Effects of High-Hydrostatic-Pressure Treatment on Polyphenols and Volatile Aromatic Compounds in Marselan Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation and Equipment
2.3. Determination Methods
2.3.1. Marselan Red-Wine-Making Process
2.3.2. High-Hydrostatic-Pressure Treatment
2.3.3. Determination of Phenolic Acids
2.3.4. Determination of Volatile Aromatic Compounds
2.3.5. Characteristic Aroma and Aroma-Profile Analysis
2.4. Sensorial Analysis
2.5. Data Processing and Analysis
3. Results
3.1. The Impact of High-Hydrostatic-Pressure Treatment on the Polyphenol Content of Marselan Red Wine
3.1.1. The Effects of HHP on the Total Polyphenol Content and Types
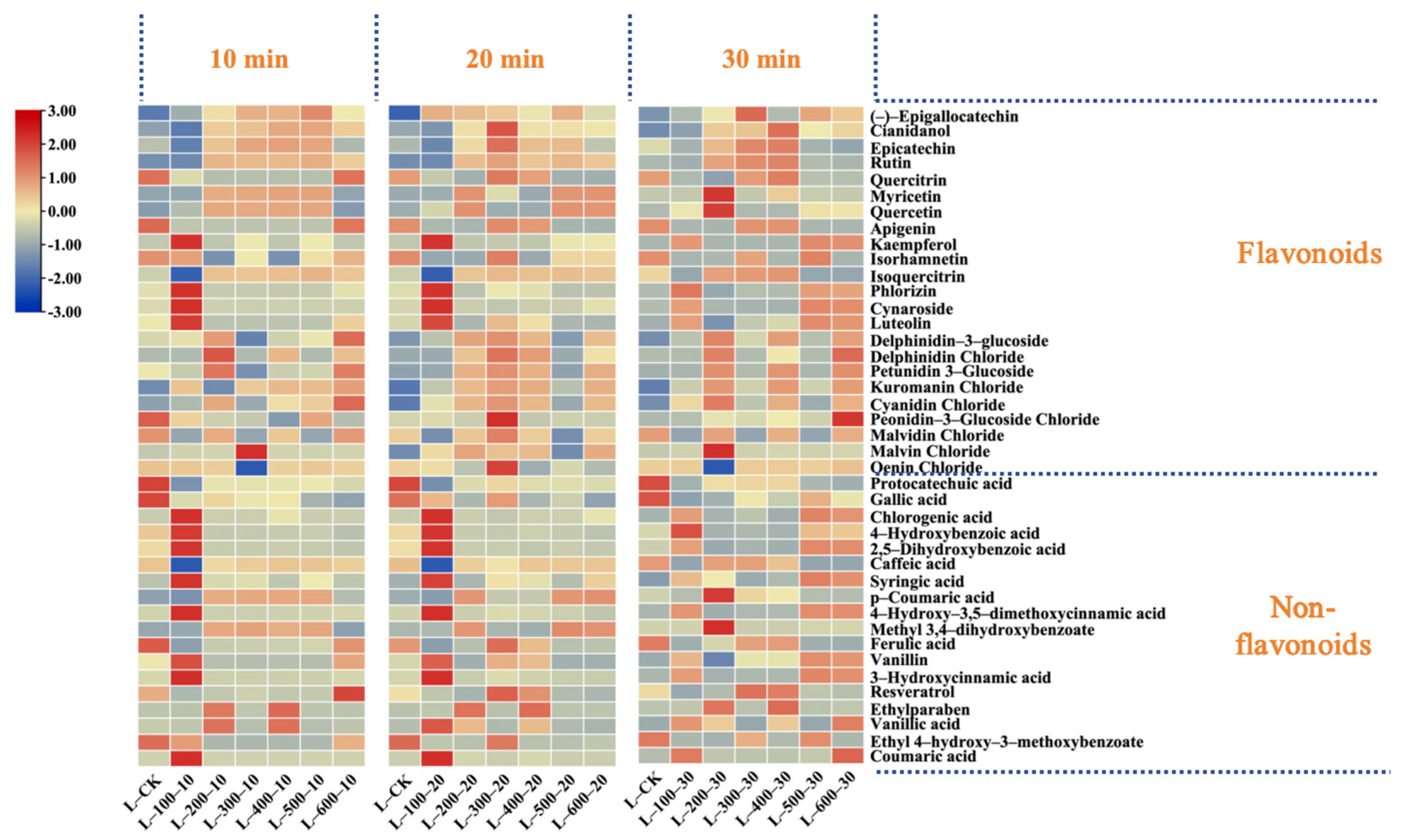
3.1.2. Effects of High-Hydrostatic-Pressure Treatment on Various Phenolic Substances
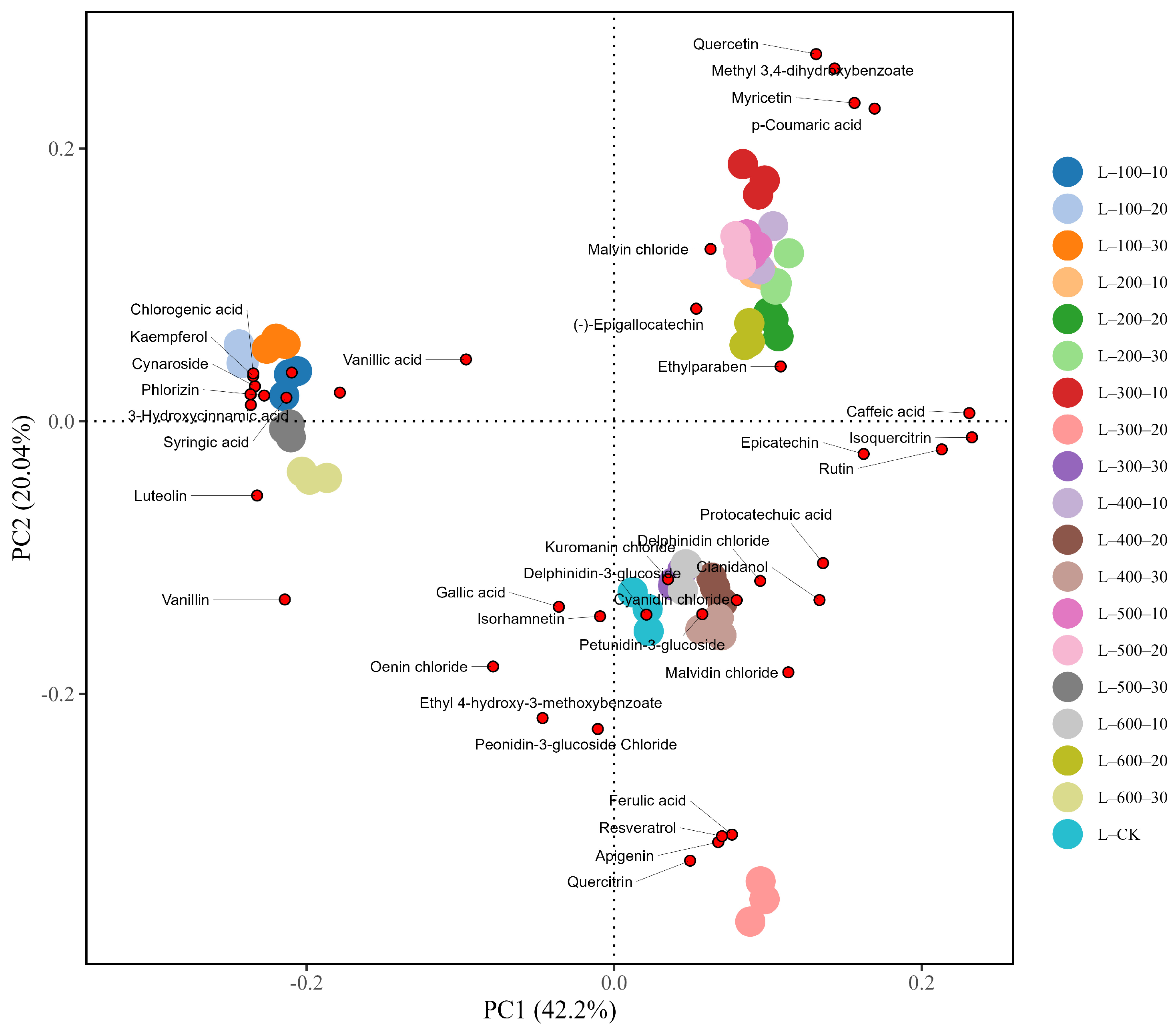
3.1.3. PCA Analysis
3.2. The Effect of High-Hydrostatic-Pressure Treatment on Volatile Aromatic Compounds
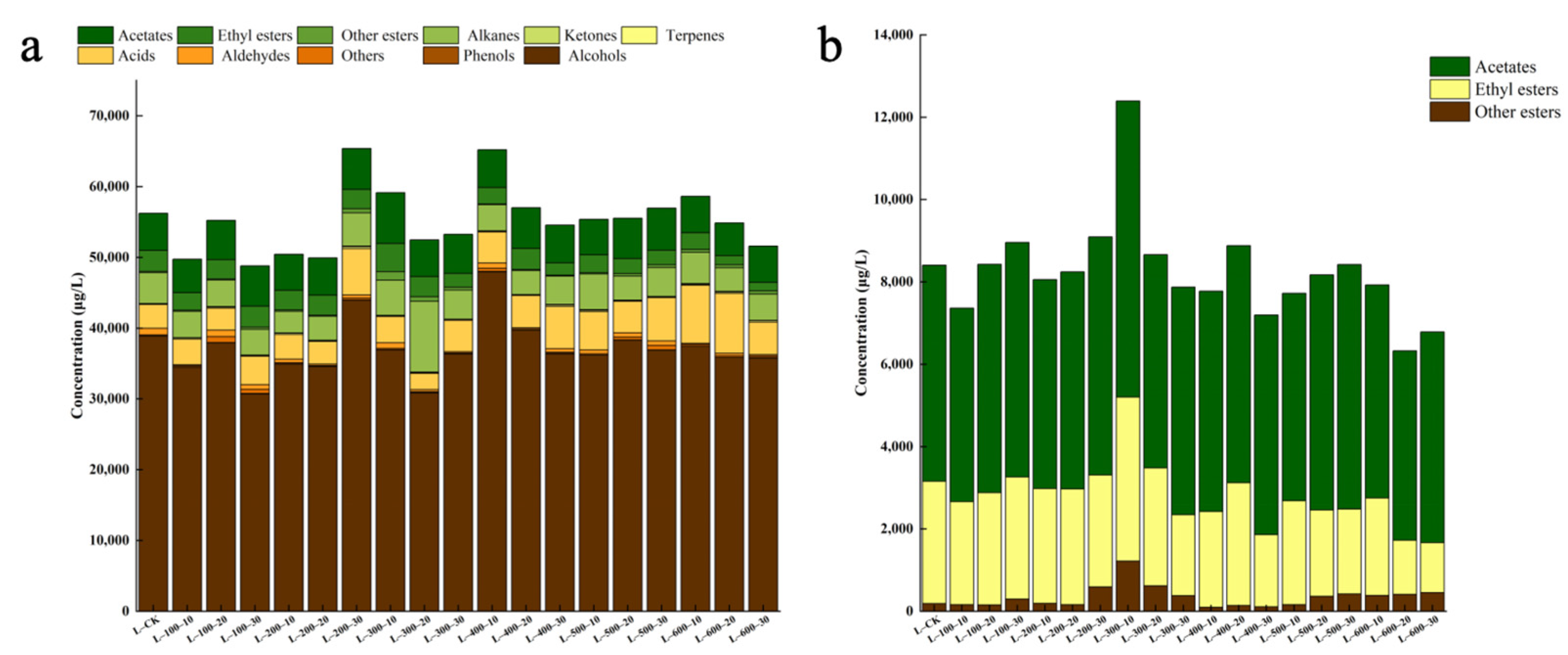
3.2.1. The Influence of High-Hydrostatic-Pressure Treatment on the Variety and Content of Volatile Aromatic Compounds
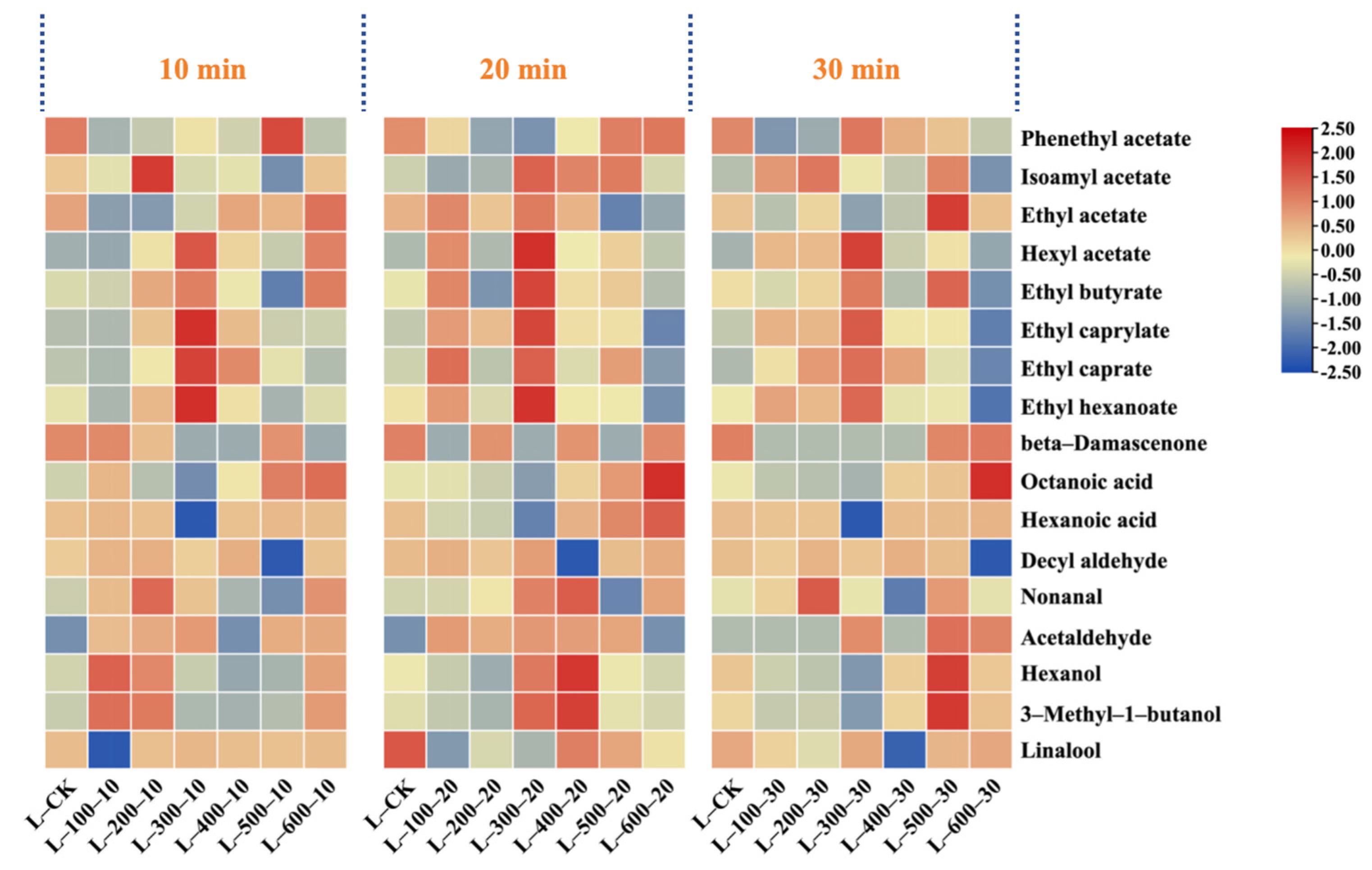
3.2.2. The Impact of High-Hydrostatic-Pressure Treatment on Major Volatile Aroma Compounds
| Compound | Concentration/(μg/L) | Threshold (μg/L) [26,27,28] | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-CK | L-100-10 | L-100-20 | L-100-30 | L-200-10 | L-200-20 | L-200-30 | L-300-10 | L-300-20 | L-300-30 | L-400-10 | L-400-20 | L-400-30 | L-500-10 | L-500-20 | L-500-30 | L-600-10 | L-600-20 | L-600-30 | ||
| Phenethyl acetate | 411.65 ± 14.26 abc | 336.7 ± 18.75 efghi | 372.15 ± 3.72 cde | 326.17 ± 28.43 fghij | 347.21 ± 12.52 defgh | 319.7 ± 13.94 ghij | 305.46 ± 34.01 ij | 367.61 ± 13.25 def | 309.6 ± 27.52 hij | 291.73 ± 26.26 j | 351.83 ± 15.34 defg | 362.42 ± 44.54 def | 387 ± 23.54 bcd | 436.03 ± 19.01 a | 420.81 ± 26.28 ab | 374.3 ± 46 cde | 344.77 ± 22.61 efghi | 424.67 ± 25.83 ab | 424.62 ± 19.46 ab | 250 |
| Isoamyl acetate | 6077.36 ± 425.42 defg | 5299.79 ± 505.57 gh | 5529.27 ± 331.76 efgh | 6796.93 ± 475.79 d | 9125.4 ± 1075.87 a | 5689.13 ± 227.57 efgh | 8842.54 ± 755.51 ab | 5152.76 ± 472.26 h | 8328.87 ± 288.52 abc | 8214.77 ± 730.14 bc | 5317.67 ± 140.69 fgh | 7826.89 ± 135.57 c | 6223.01 ± 631.57 de | 3912.16 ± 103.51 i | 7952.18 ± 993.23 c | 8564.63 ± 226.6 abc | 6160.22 ± 184.81 defg | 6163.35 ± 465.32 def | 5350.44 ± 192.91 fgh | 30 |
| Ethyl acetate | 6251.75 ± 225.41 cd | 3100.57 ± 124.02 g | 7825.94 ± 414.11 ab | 5367.53 ± 568.05 e | 3048.15 ± 30.48 g | 5672.64 ± 294.76 cde | 6133.23 ± 212.46 cd | 4115.11 ± 108.88 f | 8418.32 ± 252.55 a | 5624.09 ± 97.41 de | 6199.58 ± 647.26 cd | 6242.02 ± 286.05 cd | 5659.22 ± 226.37 cde | 5809.02 ± 307.38 cde | 2249.37 ± 155.84 h | 7310.44 ± 633.1 b | 7663.53 ± 903.52 b | 2918.16 ± 267.45 g | 6279.98 ± 439.6 c | 7500 |
| Hexyl acetate | 594.96 ± 64.35 ij | 567.05 ± 5.67 j | 1252.47 ± 57.4 d | 1806.77 ± 177.95 b | 855.65 ± 53.44 fg | 592.82 ± 42.75 ij | 1028.17 ± 54.41 e | 1561.44 ± 133.41 c | 1964.5 ± 34.03 a | 1040.92 ± 72.86 e | 908.89 ± 63.62 fg | 795.86 ± 68 gh | 697.56 ± 66.54 hi | 695.77 ± 31.88 hi | 913.86 ± 15.83 f | 862.49 ± 22.82 fg | 1296.65 ± 44.92 d | 642.92 ± 40.15 ij | 551.6 ± 9.55 j | 1500 |
| Ethyl butyrate | 697.9 ± 62.03 efgh | 686.52 ± 31.46 fghi | 865.64 ± 62.42 b | 763.31 ± 7.63 cde | 807.26 ± 32.29 bc | 566.37 ± 25.95 j | 705.96 ± 61.54 defgh | 864.11 ± 37.67 b | 988.65 ± 69.21 a | 675.85 ± 17.88 ghi | 717.79 ± 31.29 defg | 729.63 ± 71.86 defg | 656.83 ± 28.63 ghi | 574.91 ± 71.81 j | 756.07 ± 27.26 cdef | 779.26 ± 54.55 cd | 867.7 ± 26.03 b | 631.68 ± 31.58 hij | 620.78 ± 22.38 ij | 20 |
| Ethyl caprylate | 4307.13 ± 261.99 g | 4117.56 ± 288.23 g | 11,211.21 ± 917.68 c | 19,550.48 ± 2304.97 b | 8025.95 ± 526.3 e | 8657.03 ± 687.13 de | 9178.65 ± 91.79 de | 20,813.62 ± 1456.95 ab | 22,059.11 ± 1167.26 a | 9547.03 ± 626.04 d | 8405.8 ± 303.08 de | 6634.99 ± 239.23 f | 6398.36 ± 610.36 f | 4934.81 ± 85.47 g | 6586.9 ± 287.12 f | 6395.79 ± 230.6 f | 5013.57 ± 279.14 g | 2186.4 ± 178.96 h | 2011.03 ± 111.97 h | 580 |
| Ethyl caprate | 974.92 ± 44.68 i | 885.3 ± 61.97 i | 2895.57 ± 176.13 c | 3325.2 ± 185.14 b | 1373.03 ± 137.3 fg | 872.06 ± 26.16 i | 2477.56 ± 178.66 d | 3943.38 ± 197.17 a | 3159.77 ± 175.93 b | 1571.82 ± 149.94 f | 2493.99 ± 199.52 d | 1056.78 ± 104.08 hi | 2339.57 ± 101.98 d | 1253.51 ± 57.44 gh | 2083.29 ± 157.28 e | 1326.56 ± 105.29 g | 913.69 ± 48.35 i | 604.46 ± 74.28 j | 634.6 ± 54.22 j | 200 |
| Ethyl hexanoate | 4729.15 ± 451.13 fg | 3592.88 ± 164.65 i | 7394.96 ± 295.8 d | 10,261.61 ± 271.5 c | 6338 ± 385.53 e | 3963.93 ± 79.28 hi | 6375.37 ± 292.16 e | 12,394.77 ± 1101.67 b | 13,665.46 ± 236.69 a | 7247.59 ± 452.61 d | 5236.19 ± 617.34 f | 4569.33 ± 199.17 gh | 4512.01 ± 274.46 gh | 3533.56 ± 61.2 i | 4560.52 ± 373.3 gh | 4639.9 ± 202.25 fg | 4539.19 ± 433.01 gh | 2249 ± 192.15 j | 1972.25 ± 161.44 j | 14 |
| beta-Damascenone | 22.2 ± 1.35 b | 22.26 ± 1.68 b | ND | ND | 8.5 ± 0.53 e | 16.55 ± 1.58 d | ND | ND | ND | ND | ND | 15.92 ± 1.27 d | ND | 19 ± 0.95 c | ND | 19.22 ± 0.77 c | ND | 18.57 ± 1.65 c | 25.11 ± 0.5 a | 0.05 |
| Octanoic acid | 1054.48 ± 36.53 g | 1780 ± 141.28 e | 1015.72 ± 36.62 g | 638.34 ± 49.86 jkl | 916.87 ± 87.46 gh | 848.15 ± 38.87 hi | 720.61 ± 12.48 ijk | 607.09 ± 37.91 kl | 532.93 ± 33.28 l | 773.15 ± 73.75 hij | 1336.84 ± 114.22 f | 1353.03 ± 75.33 f | 1363.23 ± 138.35 f | 2560.28 ± 67.74 c | 2031.7 ± 107.51 d | 1466.37 ± 89.2 f | 2846.41 ± 113.86 b | 4533.71 ± 119.95 a | 4604.06 ± 200.69 a | 500 |
| Hexanoic acid | 1922.98 ± 120.09 de | 2603.78 ± 130.19 a | 1684.03 ± 44.56 f | ND | 1924.32 ± 99.99 de | 1658.27 ± 141.68 f | 1567.84 ± 68.34 fg | ND | 1392.79 ± 127.65 g | 1546.81 ± 61.87 fg | 1895.46 ± 94.77 e | 1969.95 ± 171.74 de | 1974.16 ± 175.47 de | 2346.08 ± 208.52 b | 2119.69 ± 132.37 cd | 1996.89 ± 163.45 de | 2101.72 ± 105.09 cde | 2289.53 ± 160.27 bc | 2484.12 ± 155.13 ab | 420 |
| Decyl aldehyde | 26.77 ± 2.14 e | 38.09 ± 2.88 b | 31.43 ± 0.54 cd | 23.72 ± 0.47 ef | 41.36 ± 3.39 ab | 23.5 ± 1.92 ef | 30.35 ± 2.59 d | 25.49 ± 0.92 e | 41.29 ± 2.98 ab | 21.1 ± 0.56 f | 42.24 ± 2.57 a | ND | 32.8 ± 3.01 cd | ND | 26.08 ± 1.71 e | 26.48 ± 2 e | 30.52 ± 1.06 d | 34.09 ± 2.91 c | ND | 10 |
| Nonanal | 84.19 ± 6.89 gh | 120.79 ± 2.42 cd | 84.75 ± 3.69 g | 84.93 ± 7.4 g | 172.78 ± 12.09 a | 93.27 ± 4.27 fg | 121.59 ± 9.65 cd | 116.8 ± 7.66 cd | 125.31 ± 11.14 c | 91.84 ± 5.11 g | 73.16 ± 5.07 hi | 139.39 ± 7.76 b | 62.04 ± 2.7 ij | 58.9 ± 4.71 j | 62.81 ± 2.26 ij | 104.41 ± 7.31 ef | 144.13 ± 10.09 b | 113.08 ± 3.92 de | 84.15 ± 6.89 gh | 15 |
| Acetaldehyde | ND | 229.71 ± 16.08 g | 624.23 ± 43.25 b | 173.09 ± 5.19 h | 427.71 ± 33.41 de | 354.24 ± 12.77 f | ND | 742.37 ± 63.43 a | 699.22 ± 36.33 a | ND | ND | 575.65 ± 52.76 c | ND | 404.85 ± 38.62 e | 432.89 ± 22.91 de | 464.87 ± 13.95 d | 438.89 ± 19.13 de | ND | 227.68 ± 8.21 g | 186 |
| Hexanol | 2104.3 ± 147.3 c | 2757.4 ± 153.53 a | 2003.64 ± 173.52 c | 1721.97 ± 51.66 d | 2589.89 ± 258.99 ab | 1899.64 ± 165.61 cd | 1868.49 ± 37.37 cd | 2077.78 ± 90.57 c | 2467.79 ± 24.68 b | 1905.93 ± 115.93 cd | 1921.48 ± 221.6 cd | 2699.52 ± 214.27 ab | 2082.46 ± 165.29 c | 1972.29 ± 71.11 cd | 2103.25 ± 200.64 c | 2542.61 ± 110.83 ab | 2483.35 ± 99.33 b | 2031.43 ± 219.73 c | 2099.9 ± 127.73 c | 1100 |
| 3-Methyl-1-butanol | 30,780.32 ± 1231.21 cd | 43,358.1 ± 2167.9 a | 29,350.09 ± 1344.99 cd | 24,963.63 ± 660.48 e | 42,270.77 ± 2958.95 ab | 28,073.56 ± 0 de | 27,938.58 ± 1744.76 de | 29,077.82 ± 2195.33 cd | 39,643.49 ± 2207.26 b | 27,748.16 ± 1209.51 de | 28,595.03 ± 2233.34 cde | 42,425.17 ± 3818.27 ab | 30,888.4 ± 3641.69 cd | 29,594.02 ± 1183.76 cd | 31,033.24 ± 537.51 cd | 40,202.6 ± 1752.39 ab | 39,472.74 ± 4121.08 b | 30,240.51 ± 2361.86 cd | 31,856.12 ± 1592.81 c | 3000 |
| Linalool | 118.59 ± 11.31 bc | ND | 83.37 ± 1.67 ij | 117.76 ± 3.53 bcd | 112.24 ± 8.77 bcde | 93.24 ± 9.46 ghi | 54.01 ± 3.74 k | 134.67 ± 10.52 a | 87.81 ± 6.33 hij | 80.46 ± 3.69 j | 112.5 ± 6.75 bcde | 112.28 ± 6.83 bcde | 11.27 ± 1.03 l | 105.96 ± 1.84 def | 106.15 ± 9.55 cdef | 104.72 ± 4.8 efg | 121.61 ± 14.34 b | 97.64 ± 0.98 fgh | 120.24 ± 13.39 b | 25 |
3.2.3. PCA Analysis
3.3. Wine Sensorial Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Sun, F.; Wang, W.; Liu, Y.; Wang, J.; Sun, J.; Mu, J.; Gao, Z. Effects of spontaneous fermentation on the microorganisms diversity and volatile compounds during ‘Marselan’ from grape to wine. LWT-Food Sci. Technol. 2020, 134, 110193. [Google Scholar] [CrossRef]
- Minnaar, P.; Nyobo, L.; Jolly, N.; Ntushelo, N.; Meiring, S. Anthocyanins and polyphenols in Cabernet Franc wines produced with Saccharomyces cerevisiae and Torulaspora delbrueckii yeast strains: Spectrophotometric analysis and effect on selected sensory attributes. Food Chem. 2018, 268, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Shi, J.; Zeng, M.; Tang, Z.; Xie, S.; Zhang, Z. Inter- and intra-varietal genetic variations co-shape the polyphenol profiles of Vitis vinifera L. grapes and wines. Food Chem. X 2023, 20, 101030. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ning, Z.; Guang-Feng, W.; Fei, H.; Yi-Bin, L.; Chang-Qing, D. Intermolecular copigmentation between anthocyanidin-3,5-O-diglucosides and three phenolic compounds: Insights from experimental and theoretical studies. Food Chem. Adv. 2022, 1, 100111. [Google Scholar] [CrossRef]
- Tao, H.; Zhao, Y.; Li, L.; He, Y.; Zhang, X.; Zhu, Y.; Hong, G. Corrigendum to “Comparative metabolomics of flavonoids in twenty vegetables reveal their nutritional diversity and potential health benefits” [Food Res. Int. 164 (2023) 112384]. Food Res. Int. 2023, 173, 113439. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, R.G.; Bhange, M.A.; Oulkar, D.P.; Sharma, A.K.; Shabeer, T.P.A. Estimation of polyphenols by using HPLC-DAD in red and white wine grape varieties grown under tropical conditions of India. J. Food Sci. Technol. 2018, 55, 4994–5002. [Google Scholar] [CrossRef]
- Longo, R.; Carew, A.; Sawyer, S.; Kemp, B.; Kerslake, F. A review on the aroma composition of Vitis vinifera L. Pinot noir wines: Origins and influencing factors. Crit. Rev. Food Sci. Nutr. 2021, 61, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiong, R.; Wei, X.; Wei, Z.Y.; Yu, K.J.; Xie, L.M.; Yu, X.; Huang, H.L. Advances in the Study of Mechanisms and Influencing Factors for the Production of Phenolic Compounds in Wine. Food Res. Dev. 2023, 44, 195–201. [Google Scholar]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, F.; Yan, X.; Qin, Y.; Jiang, J.; Liu, Y.; Song, Y. Characterization of the β-Glucosidase activity in indigenous yeast isolated from wine regions in China. J. Food Sci. 2021, 86, 2327–2345. [Google Scholar] [CrossRef]
- Bai, X.; Ling, M.; Chen, B.; Lan, Y.; Cheng, C.; Duan, C.; Shi, Y. Effect of Grape Seed Tannin Addition before Barrel Aging on the Aroma of Cabernet Sauvignon and Marselan Dry Red Wine. Food Sci. 2022, 43, 251–257. [Google Scholar]
- Xiao, Z.; Jiang, X.; Niu, Y. Research Progress on Analysis of Aroma Compounds in Fruits. J. Food Sci. Technol. 2021, 39, 14–22. [Google Scholar]
- Hurtado, A.; Dolors Guardia, M.; Picouet, P.; Jofre, A.; Maria Ros, J.; Banon, S. Stabilization of red fruit-based smoothies by high-pressure processing. Part A. Effects on microbial growth, enzyme activity, antioxidant capacity and physical stability. J. Sci. Food Agric. 2017, 97, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Ren, H.; Yang, H.; Liu, Y.; Gao, Z.; Long, F. Changes in Physicochemical Properties and Volatiles of Kiwifruit Pulp Beverage Treated with High Hydrostatic Pressure. Foods 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Ma, T.; Zhao, F.; Yu, D.; Huang, W.; Zhan, J. High hydrostatic pressure treatment: An artificial accelerating aging method which did not change the region and variety non-colored phenolic characteristic of red wine. Innov. Food Sci. Emerg. Technol. 2016, 33, 123–134. [Google Scholar] [CrossRef]
- Lin, M.; Yang, B.; Dai, M.; Xu, Y.; Li, X.; Sun, B. East meets west in alcoholic beverages: Flavor comparison, microbial metabolism and health effects. Food Biosci. 2023, 56, 103385. [Google Scholar] [CrossRef]
- Xixian, S.; Weixi, Y.; Xu, Q.; Xinke, Z.; Mengqi, L.; Li, Y.; Ying, S.; Changqing, D.; Yibin, L. Comparison of Chemical and Sensory Profiles between Cabernet Sauvignon and Marselan Dry Red Wines in China. Foods 2023, 12, 1110. [Google Scholar] [CrossRef]
- Yang, P.Y.; Zhang, B.; Jiang, Y.M.; Wang, X.Q.; Lü, Z.Z.; Han, L.T.; Zhang, X.F. Effects of High-Pressure Processing on Phenolic Compounds in Vitis davidii Grapes. Food Ferment. Ind. 2023, 49, 110–119. [Google Scholar] [CrossRef]
- Liu, F.X. Study on Mango Juice Processing Technology and Quality Based on High-Pressure Technology. Ph.D. Thesis, China Agricultural University, Beijing, China, 2014. [Google Scholar]
- Wang, Y.C.; Ma, Y.K.; Yu, H.L.; Zhang, H.N.; Ye, H.; Li, J.F. Study on the Effect of High-Pressure Processing on Alcohol-Water Association in Yellow Wine. Mod. Food Sci. Technol. 2016, 32, 221–226. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Ma, L.; Liao, X.; Hu, X. Non-volatile and volatile metabolic profiling of tomato juice processed by high-hydrostatic-pressure and high-temperature short-time. Food Chem. 2022, 371, 131161. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.E.; Ramírez, R.; Martínez-Cañas, M.A.; Frutos-Puerto, S.; Moreno, D. Accelerating Aging of White and Red Wines by the Application of Hydrostatic High Pressure and Maceration with Holm Oak (Quercus ilex) Chips. Influence on Physicochemical and Sensory Characteristics. Foods 2021, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Panosyan, A.G.; Mamikonyan, G.V.; Torosyan, M.; Gabrielyan, E.S.; Mkhitaryan, S.A.; Tirakyan, M.R.; Ovanesyan, A. Determination of the composition of volatiles in cognac (brandy) by headspace gas chromatography-mass spectrometry. J. Anal. Chem. 2001, 56, 945–952. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, B.; Yang, P.Y.; Zhang, Y.; Zhang, X.F.; Chen, J.J.; Zhang, J.Q.; Zhang, Z.L.; Du, J.M. Optimization of High-Pressure Processing of Cabernet Sauvignon Grapes Using Response Surface Methodology and Its Impact on Volatile Compounds. Food Ferment. Ind. 2023, 2023, 1–11. [Google Scholar] [CrossRef]
- De Wijk, R.A.; Kaneko, D.; Dijksterhuis, G.B.; van Bergen, G.; Vingerhoeds, M.H.; Visalli, M.; Zandstra, E.H. A preliminary investigation on the effect of immersive consumption contexts on food-evoked emotions using facial expressions and subjective ratings. Food Qual. Prefer. 2022, 99, 104572. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Zhang, Z.; He, X.; Chen, F.; Chen, J.; Zhang, J. Changes in aroma compounds and characteristics of Chardonnay dry white wine from the Eastern Foot of Helan Mountain during fermentation. Food Ferment. Ind. 2023, 49, 188–195. [Google Scholar]
- Fogarty, J. The Palgrave Handbook of Wine Industry Economics. J. Wine Econ. 2020, 15, 127–129. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbuehler, B.; Schuettler, A.; Ebert, K.; Fritsch, S.; Roecker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.Q.; Xie, H.; Hua, Y.B.; Cai, J.; Li, S.Y.; Lan, Y.B.; Li, R.N.; Duan, C.Q.; Shi, Y. Flavor Profile Evolution of Bottle Aged Rose and White Wines Sealed with Different Closures. Molecules 2019, 24, 836. [Google Scholar] [CrossRef]
- Furtado, I.; Lopes, P.; Oliveira, A.S.; Amaro, F.; Bastos, M.D.; Cabral, M.; de Pinho, P.G.; Pinto, J. The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging. Foods 2021, 10, 2070. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ossandon, M.J.; Castillo, L.; Ah-Hen, K.S.; Vega-Galvez, A. Effect of high hydrostatic pressure processing on phytochemicals, antioxidant activity, and behavior of Botrytis cinerea in white grape juice concentrate. J. Food Process. Preserv. 2020, 44, 14864. [Google Scholar] [CrossRef]
- Cascaes Teles, A.S.; Hidalgo Chavez, D.W.; Zarur Coelho, M.A.; Rosenthal, A.; Fortes Gottschalk, L.M.; Tonon, R.V. Combination of enzyme-assisted extraction and high hydrostatic pressure for phenolic compounds recovery from grape pomace. J. Food Eng. 2021, 288, 110128. [Google Scholar] [CrossRef]
- De-jiang, L.; Ji-luan, C.; Long-ying, P. Effect of ultra-high pressure and heat treatment on activity and structure of four aroma synthesis enzymes in fruits and vegetables. Sci. Technol. Food Ind. 2020, 2020, 82–89. [Google Scholar]
- Pan, X.; Wu, J.; Zhang, W.; Liu, J.; Yang, X.; Liao, X.; Hu, X.; Lao, F. Effects of sugar matrices on the release of key aroma compounds in fresh and high hydrostatic pressure processed Tainong mango juices. Food Chem. 2021, 338, 128117. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Luo, Q.; Pan, X.; Zhang, W.T.; Lao, F.; Luo, W.B.; Pang, C.Y.; Wu, J.H. Research progress on aroma quality of fruit and vegetable juices under ultra-high pressure. Sci. Technol. Food Ind. 2024, 2024, 1–16. [Google Scholar] [CrossRef]

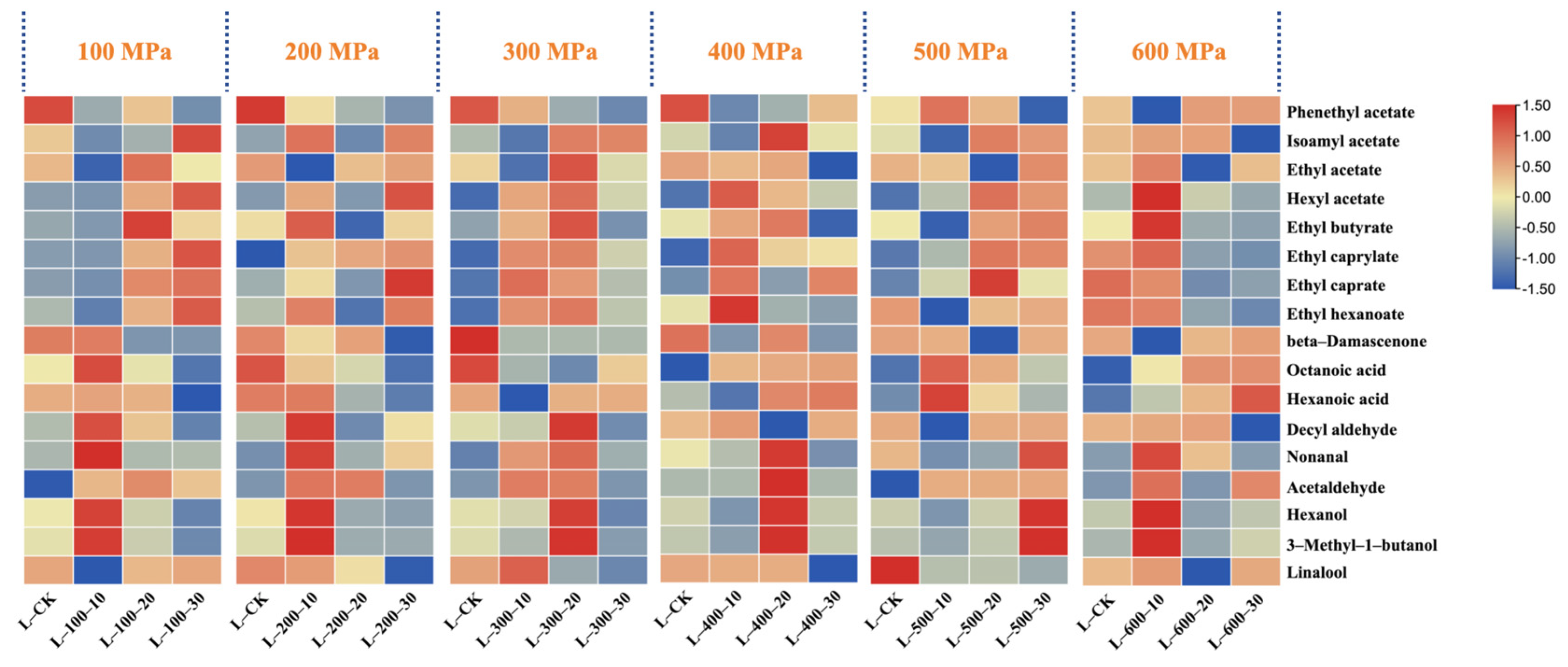
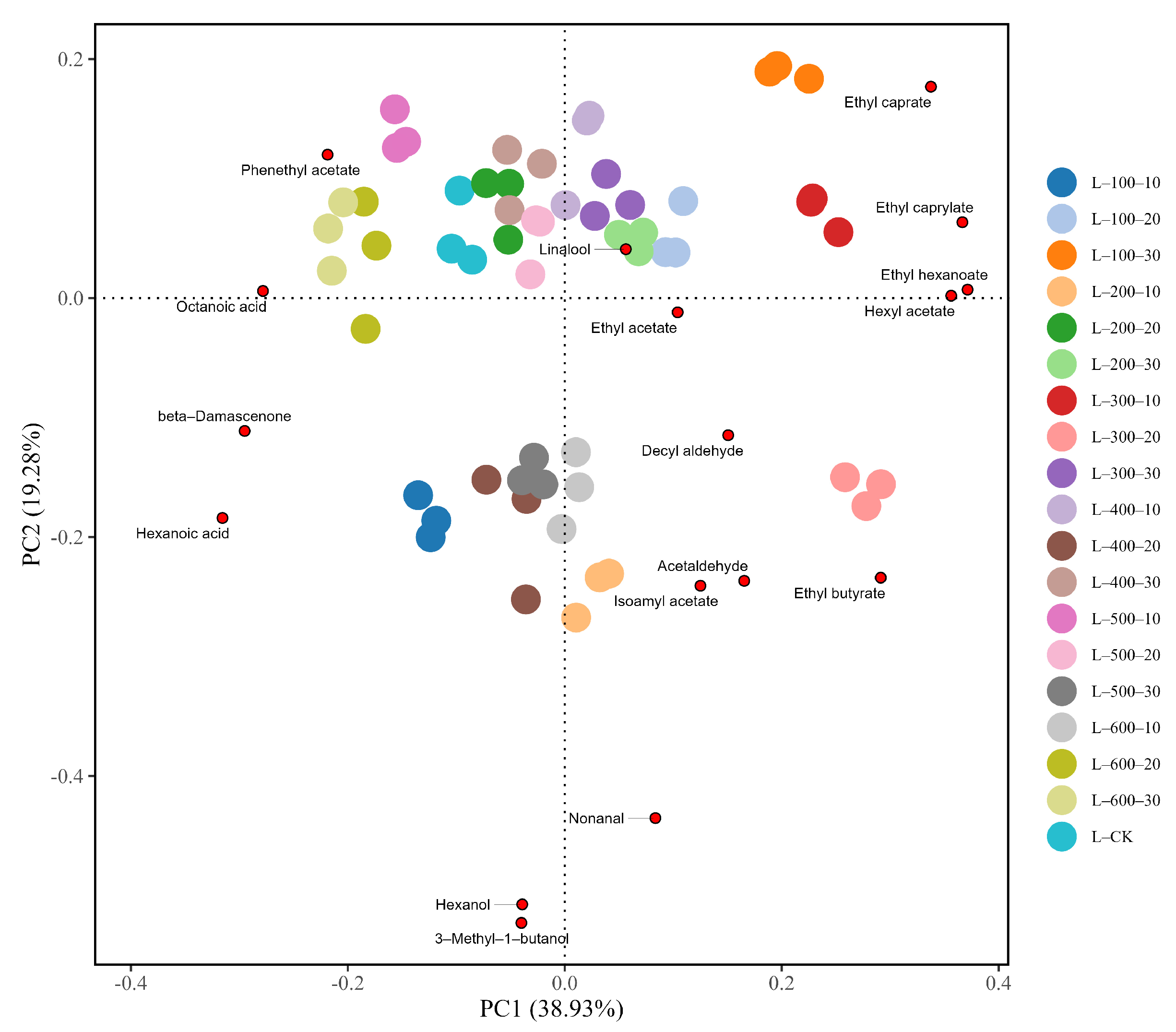
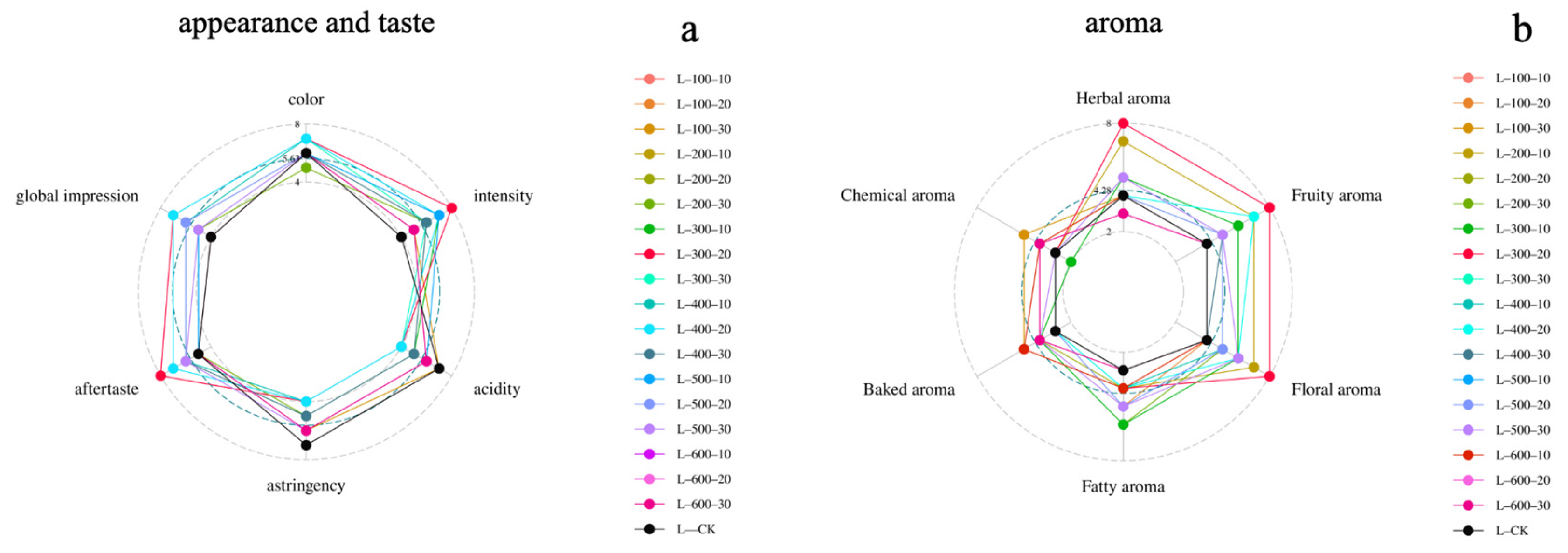
| Compound | Concentration/(mg/L) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-CK | L-100-10 | L-100-20 | L-100-30 | L-200-10 | L-200-20 | L-200-30 | L-300-10 | L-300-20 | L-300-30 | L-400-10 | L-400-20 | L-400-30 | L-500-10 | L-500-20 | L-500-30 | L-600-10 | L-600-20 | L-600-30 | ||
| Flavonoids | (-)-Epigallocatechin | 0.54 ± 0.03 g | 0.6 ± 0.01 efg | 0.74 ± 0.05 ab | 0.59 ± 0.05 fg | 0.7 ± 0.07 bcd | 0.73 ± 0.04 abc | 0.64 ± 0.03 def | 0.74 ± 0.06 abc | 0.72 ± 0.05 abc | 0.78 ± 0.07 a | 0.74 ± 0.05 abc | 0.69 ± 0.03 bcd | 0.59 ± 0.01 fg | 0.78 ± 0.02 a | 0.74 ± 0.04 ab | 0.71 ± 0.06 abc | 0.68 ± 0.03 bcd | 0.67 ± 0.02 cde | 0.68 ± 0.01 bcd |
| Cianidanol | 86.36 ± 5.25 d | 80.33 ± 6.58 d | 82.43 ± 2.18 d | 89.26 ± 3.09 d | 103.3 ± 1.03 c | 104.32 ± 3.76 c | 104.77 ± 10.94 c | 105.09 ± 2.78 c | 137.56 ± 12.61 a | 105.62 ± 8.25 c | 108.58 ± 2.87 bc | 103.88 ± 1.04 c | 117.76 ± 7.35 b | 109.16 ± 1.09 bc | 104.67 ± 4.56 c | 100.54 ± 2.66 c | 103.24 ± 6.28 c | 103.59 ± 8.09 c | 103.69 ± 2.07 c | |
| Epicatechin | 77.25 ± 6.73 defg | 70.16 ± 4.86 g | 72.18 ± 5.91 g | 74.15 ± 3.4 fg | 85.68 ± 4.28 abcd | 84.02 ± 3.03 bcde | 81.8 ± 4.98 bcdef | 88.63 ± 5.81 ab | 94.09 ± 4.89 a | 85.1 ± 7.27 abcde | 89.23 ± 2.36 ab | 86.47 ± 7.69 abcd | 85.79 ± 0.86 abcd | 88.56 ± 4.69 ab | 86.77 ± 9.66 abc | 74.18 ± 7.42 fg | 76.21 ± 5.95 efg | 78.6 ± 2.08 cdefg | 73.17 ± 6.98 fg | |
| Rutin | 1.28 ± 0.09 h | 1.14 ± 0.1 h | 1.2 ± 0.1 h | 1.03 ± 0.05 h | 8.53 ± 0.56 cde | 8.15 ± 0.67 def | 7.04 ± 0.58 g | 8.27 ± 0.5 cdef | 10.65 ± 0.65 a | 8.88 ± 1.01 bcd | 8.27 ± 0.65 cdef | 7.61 ± 0.65 efg | 9.65 ± 0.68 b | 9.19 ± 0.42 bc | 8.85 ± 1.17 bcd | 1.38 ± 0.13 h | 6.89 ± 0.6 g | 7.52 ± 0.49 fg | 1.19 ± 0.03 h | |
| Cynaroside | 0.16 ± 0.02 d | 4.99 ± 0.17 c | 6.08 ± 0.63 b | 4.65 ± 0.2 c | 0.07 ± 0 d | 0.07 ± 0.01 d | 0.07 ± 0.01 d | 0.06 ± 0.01 d | ND | 0.01 ± 0 d | 0.07 ± 0 d | 0.07 ± 0.01 d | ND | 0.07 ± 0 d | 0.06 ± 0 d | 6.45 ± 0.56 a | 0.06 ± 0 d | 0.31 ± 0.02 d | 5.96 ± 0.36 b | |
| Quercitrin | 3.03 ± 0.32 c | 0.47 ± 0.04 d | 0.51 ± 0.04 d | 0.44 ± 0.01 d | 0.14 ± 0.01 e | 0.15 ± 0 e | 0.15 ± 0.02 e | 0.14 ± 0.01 e | 4.43 ± 0.15 a | 3.1 ± 0.16 c | 0.15 ± 0.01 e | 2.91 ± 0.1 c | 4.09 ± 0.19 b | 0.16 ± 0.01 e | 0.16 ± 0.01 e | 0.54 ± 0.03 d | 2.96 ± 0.26 c | 0.14 ± 0.01 e | 0.51 ± 0.06 d | |
| Myricetin | ND | 0.02 ± 0 e | 0.04 ± 0 e | 0.02 ± 0 e | 3.11 ± 0.25 c | 3.3 ± 0.09 abc | 3.34 ± 0.26 ab | 3.25 ± 0.15 abc | 0.73 ± 0.04 d | ND | 3.42 ± 0.29 a | ND | 0.63 ± 0.02 d | 3.4 ± 0.03 a | 3.14 ± 0.08 bc | 0.04 ± 0 e | ND | 3.28 ± 0.15 abc | 0.02 ± 0 e | |
| Luteolin | 0.41 ± 0.04 g | 3.4 ± 0.3 bc | 4.25 ± 0.48 a | 3.34 ± 0.22 c | 0.05 ± 0 h | 0.06 ± 0 h | 0.05 ± 0 h | 0.05 ± 0 h | 1.34 ± 0.1 d | 0.79 ± 0.04 f | 0.06 ± 0 h | 0.83 ± 0.02 ef | 1.06 ± 0.1 e | 0.06 ± 0 h | 0.05 ± 0 h | 4.06 ± 0.18 a | 0.71 ± 0.04 f | 0.05 ± 0 h | 3.66 ± 0.29 b | |
| Quercetin | 0.07 ± 0.01 d | 1.02 ± 0.09 cd | 1.25 ± 0.09 c | 0.9 ± 0.01 cd | 11.3 ± 0.88 ab | 11.35 ± 0.63 ab | 11.01 ± 0.83 ab | 11.07 ± 1.06 ab | 0.07 ± 0 d | 0.06 ± 0.01 d | 11.67 ± 1.18 a | 0.06 ± 0.01 d | 0.06 ± 0 d | 11.62 ± 0.73 ab | 11.06 ± 1.46 ab | 1.21 ± 0.04 c | 0.05 ± 0 d | 10.6 ± 0.7 b | 1.15 ± 0.11 c | |
| Apigenin | 12.86 ± 0.68 b | 0.06 ± 0 e | 0.07 ± 0 e | 0.06 ± 0 e | 0.01 ± 0 e | 0.02 ± 0 e | 0.01 ± 0 e | ND | 13.87 ± 1.08 a | 11.78 ± 1.18 c | 0.01 ± 0 e | 10.73 ± 0.84 d | 11.62 ± 0.58 c | ND | ND | 0.07 ± 0.01 e | 10.17 ± 0.54 d | ND | 0.06 ± 0 e | |
| Kaempferol | 0.03 ± 0 d | 12.02 ± 0.21 b | 13.44 ± 1.42 a | 10.82 ± 0.97 c | ND | ND | ND | 0.58 ± 0.06 d | 0.04 ± 0 d | 0.01 ± 0 d | ND | ND | ND | 0.62 ± 0.05 d | 0.59 ± 0.06 d | 13.87 ± 1.47 a | 0.01 ± 0 d | 0.63 ± 0.08 d | 12.48 ± 0.78 b | |
| Isorhamnetin | 0.7 ± 0.06 b | 0.64 ± 0.06 c | ND | ND | 0.01 ± 0 f | 0.01 ± 0 f | 0.01 ± 0 f | 0.36 ± 0.02 e | 0.78 ± 0.04 a | 0.58 ± 0.01 d | 0.01 ± 0 f | 0.01 ± 0 f | 0.01 ± 0 f | 0.39 ± 0.02 e | 0.37 ± 0.01 e | 0.77 ± 0.02 a | 0.56 ± 0.01 d | 0.37 ± 0.04 e | ND | |
| Isoquercitrin | 2.01 ± 0.27 d | ND | ND | ND | 4.36 ± 0.15 bc | 4.15 ± 0.37 c | 4.12 ± 0.18 c | 4.16 ± 0.22 c | 4.66 ± 0.4 ab | 4.4 ± 0.23 bc | 4.42 ± 0.13 bc | 4.33 ± 0 bc | 4.6 ± 0.12 ab | 4.9 ± 0.37 a | 4.44 ± 0.29 bc | ND | 4.16 ± 0.15 c | 4.21 ± 0.19 c | ND | |
| Phlorizin | 1.08 ± 0.09 de | 13.46 ± 0.94 a | 9.16 ± 0.51 b | 14.06 ± 1.15 a | 0.62 ± 0.02 e | 0.59 ± 0.07 e | 0.6 ± 0.06 e | 0.62 ± 0.01 e | 1.31 ± 0.02 d | 1.21 ± 0.07 de | 0.61 ± 0.01 e | 1.16 ± 0.06 de | 1.11 ± 0.12 de | 0.63 ± 0.03 e | 0.59 ± 0.01 e | 8.8 ± 0.81 b | 1.13 ± 0.07 de | 0.61 ± 0.05 e | 8.09 ± 0.32 c | |
| Delphinidin-3-glucoside | 0.27 ± 0.03 fg | 0.28 ± 0 fg | 0.3 ± 0.04 ef | 0.31 ± 0.01 def | 0.29 ± 0.02 efg | 0.35 ± 0.03 abc | 0.38 ± 0.03 a | 0.26 ± 0.03 g | 0.36 ± 0.02 ab | 0.32 ± 0.01 cde | 0.27 ± 0.02 fg | 0.34 ± 0.02 abcd | 0.37 ± 0.01 ab | 0.28 ± 0.02 fg | 0.28 ± 0.02 fg | 0.3 ± 0.03 def | 0.3 ± 0.03 efg | 0.34 ± 0.03 bcd | 0.37 ± 0.03 ab | |
| Delphinidin Chloride | ND | ND | ND | ND | 0.18 ± 0.02 b | 0.13 ± 0 cd | 0.12 ± 0.01 d | ND | 0.22 ± 0.01 a | ND | 0.09 ± 0 e | 0.17 ± 0.01 b | 0.04 ± 0 f | ND | ND | ND | 0.09 ± 0.01 e | 0.1 ± 0.01 e | 0.14 ± 0.02 c | |
| Petunidin 3-Glucoside | 0.34 ± 0.03 de | 0.33 ± 0.04 de | 0.37 ± 0.01 de | 0.36 ± 0.04 de | 0.38 ± 0.03 de | 0.83 ± 0.05 c | 0.93 ± 0.05 a | 0.31 ± 0.01 e | 0.96 ± 0.02 a | 0.39 ± 0.02 d | 0.33 ± 0.02 de | 0.83 ± 0.1 c | 0.91 ± 0.07 ab | 0.34 ± 0.02 de | 0.34 ± 0.02 de | 0.37 ± 0.02 de | 0.38 ± 0.02 de | 0.84 ± 0.02 bc | 0.93 ± 0.06 a | |
| Kuromanin Chloride | ND | 0.11 ± 0 gh | 0.12 ± 0.01 efg | 0.12 ± 0 efgh | ND | 0.24 ± 0.01 d | 0.27 ± 0.02 ab | 0.1 ± 0 h | 0.28 ± 0.02 a | 0.12 ± 0 efg | 0.12 ± 0.01 fgh | 0.26 ± 0.01 bc | 0.27 ± 0.02 abc | 0.11 ± 0.01 fgh | 0.11 ± 0 fgh | 0.12 ± 0 ef | 0.13 ± 0 e | 0.26 ± 0.01 cd | 0.27 ± 0.01 abc | |
| Cyanidin Chloride | 0.61 ± 0.06 i | 0.82 ± 0.06 hi | 2 ± 0.22 d | 1.58 ± 0.14 e | 1.86 ± 0.16 d | 3.09 ± 0.14 b | 2.52 ± 0.24 c | 0.7 ± 0.03 i | 3.76 ± 0.33 a | 1.05 ± 0.09 gh | 1.37 ± 0.1 ef | 3.04 ± 0.2 b | 1.97 ± 0.17 d | 1.53 ± 0.08 e | 1.15 ± 0.09 fg | 0.84 ± 0.05 hi | 2.59 ± 0.21 c | 3 ± 0.14 b | 1.93 ± 0.1 d | |
| Peonidin-3-Glucoside Chloride | 0.57 ± 0.01 defgh | 0.54 ± 0.02 fgh | 0.58 ± 0.07 cdefg | 0.57 ± 0.04 defgh | 0.53 ± 0.05 gh | 0.54 ± 0.02 fgh | 0.63 ± 0.04 cd | 0.53 ± 0.03 gh | 1.19 ± 0.09 a | 0.61 ± 0.05 cde | 0.52 ± 0.01 h | 0.52 ± 0.03 gh | 0.64 ± 0.02 c | 0.55 ± 0.03 efgh | 0.55 ± 0.01 efgh | 0.6 ± 0.04 cdef | 0.52 ± 0.03 gh | 0.54 ± 0.01 fgh | 0.86 ± 0.01 b | |
| Malvidin Chloride | 0.12 ± 0.01 bc | ND | ND | ND | 0.1 ± 0.01 f | 0.13 ± 0.01 b | 0.12 ± 0.01 cd | ND | 0.19 ± 0.01 a | ND | 0.08 ± 0.01 g | 0.12 ± 0.01 bc | 0.11 ± 0.01 ef | ND | ND | ND | 0.12 ± 0 cde | 0.12 ± 0.01 bc | 0.11 ± 0.01 def | |
| Malvin Chloride | ND | 0.12 ± 0.01 c | 0.11 ± 0.01 c | 0.11 ± 0.01 c | 0.13 ± 0.01 c | 0.16 ± 0.01 c | 7.67 ± 0.67 a | 6.86 ± 0.12 b | 0.13 ± 0.01 c | 0.11 ± 0.01 c | 0.13 ± 0.01 c | 0.14 ± 0.01 c | 0.15 ± 0.01 c | 0.11 ± 0.01 c | ND | 0.11 ± 0 c | 0.13 ± 0.01 c | 0.15 ± 0.02 c | 0.15 ± 0.01 c | |
| Oenin Chloride | 7.65 ± 0.67 cd | 7.23 ± 0.64 cdef | 7.53 ± 1 cde | 7.64 ± 0.4 cd | 6.83 ± 0.84 def | 6.91 ± 0.18 cdef | 0.15 ± 0.01 g | 0.11 ± 0.01 g | 9.29 ± 1.1 a | 7.83 ± 0.14 bc | 6.64 ± 0.33 ef | 6.65 ± 0.35 ef | 7.72 ± 0.48 cd | 7.15 ± 0.19 cdef | 7.14 ± 0.76 cdef | 7.51 ± 0.53 cdef | 6.6 ± 0.11 f | 6.8 ± 0.66 def | 8.76 ± 0.46 ab | |
| total content | 195.37 ± 7.04 | 197.75 ± 9.06 | 202.36 ± 15.81 | 210.01 ± 13.12 | 228.17 ± 26.90 | 229.32 ± 30.34 | 226.39 ± 7.84 | 231.91 ± 22.84 | 286.63 ± 29.09 | 232.75 ± 11.64 | 236.79 ± 16.41 | 230.82 ± 15.14 | 249.15 ± 22.82 | 239.61 ± 24.318 | 231.09 ± 15.15 | 222.48 ± 8.89 | 217.68 ± 15.69 | 222.73 ± 5.89 | 224.19 ± 15.69 | |
| Non-Flavonoids | Protocatechuic acid | 1.9 ± 0.12 a | 0.13 ± 0.01 e | 0.13 ± 0.01 e | 0.1 ± 0.01 e | 0.6 ± 0.04 c | 0.56 ± 0.01 cd | 0.57 ± 0.05 cd | 0.58 ± 0.06 c | 0.78 ± 0.05 b | 0.62 ± 0.05 c | 0.6 ± 0.05 c | 0.57 ± 0.04 cd | 0.63 ± 0.04 c | 0.63 ± 0.05 c | 0.58 ± 0.03 cd | 0.13 ± 0.01 e | 0.51 ± 0.01 d | 0.56 ± 0.01 cd | 0.08 ± 0.01 e |
| Gallic acid | 59.7 ± 4.14 a | 46.58 ± 3.05 efgh | 54.41 ± 2.18 bc | 44.23 ± 4.68 fgh | 49.34 ± 3.73 cdef | 47 ± 4.31 efgh | 45.32 ± 1.63 efgh | 47.6 ± 2.52 efgh | 56.43 ± 1.49 ab | 49.99 ± 3.6 cde | 48.26 ± 3.38 defg | 46.91 ± 5.22 efgh | 47.41 ± 0.82 efgh | 43.58 ± 2.72 gh | 48.9 ± 0.85 def | 53.32 ± 2.44 bcd | 42.4 ± 1.94 h | 45.5 ± 2.36 efgh | 49.22 ± 4.43 cdef | |
| Chlorogenic acid | 0.02 ± 0 e | 0.58 ± 0.05 bc | 0.66 ± 0.01 a | 0.55 ± 0.05 c | 0.03 ± 0 e | 0.02 ± 0 e | 0.02 ± 0 e | 0.02 ± 0 e | ND | 0.08 ± 0.01 d | 0.08 ± 0.01 d | 0.02 ± 0 e | ND | 0.02 ± 0 e | 0.02 ± 0 e | 0.67 ± 0.02 a | 0.02 ± 0 e | 0.08 ± 0 d | 0.59 ± 0.02 b | |
| 4-Hydroxybenzoic acid | 0.48 ± 0.02 e | 1.32 ± 0.03 c | 1.92 ± 0.15 b | 3.18 ± 0.3 a | 0.17 ± 0.02 f | 0.17 ± 0.01 f | 0.13 ± 0.01 f | 0.17 ± 0.02 f | 0.16 ± 0.02 f | 0.17 ± 0 f | 0.17 ± 0.01 f | 0.19 ± 0.01 f | 0.14 ± 0.01 f | 0.18 ± 0.01 f | 0.16 ± 0.01 f | 1.25 ± 0.1 c | 0.13 ± 0.01 f | 0.12 ± 0.01 f | 1.09 ± 0.04 d | |
| 2,5-Dihydroxybenzoic acid | 0.55 ± 0.01 e | 2.41 ± 0.21 c | 2.82 ± 0.07 a | 2.21 ± 0.12 d | 0.19 ± 0.01 f | 0.19 ± 0.01 f | 0.12 ± 0.01 f | 0.19 ± 0.02 f | 0.19 ± 0.02 f | 0.21 ± 0.02 f | 0.2 ± 0.02 f | 0.15 ± 0.01 f | 0.17 ± 0.02 f | 0.18 ± 0.01 f | 0.19 ± 0.02 f | 2.74 ± 0.32 ab | 0.15 ± 0 f | 0.16 ± 0.01 f | 2.66 ± 0.14 b | |
| Caffeic acid | 1.6 ± 0.06 ab | 0.02 ± 0 e | 0.03 ± 0 e | 0.02 ± 0 e | 1.41 ± 0.08 c | 1.45 ± 0.16 bc | 1.55 ± 0.06 abc | 1.53 ± 0.06 abc | 1.24 ± 0.13 d | 1.53 ± 0.08 abc | 1.6 ± 0.19 ab | 1.52 ± 0.12 abc | 1.1 ± 0.1 d | 1.62 ± 0.09 a | 1.5 ± 0.15 abc | 0.02 ± 0 e | 1.5 ± 0.01 abc | 1.52 ± 0.13 abc | 0.03 ± 0 e | |
| Syringic acid | 4.66 ± 0.34 i | 11.05 ± 1.01 a | 10.92 ± 0.38 a | 8.55 ± 0.6 c | 5.18 ± 0.49 fghi | 4.79 ± 0.08 hi | 7.08 ± 0.31 d | 5.29 ± 0.32 fghi | 6.31 ± 0.54 de | 5.08 ± 0.4 fghi | 4.74 ± 0.22 hi | 5.84 ± 0.2 ef | 5.48 ± 0.52 fgh | 5.64 ± 0.41 efg | 4.98 ± 0.39 ghi | 10.82 ± 0.57 ab | 4.74 ± 0.17 hi | 6.71 ± 0.66 d | 10.13 ± 0.41 b | |
| p-Coumaric acid | 0.17 ± 0 cd | ND | ND | ND | 2.96 ± 0.16 a | 2.99 ± 0.27 a | 3.16 ± 0.2 a | 3.04 ± 0.32 a | 0.53 ± 0.05 b | 0.61 ± 0.06 b | 3.1 ± 0.25 a | 0.53 ± 0.01 b | 0.43 ± 0.04 bc | 3.13 ± 0.24 a | 3 ± 0.21 a | ND | 0.56 ± 0.04 b | 3.16 ± 0.31 a | ND | |
| 4-Hydroxy-3,5-dimethoxycinnamic acid | 0.12 ± 0 d | 4.05 ± 0.23 bc | 4.5 ± 0.32 a | 3.82 ± 0.35 c | 0.09 ± 0 d | 0.08 ± 0 d | ND | 0.09 ± 0.01 d | 0.21 ± 0.01 d | 0.16 ± 0.01 d | 0.09 ± 0 d | 0.14 ± 0.01 d | 0.17 ± 0.01 d | 0.11 ± 0.01 d | 0.09 ± 0 d | 4.25 ± 0.41 ab | 0.14 ± 0.01 d | ND | 4.22 ± 0.32 b | |
| Methyl 3,4-dihydroxybenzoate | 0.16 ± 0.01 f | 0.16 ± 0.01 f | 0.17 ± 0.02 f | 0.15 ± 0.01 f | 7.46 ± 0.34 cd | 7.09 ± 0.43 de | 7.74 ± 0.77 bc | 8.14 ± 0.28 ab | 0.15 ± 0.01 f | 0.08 ± 0 f | 6.84 ± 0.34 e | ND | 0.09 ± 0.01 f | 7.77 ± 0.13 bc | 8.27 ± 0.46 a | 0.18 ± 0.01 f | ND | 7.41 ± 0.26 cd | 0.18 ± 0.02 f | |
| Ferulic acid | 14.56 ± 1.29 b | 0.12 ± 0 f | 0.13 ± 0.01 f | 0.12 ± 0.01 f | 1.08 ± 0.08 e | 1.13 ± 0.06 e | 1.27 ± 0.06 e | 1.15 ± 0.1 e | 28.05 ± 1.84 a | 8.15 ± 0.64 cd | 1.22 ± 0.06 e | 8.09 ± 0.21 cd | 8.87 ± 0.41 c | 1.35 ± 0.13 e | 1.17 ± 0.1 e | 0.13 ± 0.01 f | 7.32 ± 0.48 d | 1.27 ± 0.03 e | 0.09 ± 0 f | |
| Vanillin | 0.22 ± 0.01 g | 1.16 ± 0.06 c | 1.29 ± 0.11 ab | 1.03 ± 0 d | 0.02 ± 0 h | 0.01 ± 0 h | 0.02 ± 0 h | 0.02 ± 0 h | 0.7 ± 0.03 e | 0.61 ± 0.04 f | 0.03 ± 0 h | 0.61 ± 0.01 f | 0.6 ± 0.05 f | 0.02 ± 0 h | 0.02 ± 0 h | 1.36 ± 0.05 a | 0.59 ± 0.04 f | 0.02 ± 0 h | 1.28 ± 0.15 b | |
| 3-Hydroxycinnamic acid | 0.02 ± 0 d | 0.61 ± 0 b | 0.67 ± 0.08 a | 0.56 ± 0.04 c | ND | ND | ND | ND | 0.04 ± 0 d | 0.02 ± 0 d | ND | 0.02 ± 0 d | 0.03 ± 0 d | ND | ND | 0.67 ± 0.04 a | 0.02 ± 0 d | ND | 0.62 ± 0.06 b | |
| Resveratrol | 1.64 ± 0.11 e | 0.66 ± 0.02 hi | 0.95 ± 0.05 f | 0.6 ± 0.01 i | 0.78 ± 0.05 fghi | 0.71 ± 0.05 ghi | 0.81 ± 0.08 fghi | 0.86 ± 0.07 fgh | 4.15 ± 0.25 a | 3.5 ± 0.13 b | 0.79 ± 0.06 fghi | 2.98 ± 0.28 d | 3.2 ± 0.2 c | 0.8 ± 0.07 fghi | 0.82 ± 0.05 fghi | 0.94 ± 0.04 fg | 2.92 ± 0.32 d | 0.76 ± 0.02 fghi | 0.84 ± 0.04 fgh | |
| Ethylparaben | 0.01 ± 0 c | ND | 0.02 ± 0 c | 0.02 ± 0 c | 0.53 ± 0.05 b | 0.54 ± 0.03 b | 0.56 ± 0.03 ab | 0.02 ± 0 c | ND | ND | 0.59 ± 0.05 a | 0.57 ± 0.05 ab | 0.6 ± 0.04 a | 0.01 ± 0 c | 0.01 ± 0 c | ND | ND | 0.02 ± 0 c | 0.02 ± 0 c | |
| Vanillic acid | 0.03 ± 0 e | 0.02 ± 0 e | 0.74 ± 0.04 a | 0.58 ± 0.06 c | 0.36 ± 0.01 d | 0.35 ± 0.04 d | 0.38 ± 0.03 d | ND | 0.02 ± 0 e | 0.02 ± 0 e | 0.37 ± 0.04 d | 0.35 ± 0.02 d | 0.38 ± 0 d | ND | 0.02 ± 0 e | 0.02 ± 0 e | 0.01 ± 0 e | ND | 0.68 ± 0.09 b | |
| Ethyl 4-hydroxy-3-methoxybenzoate | 0.55 ± 0.05 a | 0.39 ± 0.03 c | 0.01 ± 0 e | 0.01 ± 0 e | 0.01 ± 0 e | ND | ND | 0.02 ± 0 e | 0.5 ± 0.01 b | 0.37 ± 0.03 c | 0.02 ± 0 e | ND | 0.03 ± 0 e | 0.02 ± 0 e | ND | 0.48 ± 0.04 b | 0.33 ± 0.01 d | 0.02 ± 0 e | 0.02 ± 0 e | |
| Coumaric acid | ND | 0.02 ± 0 de | 0.48 ± 0 a | 0.36 ± 0.03 c | ND | 0.01 ± 0 de | 0.02 ± 0 de | ND | ND | 0.02 ± 0 de | ND | 0.02 ± 0 de | 0.02 ± 0 de | ND | ND | 0.03 ± 0 d | ND | ND | 0.42 ± 0.05 b | |
| Total content | 86.40 ± 10.79 | 69.29 ± 7.49 | 79.86 ± 3.19 | 66.13 ± 3.49 | 70.21 ± 3.90 | 67.11 ± 4.08 | 68.74 ± 1.82 | 68.73 ± 5.99 | 99.45 ± 8.14 | 71.24 ± 6.79 | 68.70 ± 3.43 | 68.49 ± 3.62 | 69.35 ± 2.50 | 65.06 ± 4.55 | 69.72 ± 2.09 | 77.02 ± 2.77 | 61.34 ± 3.83 | 67.29 ± 7.49 | 72.17 ± 5.45 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.; Zhao, D.; Chang, T.; Chen, X.; Kai, J.; Luo, Y.; Peng, B.; Yang, B.; Ge, Q. Effects of High-Hydrostatic-Pressure Treatment on Polyphenols and Volatile Aromatic Compounds in Marselan Wine. Foods 2024, 13, 2468. https://doi.org/10.3390/foods13152468
Yi Z, Zhao D, Chang T, Chen X, Kai J, Luo Y, Peng B, Yang B, Ge Q. Effects of High-Hydrostatic-Pressure Treatment on Polyphenols and Volatile Aromatic Compounds in Marselan Wine. Foods. 2024; 13(15):2468. https://doi.org/10.3390/foods13152468
Chicago/Turabian StyleYi, Zicheng, Danqing Zhao, Tengwen Chang, Xiang Chen, Jianrong Kai, Yang Luo, Bangzhu Peng, Binkun Yang, and Qian Ge. 2024. "Effects of High-Hydrostatic-Pressure Treatment on Polyphenols and Volatile Aromatic Compounds in Marselan Wine" Foods 13, no. 15: 2468. https://doi.org/10.3390/foods13152468
APA StyleYi, Z., Zhao, D., Chang, T., Chen, X., Kai, J., Luo, Y., Peng, B., Yang, B., & Ge, Q. (2024). Effects of High-Hydrostatic-Pressure Treatment on Polyphenols and Volatile Aromatic Compounds in Marselan Wine. Foods, 13(15), 2468. https://doi.org/10.3390/foods13152468








