical and flavour qualities of white pepper (Piper nigrum L.) derived from grafted and non-grafted plants. Eur. Food Res. Technol. 2020, 246, 2601–2610. [Google Scholar] [CrossRef]
- Fu, Y.T.; Chen, S.; Wang, X.J.; Wang, L.; Wang, Z.X.; Cheng, Y.F.; Liu, Y.Y.; Zhang, L.; Liu, S.X.; Kang, J.M.; et al. Insights into the Correlation between Microbial Community Succession and Pericarp Degradation during Pepper (Piper nigrum L.) Peeling Process via Retting. Foods 2024, 13, 1615. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, C.; Li, Y.-l.; Li, M.-f.; Luo, W.-y.; Tan, G.-y. Design and Experimental Analysis of Pepper Fruit Preprocessing Machine. Adv. Mater. Res. 2015, 1061–1062, 687–691. [Google Scholar] [CrossRef]
- Thirupathi, V.; Viswanathan, R. A Hand Operated White Pepper Peeling Machine. Ama-Agric. Mech. Asia Afr. Lat. Am. 2009, 40, 57–59. [Google Scholar]
- Xiang, N.; Zhao, Y.; Zhang, B.; Gu, Q.; Chen, W.; Guo, X. Volatiles Accumulation during Young Pomelo (Citrus maxima (Burm.) Merr.) Fruits Development. Int. J. Mol. Sci. 2022, 23, 5665. [Google Scholar] [CrossRef]
- Rotsatchakul, P.; Chaiseri, S.; Cadwallader, K.R. Identification of characteristic aroma components of Thai fried chili paste. J. Agric. Food Chem. 2008, 56, 528–536. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, L.; Zhang, C.; Feng, T.; Zhuang, H. Analysis of Volatile Flavor Compounds of Corn Under Different Treatments by GC-MS and GC-IMS. Front. Chem. 2022, 10, 725208. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.F.; Sauri-Duch, E.; Sosa-Moguel, O.; Pino, J.A. Characterization of odor-active compounds in mango ‘Ataulfo’ (Mangifera indica L.) fruit. Chem. Pap. 2020, 74, 4025–4032. [Google Scholar] [CrossRef]
- Multari, S.; Marsol-Vall, A.; Yang, B.; Suomela, J.-P. Effects of Aromatic Herb Flavoring on Carotenoids and Volatile Compounds in Edible Oil From Blue Sweet Lupin (Lupinus angustifolius). Eur. J. Lipid Sci. Technol. 2018, 120, 1800227. [Google Scholar] [CrossRef]
- Cui, L.; Liu, C.Q.; Li, D.J. Changes in Volatile Compounds of Sweet Potato Tips During Fermentation. Agric. Sci. China 2010, 9, 1689–1695. [Google Scholar] [CrossRef]
- Zhang, B.; Li, K.; Cheng, H.; Hu, J.; Qi, X.; Guo, X. Effect of thermal treatments on volatile profiles and fatty acid composition in sweet corn (Zea mays L.). Food Chem.-X 2023, 18, 100743. [Google Scholar] [CrossRef] [PubMed]
- Schimitberger, V.M.B.; Pratti, D.L.D.; Cavalcanti, L.C.; Ramalho, V.F.; da Costa, A.P.F.; Scherer, R.; Kuster, R.M.; Ramos, A.C.; da Silva, A.G. Volatile compounds profile changes from unripe to ripe fruits of Brazilian pepper (Schinus terebinthifolia Raddi). Ind. Crops Prod. 2018, 119, 125–131. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine-The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Madmony, A.; Tognetti, R.; Zamponi, L.; Capretti, P.; Michelozzi, M. Monoterpene responses to interacting effects of drought stress and infection by the fungus Heterobasidion parviporum in two clones of Norway spruce (Picea abies). Environ. Exp. Bot. 2018, 152, 137–148. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Wang, S. Effect of mechanical vibration on postharvest quality and volatile compounds of blueberry fruit. Food Chem. 2021, 349, 129216. [Google Scholar] [CrossRef]
- Xu, F.; Lu, F.; Xiao, Z.; Li, Z. Influence of drop shock on physiological responses and genes expression of apple fruit. Food Chem. 2020, 303, 125424. [Google Scholar] [CrossRef]
- Garrido-Banuelos, G.; Miljkovic, A.; Morange, C.; Mihnea, M.; Lopez-Sanchez, P. Assessing the volatile composition of seaweed (Laminaria digitata) suspensions as function of thermal and mechanical treatments. LWT Food Sci. Technol. 2022, 162, 113483. [Google Scholar] [CrossRef]
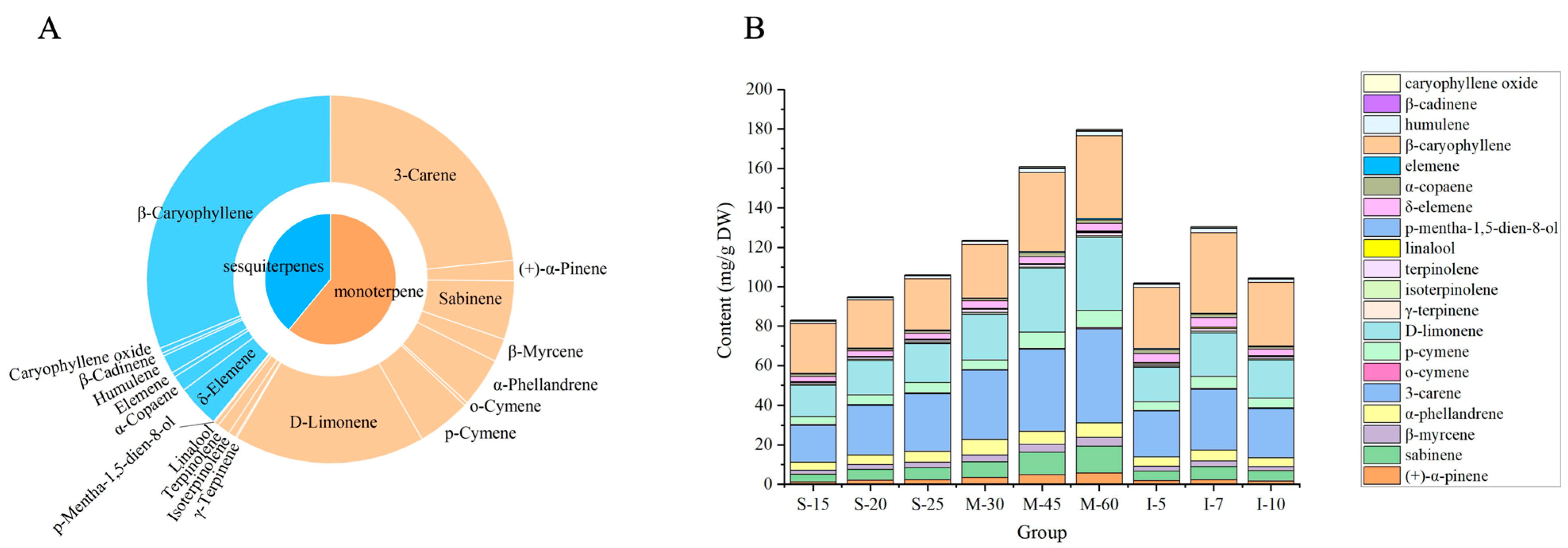


| NO. | Compound | Type | RT (min) | S-15 | S-20 | S-25 | M-30 | M-45 | M-60 | I-5 | I-7 | I-10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | (+)-α-pinene | monoterpene | 9.804 | 1.32 ± 0.04 f | 2.18 ± 0.17 de | 2.36 ± 0.14 d | 3.52 ± 0.60 c | 4.97 ± 0.35 b | 5.83 ± 0.12 a | 1.91 ± 0.27 de | 2.34 ± 0.13 d | 1.73 ± 0.02 ef |
| C2 | sabinene | monoterpene | 11.279 | 3.85 ± 0.20 g | 5.40 ± 0.43 ef | 5.95 ± 0.29 de | 7.93 ± 0.95 c | 11.42 ± 0.80 b | 13.45 ± 0.15 a | 4.93 ± 0.84 f | 6.75 ± 0.25 d | 5.19 ± 0.03 ef |
| C3 | β-myrcene | monoterpene | 11.699 | 1.91 ± 0.14 g | 2.47 ± 0.21 def | 2.83 ± 0.25 d | 3.46 ± 0.31 c | 4.01 ± 0.27 b | 4.49 ± 0.10 a | 2.31 ± 0.28 efg | 2.67 ± 0.28 de | 2.15 ± 0.01 fg |
| C4 | α-phellandrene | monoterpene | 12.244 | 4.06 ± 0.32 d | 4.81 ± 0.36 d | 5.55 ± 0.29 c | 7.83 ± 0.59 a | 6.39 ± 0.55 b | 7.43 ± 0.12 a | 4.74 ± 0.53 d | 5.65 ± 0.54 c | 4.47 ± 0.05 d |
| C5 | 3-carene | monoterpene | 12.319 | 18.79 ± 1.43 g | 25.29 ± 1.98 ef | 29.17 ± 2.27 de | 34.96 ± 3.05 c | 41.52 ± 3.12 b | 47.47 ± 0.72 a | 23.30 ± 3.03 f | 30.56 ± 2.83 d | 24.91 ± 0.14 f |
| C6 | o-cymene | monoterpene | 12.683 | 0.29 ± 0.01 e | 0.29 ± 0.02 e | 0.35 ± 0.01 d | 0.30 ± 0.02 e | 0.50 ± 0.05 b | 0.57 ± 0.03 a | 0.30 ± 0.03 e | 0.41 ± 0.04 c | 0.31 ± 0.02 de |
| C7 | p-cymene | monoterpene | 12.848 | 4.24 ± 0.21 d | 4.62 ± 0.35 cd | 5.25 ± 0.24 c | 4.79 ± 0.33 cd | 8.28 ± 0.58 a | 8.82 ± 0.73 a | 4.44 ± 0.39 cd | 6.19 ± 0.55 b | 4.99 ± 0.22 cd |
| C8 | D-limonene | monoterpene | 13.002 | 15.62 ± 0.97 f | 17.46 ± 1.34 ef | 19.64 ± 1.05 de | 23.14 ± 1.67 c | 32.18 ± 2.46 b | 36.95 ± 0.63 a | 17.27 ± 1.55 ef | 22.01 ± 2.63 cd | 19.13 ± 0.10 e |
| C9 | γ-terpinene | monoterpene | 13.938 | ND | 0.16 ± 0.01 c | 0.16 ± 0.00 bc | 0.24 ± 0.02 a | ND | ND | 0.17 ± 0.00 b | ND | ND |
| C10 | isoterpinolene | monoterpene | 14.675 | 0.54 ± 0.02 c | 0.62 ± 0.04 bc | 0.64 ± 0.02 bc | 0.83 ± 0.05 a | 0.69 ± 0.01 b | 0.87 ± 0.09 a | 0.54 ± 0.02 c | 0.85 ± 0.16 a | 0.52 ± 0.02 c |
| C11 | terpinolene | monoterpene | 14.799 | 0.90 ± 0.03 c | 0.99 ± 0.08 bc | 1.10 ± 0.04 bc | 1.64 ± 0.09 a | 1.18 ± 0.01 b | 1.45 ± 0.10 a | 0.97 ± 0.04 c | 1.48 ± 0.29 a | 0.95 ± 0.03 c |
| C12 | linalool | monoterpene | 15.262 | 0.27 ± 0.02 d | 0.29 ± 0.02 d | 0.30 ± 0.02 d | 0.40 ± 0.02 c | 0.49 ± 0.03 b | 0.56 ± 0.03 a | 0.56 ± 0.05 a | 0.54 ± 0.02 a | 0.44 ± 0.03 bc |
| C13 | p-mentha-1,5-dien-8-ol | monoterpene | 16.772 | ND | ND | 0.16 ± 0.01 c | ND | 0.24 ± 0.00 b | 0.28 ± 0.04 a | 0.16 ± 0.01 c | ND | 0.17 ± 0.00 c |
| C14 | δ-elemene | sesquiterpene | 19.377 | 2.71 ± 0.30 d | 2.93 ± 0.27 cd | 3.03 ± 0.16 cd | 3.93 ± 0.23 b | 3.36 ± 0.23 c | 3.86 ± 0.28 b | 4.48 ± 0.10 a | 4.88 ± 0.49 a | 3.40 ± 0.02 c |
| C15 | α-copaene | sesquiterpene | 19.866 | 1.30 ± 0.03 c | 1.06 ± 0.04 d | 1.12 ± 0.04 cd | 1.08 ± 0.08 d | 2.03 ± 0.16 a | 1.98 ± 0.17 ab | 2.05 ± 0.06 a | 1.81 ± 0.21 b | 1.24 ± 0.01 cd |
| C16 | elemene | sesquiterpene | 19.994 | 0.37 ± 0.01 d | 0.33 ± 0.03 d | 0.35 ± 0.02 d | 0.36 ± 0.05 d | 0.63 ± 0.04 a | 0.64 ± 0.03 a | 0.55 ± 0.01 b | 0.55 ± 0.05 b | 0.43 ± 0.00 c |
| C17 | β-caryophyllene | sesquiterpene | 20.368 | 25.05 ± 2.16 c | 24.30 ± 1.94 c | 26.15 ± 1.76 c | 27.17 ± 3.36 c | 39.95 ± 1.21 a | 41.96 ± 1.17 a | 30.88 ± 0.72 b | 40.74 ± 3.53 a | 32.16 ± 0.18 b |
| C18 | humulene | sesquiterpene | 20.724 | 1.33 ± 0.13 c | 1.27 ± 0.11 c | 1.36 ± 0.11 c | 1.42 ± 0.19 bc | 2.17 ± 0.09 a | 2.26 ± 0.08 a | 1.62 ± 0.04 b | 2.13 ± 0.22 a | 1.64 ± 0.02 b |
| C19 | β-cadinene | sesquiterpene | 21.267 | 0.23 ± 0.01 bc | 0.19 ± 0.00 d | 0.21 ± 0.02 cd | 0.24 ± 0.05 bc | 0.40 ± 0.01 a | 0.39 ± 0.02 a | 0.39 ± 0.02 a | 0.36 ± 0.03 a | 0.26 ± 0.00 b |
| C20 | caryophyllene oxide | sesquiterpene | 21.904 | 0.32 ± 0.03 e | 0.33 ± 0.02 e | 0.39 ± 0.04 d | 0.23 ± 0.01 f | 0.46 ± 0.04 c | 0.54 ± 0.02 b | 0.44 ± 0.03 c | 0.60 ± 0.03 a | 0.34 ± 0.01 e |
| total monoterpenes | 51.68 ± 3.39 | 64.43 ± 5.01 | 73.35 ± 4.63 | 88.94 ± 7.70 | 111.75 ± 8.23 | 128.00 ± 2.86 | 61.53 ± 7.04 | 79.38 ± 7.72 | 64.92 ± 0.67 | |||
| total sesquiterpenes | 31.26 ± 2.67 | 30.41 ± 2.41 | 32.56 ± 2.15 | 34.36 ± 3.97 | 48.95 ± 1.78 | 51.57 ± 0.77 | 40.33 ± 0.98 | 51.03 ± 4.56 | 39.41 ± 0.24 | |||
| total volatiles | 82.94 ± 6.06 | 94.84 ± 7.42 | 105.91 ± 6.78 | 123.30 ± 11.67 | 160.70 ± 10.01 | 179.57 ± 3.63 | 101.86 ± 8.02 | 130.41 ± 12.28 | 104.33 ± 0.91 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, P.; Wei, L.; Zhang, B.; Shen, D.; Zhao, Z.; Guo, X. Impact of Mechanical and Manual Peeling on the Volatile Profile of White Pepper (Piper nigrum L.). Foods 2024, 13, 2458. https://doi.org/10.3390/foods13152458
Zhang Y, Yu P, Wei L, Zhang B, Shen D, Zhao Z, Guo X. Impact of Mechanical and Manual Peeling on the Volatile Profile of White Pepper (Piper nigrum L.). Foods. 2024; 13(15):2458. https://doi.org/10.3390/foods13152458
Chicago/Turabian StyleZhang, Yuan, Peiyao Yu, Lijiao Wei, Bing Zhang, Dezhan Shen, Zhenhua Zhao, and Xinbo Guo. 2024. "Impact of Mechanical and Manual Peeling on the Volatile Profile of White Pepper (Piper nigrum L.)" Foods 13, no. 15: 2458. https://doi.org/10.3390/foods13152458
APA StyleZhang, Y., Yu, P., Wei, L., Zhang, B., Shen, D., Zhao, Z., & Guo, X. (2024). Impact of Mechanical and Manual Peeling on the Volatile Profile of White Pepper (Piper nigrum L.). Foods, 13(15), 2458. https://doi.org/10.3390/foods13152458






