Influence of the Culture Conditions on Camellia sinensis Cell Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
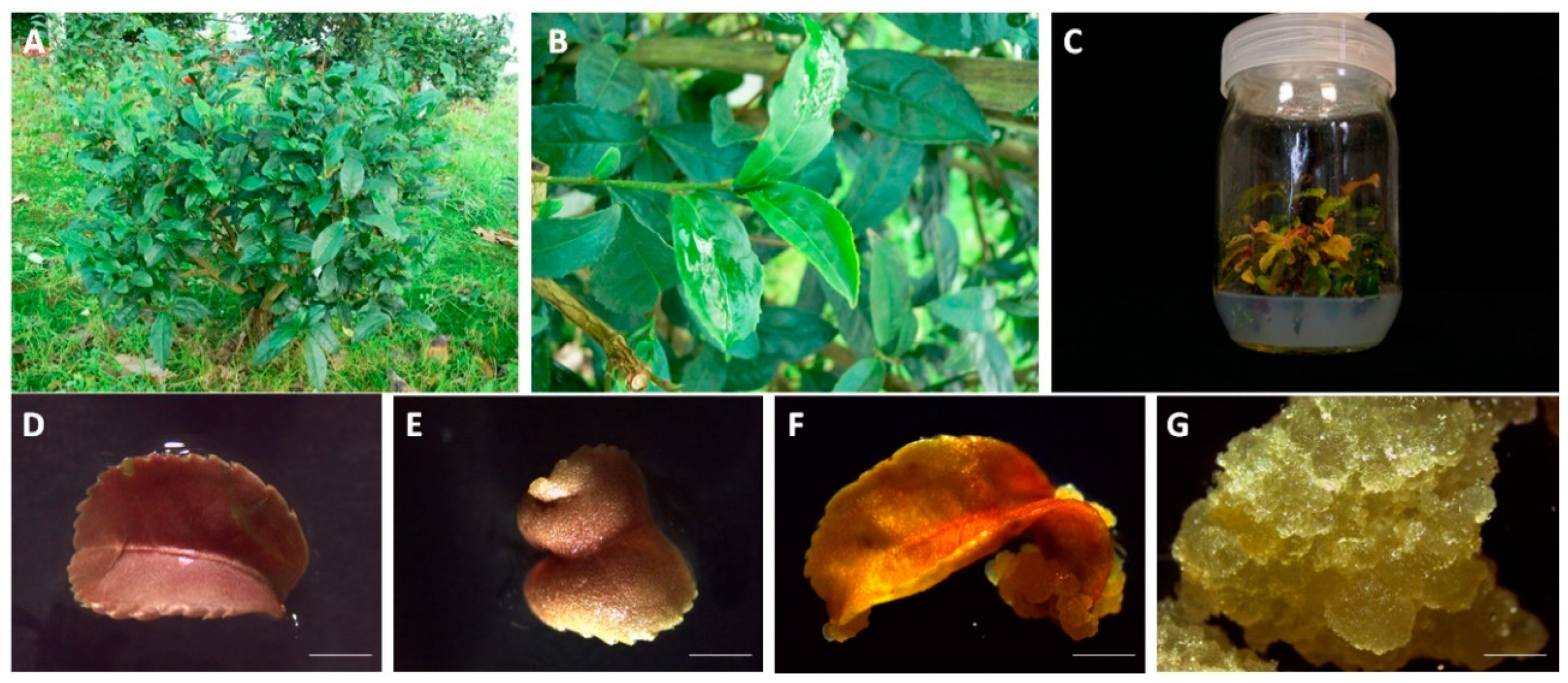
2.2. Plant Material and Callus Induction
2.3. Selection and Screening of LSC-5 Callus Lines under Light Treatment
2.4. Establishment of Tea CSCs
2.5. Effect of PGR Concentration, Inoculum Size, and Cell Line Age on Biomass and Metabolite Productivity
2.6. Long-Term Stability of TC Production by Tea CSCs
2.7. Elicitor Treatments of Target Metabolites
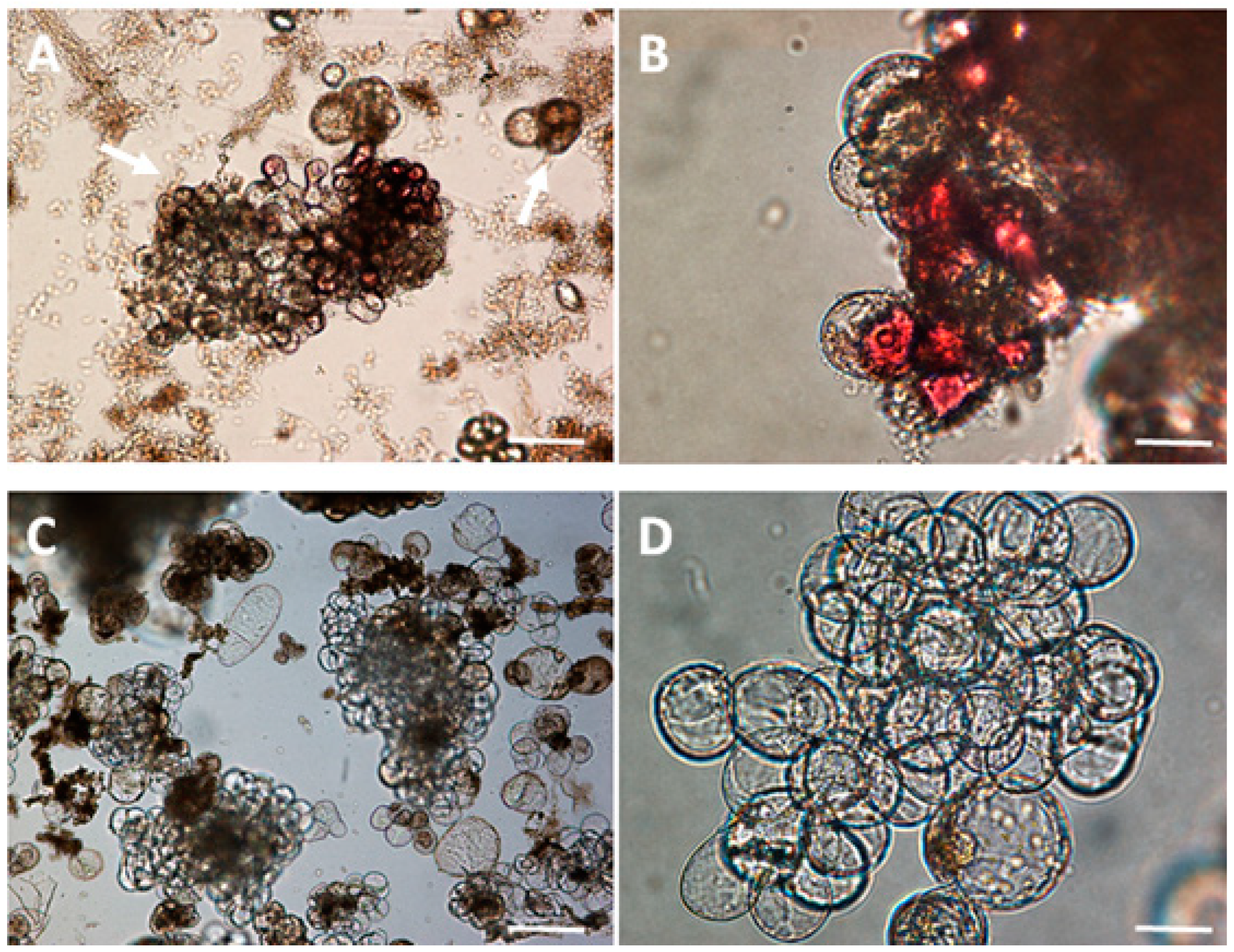
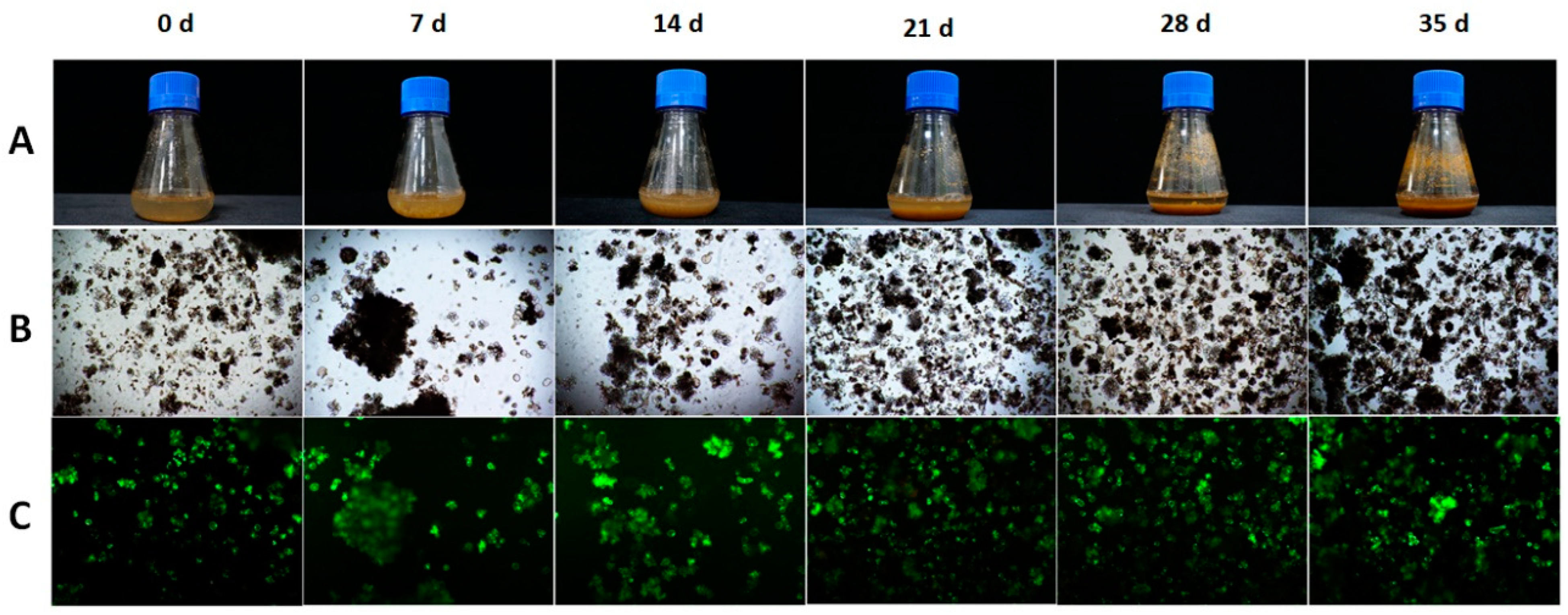
2.8. Microscopic Observation and Cell Staining
2.9. Biomass Measurements
2.10. Preparation of Extracts for HPLC Analysis
2.11. HPLC Analysis
2.12. Statistical Analysis
3. Results and Discussion
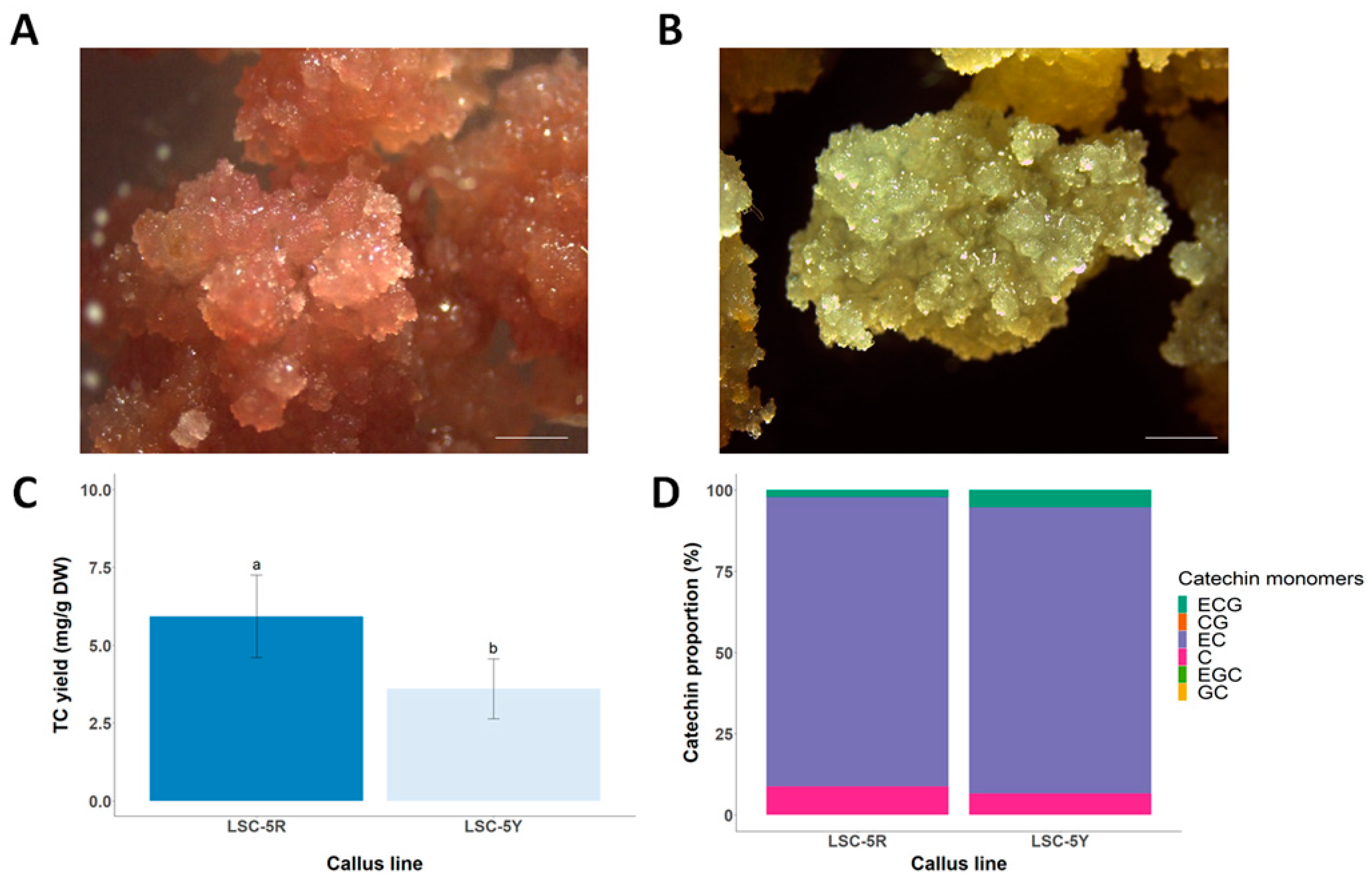
3.1. Selection of LSC-5 Callus Lines under Light Treatment
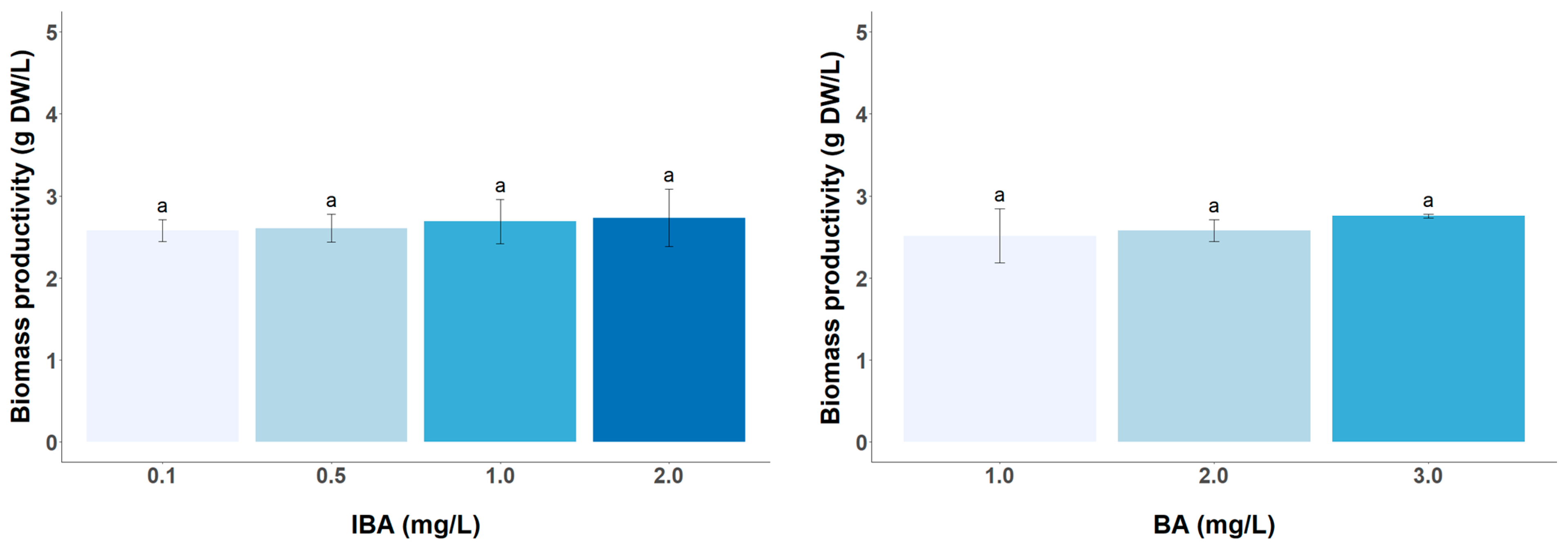
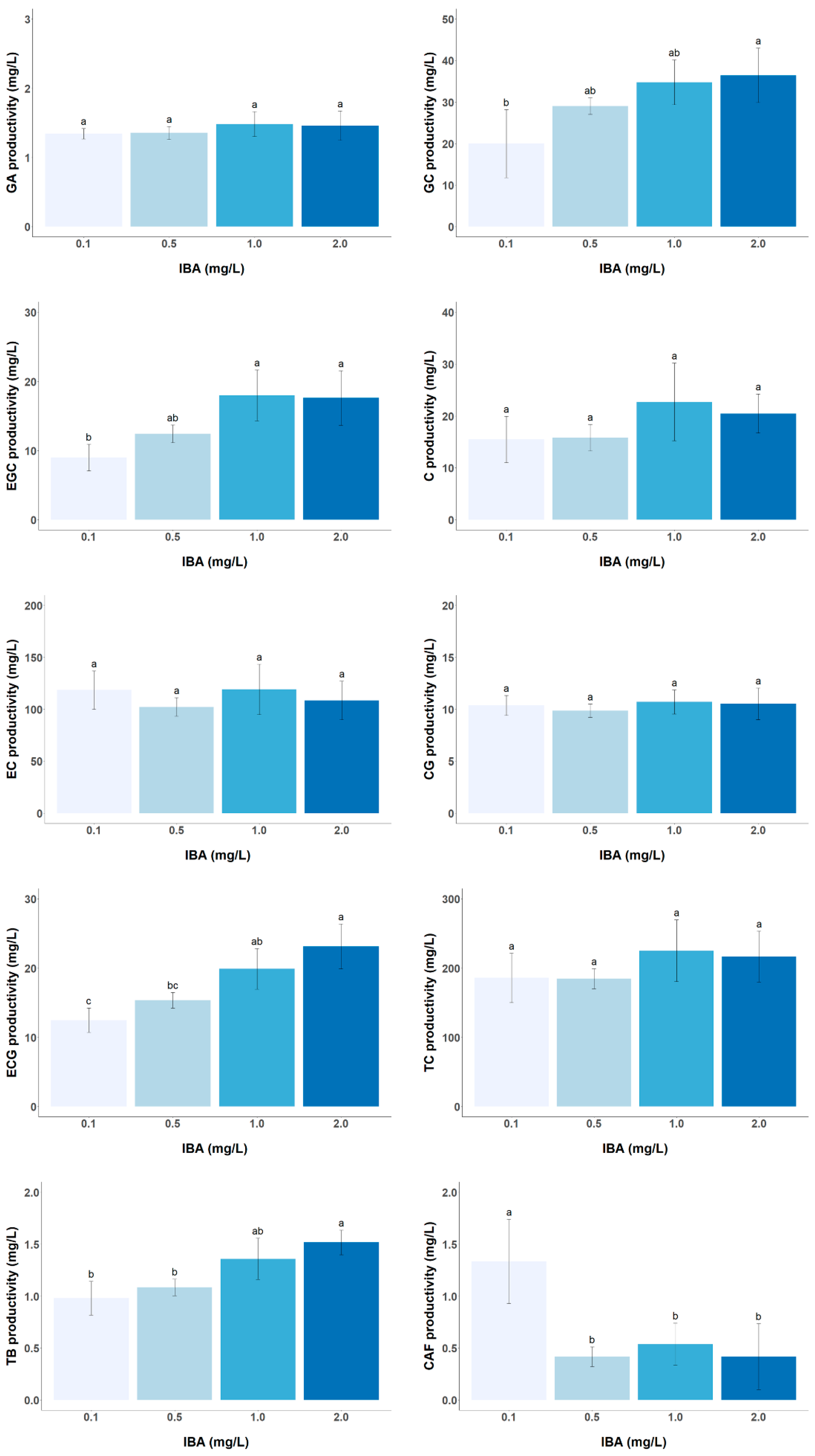
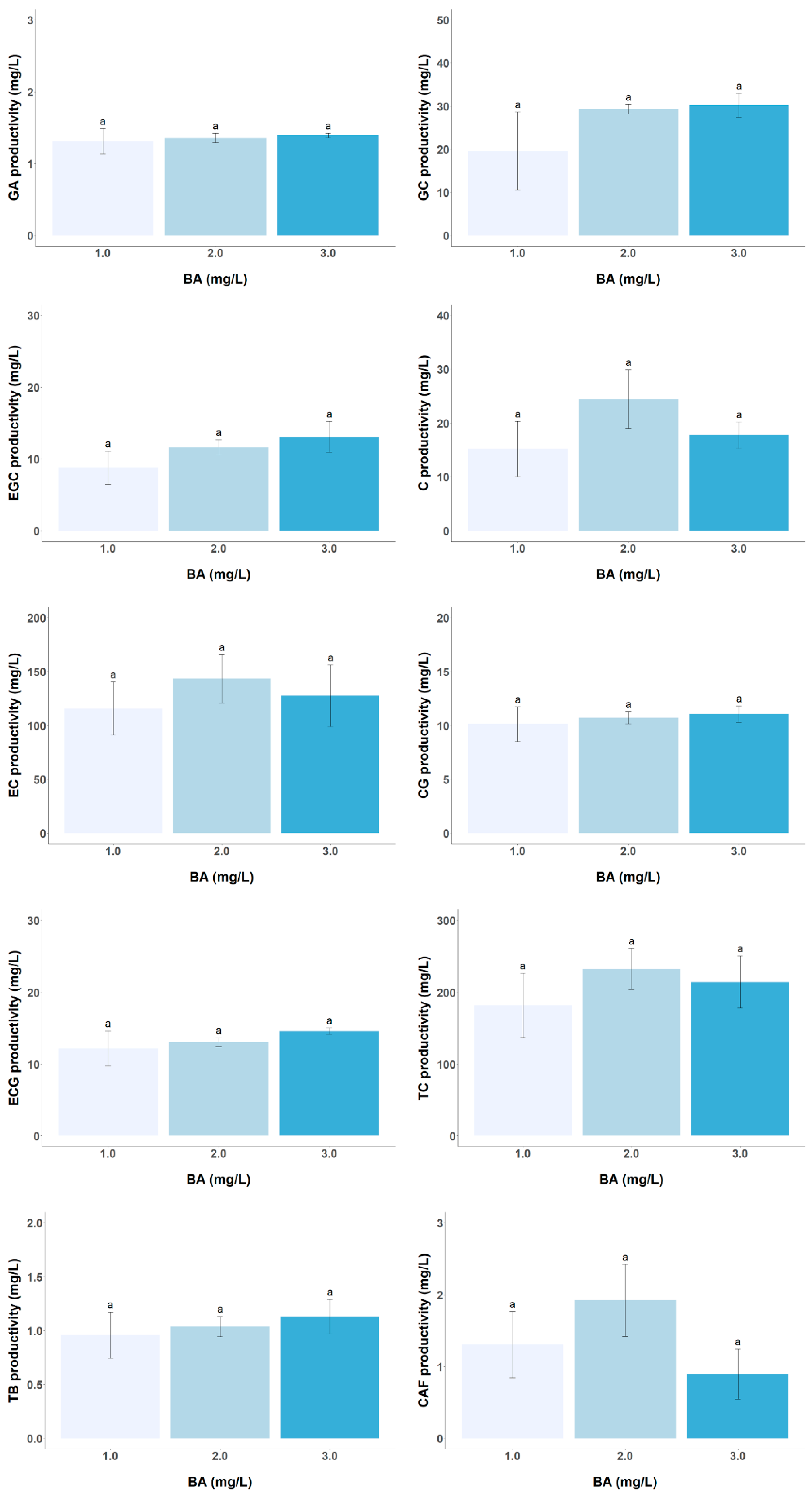
3.2. Effect of PGR Concentration on the Biomass and Target Metabolite Productivity
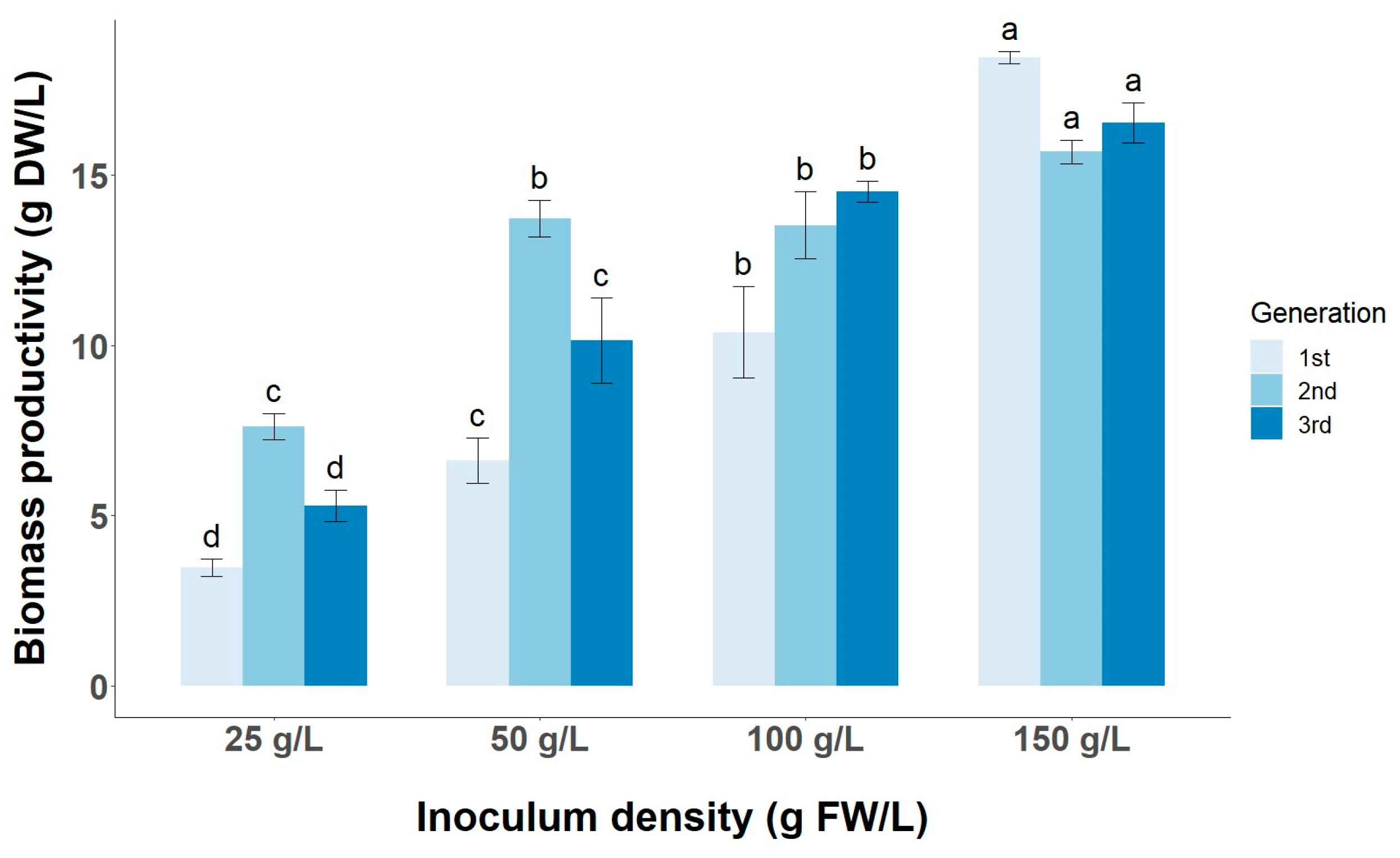
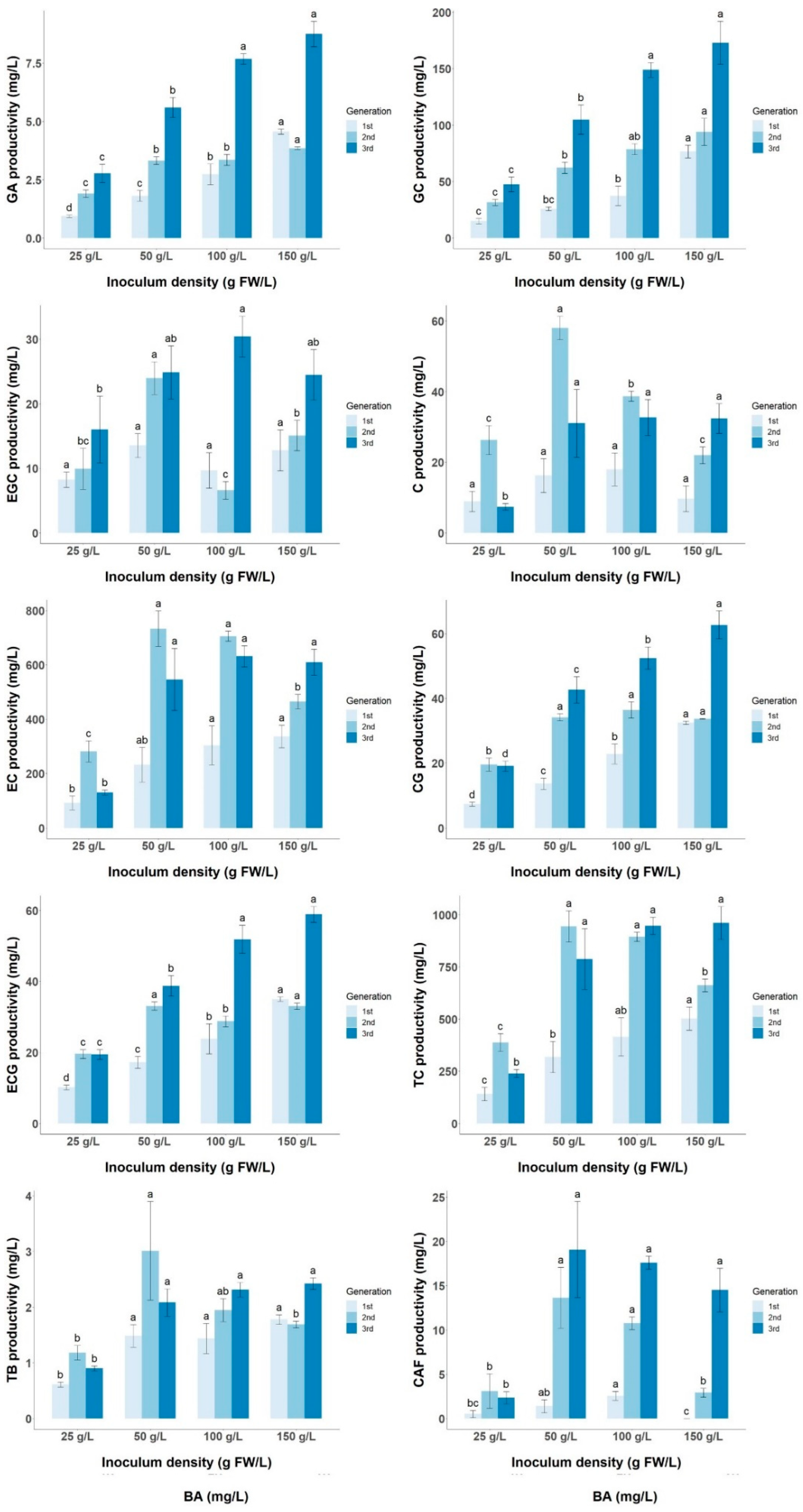
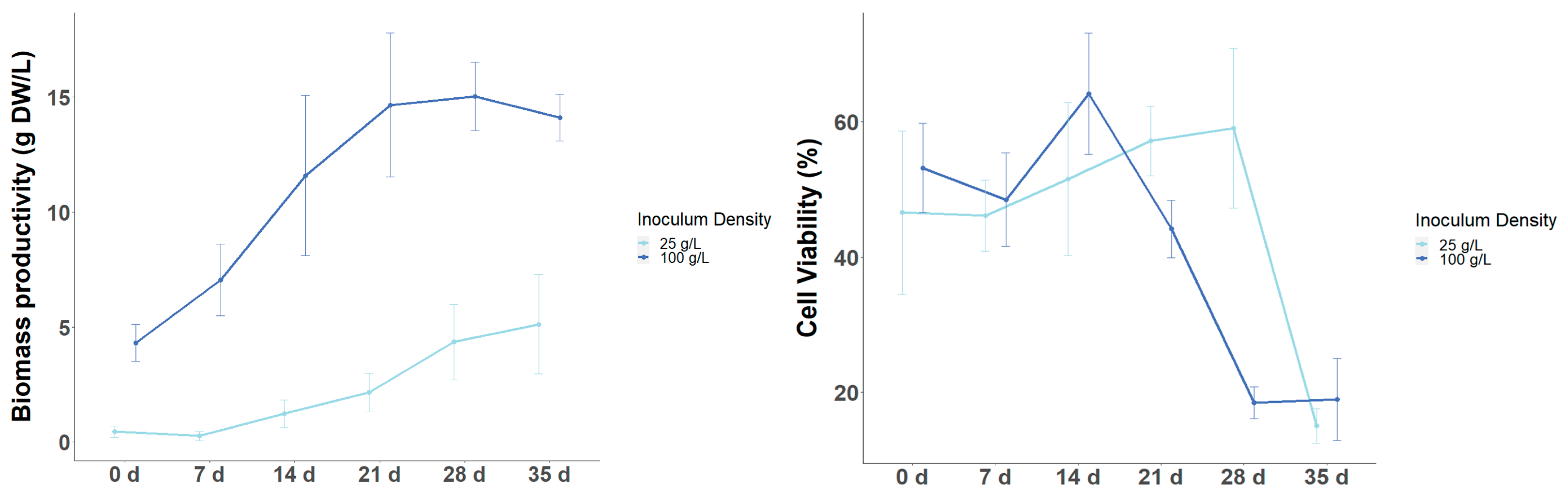
3.3. Effect of Inoculum Size on Biomass and Target Metabolite Productivity
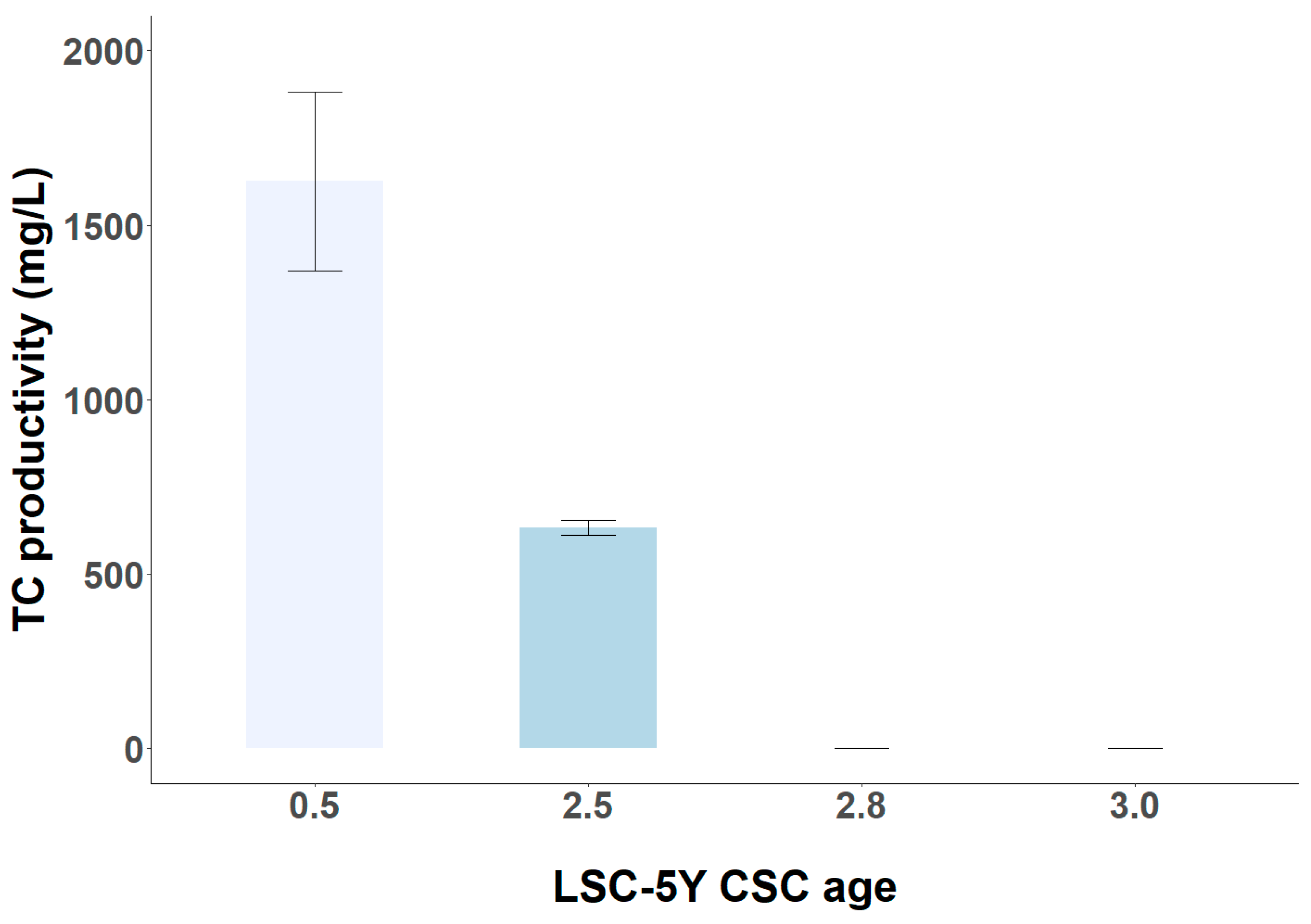
3.4. Short-Term and Long-Term Stability of TC Productivity in LSC-5Y Cell Line
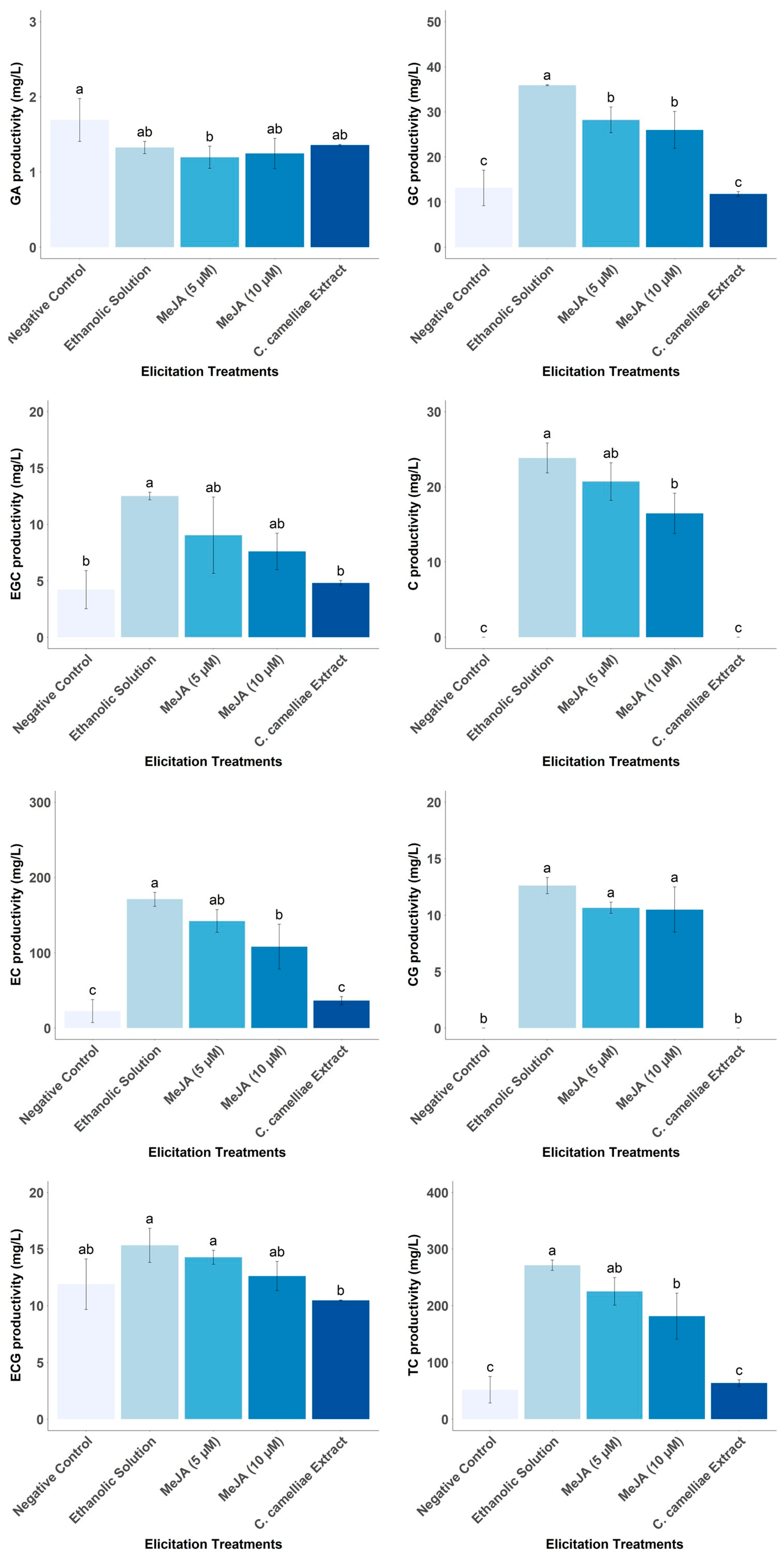
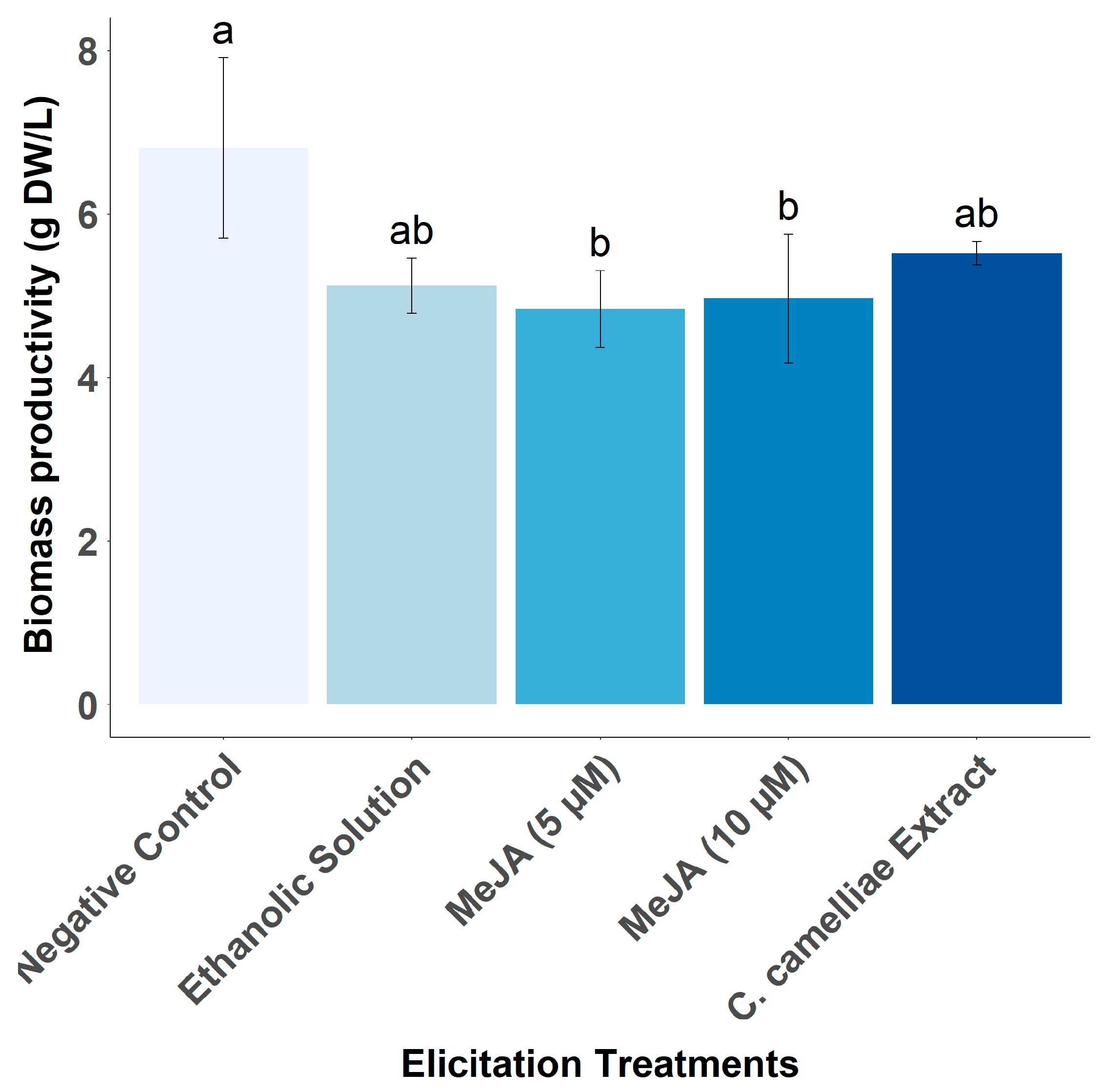
3.5. Effect of Different Elicitors on Catechin Productivity by LSC-5Y CSC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deka, H.; Barman, T.; Dutta, J.; Devi, A.; Tamuly, P.; Kumar Paul, R.; Karak, T. Catechin and Caffeine Content of Tea (Camellia sinensis L.) Leaf Significantly Differ with Seasonal Variation: A Study on Popular Cultivars in North East India. J. Food Compos. Anal. 2021, 96, 103684. [Google Scholar] [CrossRef]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Coll. Nutr. 2022, 41, 65–93. [Google Scholar] [CrossRef] [PubMed]
- FAO. Current Global Market Situation and Medium Term Outlook; FAO: Rome, Italy, 2024. [Google Scholar]
- Barreira, S.; Moutinho, C.; Silva, A.M.N.; Neves, J.; Seo, E.J.; Hegazy, M.E.F.; Efferth, T.; Gomes, L.R. Phytochemical Characterization and Biological Activities of Green Tea (Camellia sinensis) Produced in the Azores, Portugal. Phytomed. Plus 2021, 1, 100001. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH Assay Comparison to Assess Antioxidant Capacity of Tea Infusions: Relationship between Total Polyphenol and Individual Catechin Content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Samynathan, R.; Kiruthikaa, S.; Chithraanjane, N.; Harsha, M.; Ilango, R.V.J.; Shanmugam, A.; Venkidasamy, B.; Thiruvengadam, M. The Effect of Abiotic and Biotic Stresses on the Production of Bioactive Compounds in Tea (Camellia sinensis (L.) O. Kuntze). Plant Gene 2021, 27, 100316. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Motta, M.; Marcone, M.; Baptista, J. Variability of Antioxidant Properties, Catechins, Caffeine, L-Theanine and Other Amino Acids in Different Plant Parts of Azorean Camellia sinensis. Curr. Res. Food Sci. 2020, 3, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Vasil, I.K. A History of Plant Biotechnology: From the Cell Theory of Schleiden and Schwann to Biotech Crops. Plant Cell Rep. 2008, 27, 1423–1440. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Zhu, Q.; Tukhvatshin, M.; Cheng, B.; Tan, M.; Liu, J.; Wang, X.; Huang, J.; Gao, S.; Lin, D.; et al. Light Control of Catechin Accumulation Is Mediated by Photosynthetic Capacity in Tea Plant (Camellia sinensis). BMC Plant Biol. 2021, 21, 478. [Google Scholar] [CrossRef] [PubMed]
- Forrest, G.I. Studies on the Polyphenol Metabolism of Tissue Cultures Derived from the Tea Pant (Camellia sinensis L.). Biochem. J. 1969, 113, 765–781. [Google Scholar] [CrossRef]
- Orihara, Y.; Furuya, T. Production of Theanine and Other γ-Glutamyl Derivatives by Camellia sinensis Cultured Cells. Plant Cell Rep. 1990, 9, 65–68. [Google Scholar] [CrossRef]
- Muthaiya, M.J.; Nagella, P.; Thiruvengadam, M.; Mandal, A.A. Enhancement of the Productivity of Tea (Camellia sinensis) Secondary Metabolites in Cell Suspension Cultures Using Pathway Inducers. J. Crop Sci. Biotechnol. 2013, 16, 143–149. [Google Scholar] [CrossRef]
- Grover, A.; Yadav, J.S.; Biswas, R.; Pavan, C.S.S.; Mishra, P.; Bisaria, V.S.; Sundar, D. Production of Monoterpenoids and Aroma Compounds from Cell Suspension Cultures of Camellia sinensis. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 108, 323–331. [Google Scholar] [CrossRef]
- Shibasaki-Kitakawa, N.; Takeishi, J.; Yonemoto, T. Improvement of Catechin Productivity in Suspension Cultures of Tea Callus Cells. Biotechnol. Prog. 2003, 19, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki-Kitakawa, N.; Iizuka, Y.; Takahashi, A.; Yonemoto, T. A Kinetic Model for Flavonoid Production in Tea Cell Culture. Bioprocess. Biosyst. Eng. 2017, 40, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, Y.; Zhang, S.; Wu, T. Induction of Callus and Establishment of Cell Suspension and Effects on Tea Polyphenols in the Cell Suspension System of Tea Plants by PGRs. Sci. Hortic. 2023, 310, 111770. [Google Scholar] [CrossRef]
- San-José, M.C.; Vieitez, A.M. In Vitro Regeneration of Camellia reticulata Cultivar ‘Captain Rawes’ from Adult Material. Sci. Hortic. 1990, 43, 155–162. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Wang, Z.; Liu, Y.; Sun, M.; Yang, D.; Wei, C.; Shan, Y.; Xia, T. Light-Induced Expression of Genes Involved in Phenylpropanoid Biosynthetic Pathways in Callus of Tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2012, 133, 72–83. [Google Scholar] [CrossRef]
- Widholm, J.M. The Use of Fluorescein Diacetate and Phenosafranine for Determining Viability of Cultured Plant Cells. Biotech. Histochem. 1972, 47, 189–194. [Google Scholar] [CrossRef]
- ISO 14502-2:2005; Determination of Substances Characteristic of Green and Black Tea—Part 2: Content of Catechins in Green Tea—Method Using High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2006.
- Gai, Q.Y.; Jiao, J.; Wang, X.; Fu, Y.J.; Lu, Y.; Liu, J.; Wang, Z.Y.; Xu, X.J. Establishment of Cajanus cajan (Linn.) Millsp. Cell Suspension Cultures as an Effective In Vitro Platform for the Production of Pharmacologically Active Phenolic Compounds. Ind. Crops Prod. 2020, 158, 112977. [Google Scholar] [CrossRef]
- Qu, J.G.; Zhang, W.; Jin, M.F.; Yu, X.J. Effect of Homogeneity on Cell Growth and Anthocyanin Biosynthesis in Suspension Cultures of Vitis vinifera. Chin. J. Biotechnol. 2006, 22, 805–810. [Google Scholar] [CrossRef]
- Ossipov, V.; Zubova, M.; Nechaeva, T.; Zagoskina, N.; Salminen, J.P. The Regulating Effect of Light on the Content of Flavan-3-Ols and Derivatives of Hydroxybenzoic Acids in the Callus Culture of the Tea Plant, Camellia sinensis L. Biochem. Syst. Ecol. 2022, 101, 104383. [Google Scholar] [CrossRef]
- Jang, E.B.; Ho, T.T.; Park, S.Y. Effect of Light Quality and Tissue Origin on Phenolic Compound Accumulation and Antioxidant Activity in Camellia japonica Calli. Vitr. Cell. Dev. Biol. Plant 2020, 56, 567–577. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, X.; Zhu, C.; Zhang, S.; Li, X.; Fu, H.; Chen, C.; Lin, Y.; Lai, Z. De Novo Transcriptome Combined with Spectrophotometry and Gas Chromatography-Mass Spectrometer (GC-MS) Reveals Differentially Expressed Genes during Accumulation of Secondary Metabolites in Purple-Leaf Tea (Camellia sinensis cv Hongyafoshou). J. Hortic. Sci. Biotechnol. 2019, 94, 349–367. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of Catechin, Epicatechin, and Rutin: Optimization of a Novel Complete Antidiabetic Formulation Using a Mixture Design Approach. J. Nutr. Biochem. 2021, 88, 108520. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, K.; Shamala, L.F.; Wei, S. Influence of Media Supplements on Inhibition of Oxidative Browning and Bacterial Endophytes of Camellia sinensis var. Sinensis. 3 Biotech 2018, 8, 356. [Google Scholar] [CrossRef]
- Dong, Y.; Fu, C.; Su, P.; Xu, X.; Yuan, J.; Wang, S.; Zhang, M.; Zhao, C.; Yu, L. Mechanisms and Effective Control of Physiological Browning Phenomena in Plant Cell Cultures. Physiol. Plant 2016, 156, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Sakurai, M.; Seki, M.; Furusaki, S. Use of Auxin and Cytokinin to Regulate Anthocyanin Production and Composition in Suspension Cultures of Strawberry Cell. J. Sci. Food Agric. 1994, 65, 271–276. [Google Scholar] [CrossRef]
- De-Eknamkul, W.; Ellis, B.E. Effects of Auxins and Cytokinins on Growth and Rosmarinic Acid Formation in Cell Suspension Cultures of Anchusa Officinalis. Plant Cell Rep. 1985, 4, 50–53. [Google Scholar] [CrossRef]
- Luczkiewicz, M.; Kokotkiewicz, A.; Glod, D. Plant Growth Regulators Affect Biosynthesis and Accumulation Profile of Isoflavone Phytoestrogens in High-Productive in Vitro Cultures of Genista Tinctoria. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 419–429. [Google Scholar] [CrossRef]
- Nagella, P.; Murthy, H.N. Establishment of Cell Suspension Cultures of Withania Somnifera for the Production of Withanolide A. Bioresour. Technol. 2010, 101, 6735–6739. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rekha, K.; Rajakumar, G.; Lee, T.J.; Kim, S.H.; Chung, I.-M. Enhanced Production of Anthraquinones and Phenolic Compounds and Biological Activities in the Cell Suspension Cultures of Polygonum Multiflorum. Int. J. Mol. Sci. 2016, 17, 1912. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and Related Purine Alkaloids: Biosynthesis, Catabolism, Function and Genetic Engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Orihara, Y.; Tsuda, Y. Caffeine and Theanine from Cultured Cells of Camellia sinensis. Phytochemistry 1990, 29, 2539–2543. [Google Scholar] [CrossRef]
- Akalezi, C.O.; Liu, S.; Li, Q.S.; Yu, J.T.; Zhong, J.J. Combined Effects of Initial Sucrose Concentration and Inoculum Size on Cell Growth and Ginseng Saponin Production by Suspension Cultures of Panax ginseng. Process Biochem. 1999, 34, 639–642. [Google Scholar] [CrossRef]
- Zhong, J.J.; Yoshida, T. High-Density Cultivation of Perilla Frutescens Cell Suspensions for Anthocyanin Production: Effects of Sucrose Concentration and Inoculum Size. Enzym. Microb. Technol. 1995, 17, 1073–1079. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wu, J.Y.; He, G.Y. Effects of Inoculum Size and Age on Biomass Growth and Paclitaxel Production of Elicitor-Treated Taxus yunnanensis Cell Cultures. Appl. Microbiol. Biotechnol. 2002, 60, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Beum, J.K.; Gibson, D.M.; Shuler, M.L. Effect of Subculture and Elicitation on Instability of Taxol Production in Taxus Sp. Suspension Cultures. Biotechnol. Prog. 2004, 20, 1666–1673. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W.; Yu, X.; Jin, M. Instability of Anthocyanin Accumulation in Vitis vinifera L. Var. Gamay Fréaux Suspension Cultures. Biotechnol. Bioprocess. Eng. 2005, 10, 155–161. [Google Scholar] [CrossRef]
- Whitmer, S.; Canel, C.; Van Der Heijden, R.; Verpoorte, R. Long-Term Instability of Alkaloid Production by Stably Transformed Cell Lines of Catharanthus Roseus. Plant Cell Tissue Organ. Cult. 2003, 74, 73–80. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary Metabolism of Pharmaceuticals in the Plant In Vitro Cultures: Strategies, Approaches, and Limitations to Achieving Higher Yield. Plant Cell Tissue Organ. Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Denton-Giles, M.; Bradshaw, R.E.; Dijkwel, P.P. Ciborinia camelliae (Sclerotiniaceae) Induces Variable Plant Resistance Responses in Selected Species of Camellia. Phytopathology 2013, 103, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of Salicylic Acid and Methyl Jasmonate in the Production of Phenolic Compounds in Plant Cell Suspension Cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The Effects of Jasmonic Acid and Methyl Jasmonate on Rosmarinic Acid Production in Mentha × Piperita Cell Suspension Cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kugimiya, T. Effects of Ethanol on Growth of Rice Seedlings. Plant Growth Regul. 2001, 35, 93–96. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Sako, K.; Matsui, A.; Suzuki, Y.; Mostofa, M.G.; Van Ha, C.; Tanaka, M.; Tran, L.-S.P.; Habu, Y.; Seki, M. Ethanol Enhances High-Salinity Stress Tolerance by Detoxifying Reactive Oxygen Species in Arabidopsis Thaliana and Rice. Front. Plant Sci. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Low Temperature Acclimation Mediated by Ethanol Production Is Essential for Chilling Tolerance in Rice Roots. Plant Signal Behav. 2008, 3, 202–203. [Google Scholar] [CrossRef]
- Das, A.K.; Anik, T.R.; Rahman, M.M.; Keya, S.S.; Islam, M.R.; Rahman, M.A.; Sultana, S.; Ghosh, P.K.; Khan, S.; Ahamed, T.; et al. Ethanol Treatment Enhances Physiological and Biochemical Responses to Mitigate Saline Toxicity in Soybean. Plants 2022, 11, 272. [Google Scholar] [CrossRef]
- Xiong, L.; Li, J.; Li, Y.; Yuan, L.; Liu, S.; Huang, J.; Liu, Z. Dynamic Changes in Catechin Levels and Catechin Biosynthesis-Related Gene Expression in Albino Tea Plants (Camellia sinensis L.). Plant Physiol. Biochem. 2013, 71, 132–143. [Google Scholar] [CrossRef]
- Tassoni, A.; Durante, L.; Ferri, M. Combined Elicitation of Methyl-Jasmonate and Red Light on Stilbene and Anthocyanin Biosynthesis. J. Plant Physiol. 2012, 169, 775–781. [Google Scholar] [CrossRef]

| Chemical Name | Formula | CAS | Catalog Number | Company |
|---|---|---|---|---|
| Acetic acid | C2H4O2 | [64-19-7] | A465250 | Fisher-Scientific (Geel, Belgium) |
| Acetonitrile | C2H3N | [75-05-8] | A998SK | Fisher-Scientific (Geel, Belgium) |
| 6-Benzylaminopurine | C12H11N5 | [1214-39-7] | B0904 | Duchefa Biochemie (Haarlem, The Netherlands) |
| Caffeine | C8H10N4O2 | [58-08-2] | 10263800 | Fisher-Scientific (Geel, Belgium) |
| (+)-Catechin | C₁₅H₁₄O₆ | [154-23-4] | 0976 S | Extrasynthese (Genay, France) |
| (−)-Catechin gallate | C₂₂H₁₈O₁₀ | [130405-40-2] | 0972 S | Extrasynthese (Genay, France) |
| 2,4-Dichlorophenoxyacetic acid | C8H6Cl2O3 | [94-75-7] | D7299 | Sigma-Aldrich (Merck, Darmstadt, Germany) |
| 6-γ,γ-Dimethylallylamino purine | C10H13N5 | [2365-40-4] | D0906 | Duchefa Biochemie (Haarlem, The Netherlands) |
| (−)-Epicatechin | C₁₅H₁₄O₆ | [490-46-0] | 0977 S | Extrasynthese (Genay, France) |
| (−)-Epigallocatechin | C₁₅H₁₄O₇ | [970-74-1] | 0979 S | Extrasynthese (Genay, France) |
| (−)-Epigallocatechin gallate | C₂₂H₁₈O₁₁ | [989-51-5] | 0981 S | Extrasynthese (Genay, France) |
| (−)-Epicatechin gallate | C₂₂H₁₈O₁₀ | [1257-08-5] | 0978 S | Extrasynthese (Genay, France) |
| Gallic acid | C7H6O5 | [149-91-7] | 398225 | Sigma-Aldrich (Merck, Darmstadt, Germany) |
| (+)-Gallocatechin | C₁₅H₁₄O₇ | [970-73-0] | 0990 S | Extrasynthese (Genay, France) |
| (−)-Gallocatechin gallate | C₂₂H₁₈O₁₁ | [4233-96-9] | 0974 S | Extrasynthese (Genay, France) |
| Indole-3-butyric acid | C12H13NO2 | [133-32-4] | I0902 | Duchefa Biochemie (Haarlem, The Netherlands) |
| Kinetin | C10H9N5O | [525-79-1] | K0905 | Duchefa Biochemie (Haarlem, The Netherlands) |
| Methanol | CH4O | [67-56-1] | 10675112 | Fisher-Scientific (Geel, Belgium) |
| Methyl jasmonate | C13H20O3 | [39924-52-2] | 392707 | Sigma-Aldrich (Merck, Darmstadt, Germany) |
| Theobromine | C7H8N4O2 | [83-67-0] | 11493930 | Fisher-Scientific (Geel, Belgium) |
| Theophylline | C7H8N4O2 | [58-55-9] | 10712481 | Acros Organics (Geel, Belgium) |
| Trans-zeatin | C10H13N5O | [1637-39-4] | Z0917 | Duchefa Biochemie (Haarlem, The Netherlands) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban-Campos, P.; Vela, P.; Rodríguez-Solana, R.; López-Sánchez, J.I.; Salinero, C.; Pérez-Santín, E. Influence of the Culture Conditions on Camellia sinensis Cell Cultures. Foods 2024, 13, 2461. https://doi.org/10.3390/foods13152461
Esteban-Campos P, Vela P, Rodríguez-Solana R, López-Sánchez JI, Salinero C, Pérez-Santín E. Influence of the Culture Conditions on Camellia sinensis Cell Cultures. Foods. 2024; 13(15):2461. https://doi.org/10.3390/foods13152461
Chicago/Turabian StyleEsteban-Campos, Pilar, Pilar Vela, Raquel Rodríguez-Solana, José Ignacio López-Sánchez, Carmen Salinero, and Efrén Pérez-Santín. 2024. "Influence of the Culture Conditions on Camellia sinensis Cell Cultures" Foods 13, no. 15: 2461. https://doi.org/10.3390/foods13152461






