The Contribution of Trichoderma viride and Metallothioneins in Enhancing the Seed Quality of Avena sativa L. in Cd-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Metal Resistance of T. viride and Minimal Inhibitory Concentration
2.3. Growth of A. sativa in the Presence of Fungi
2.4. Effect of Heavy Metals on the Germination and Growth of A. sativa Seedlings
2.5. Effect of T. viride on the Growth of A. sativa in the Presence of Heavy Metals
2.6. Pot Experiment
2.7. Level of Heavy Metals in A. sativa L. Plants
2.8. Identification of Metal-Responsive Elements in the Promoters of A. sativa Metallothioneins
2.9. Functional Analysis of AsMT1-4 in E. coli
2.10. Gene Expression
2.11. Statistical Analysis
3. Results
3.1. Tolerance of T. viride to Cd, Cu, and Zn
3.2. Seed Germination and Seedling Growth of A. sativa in the Presence of T. viride
3.3. Effect of Cadmium and T. viride on the Seed Germination and Seedling Growth of A. sativa
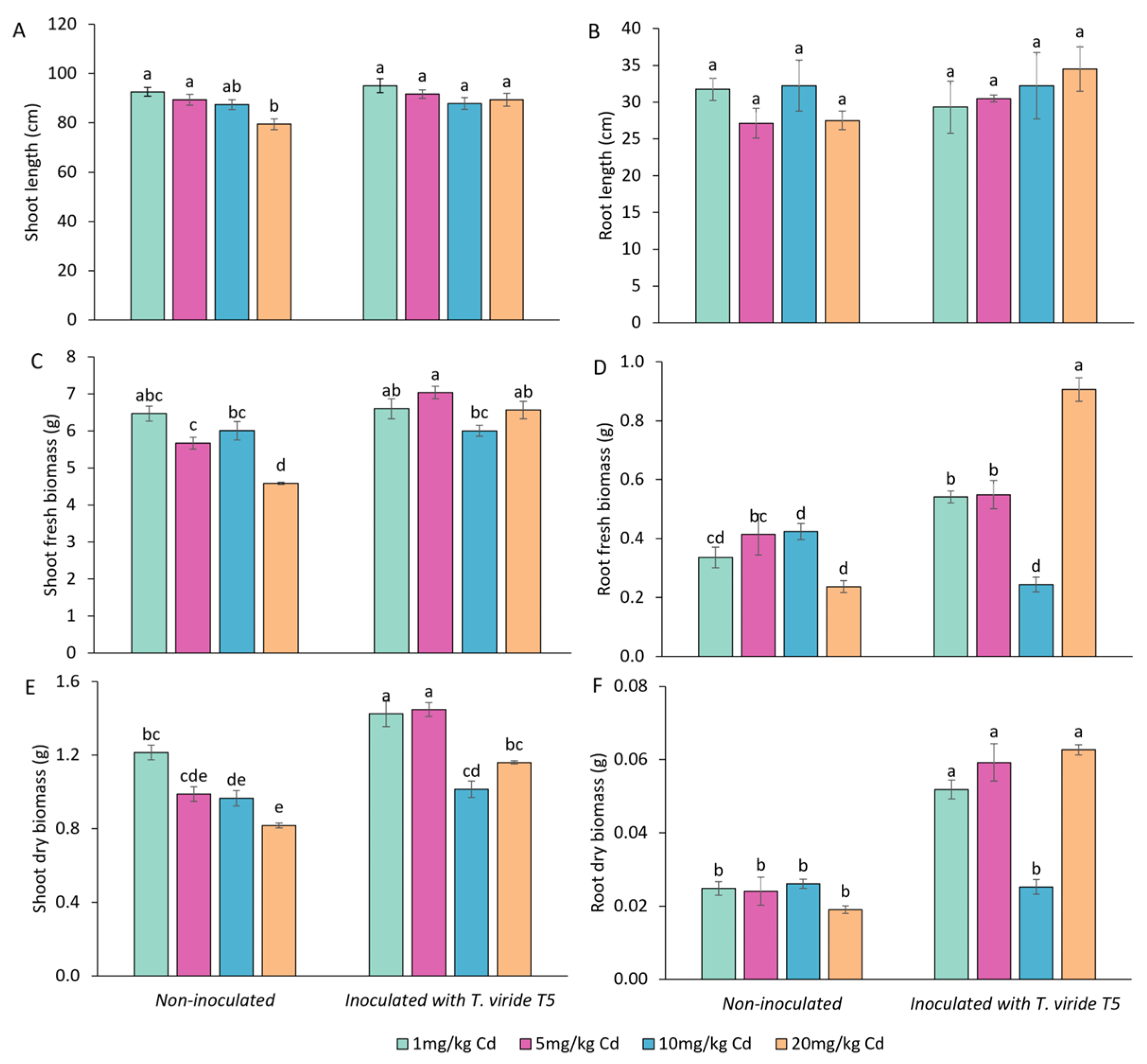
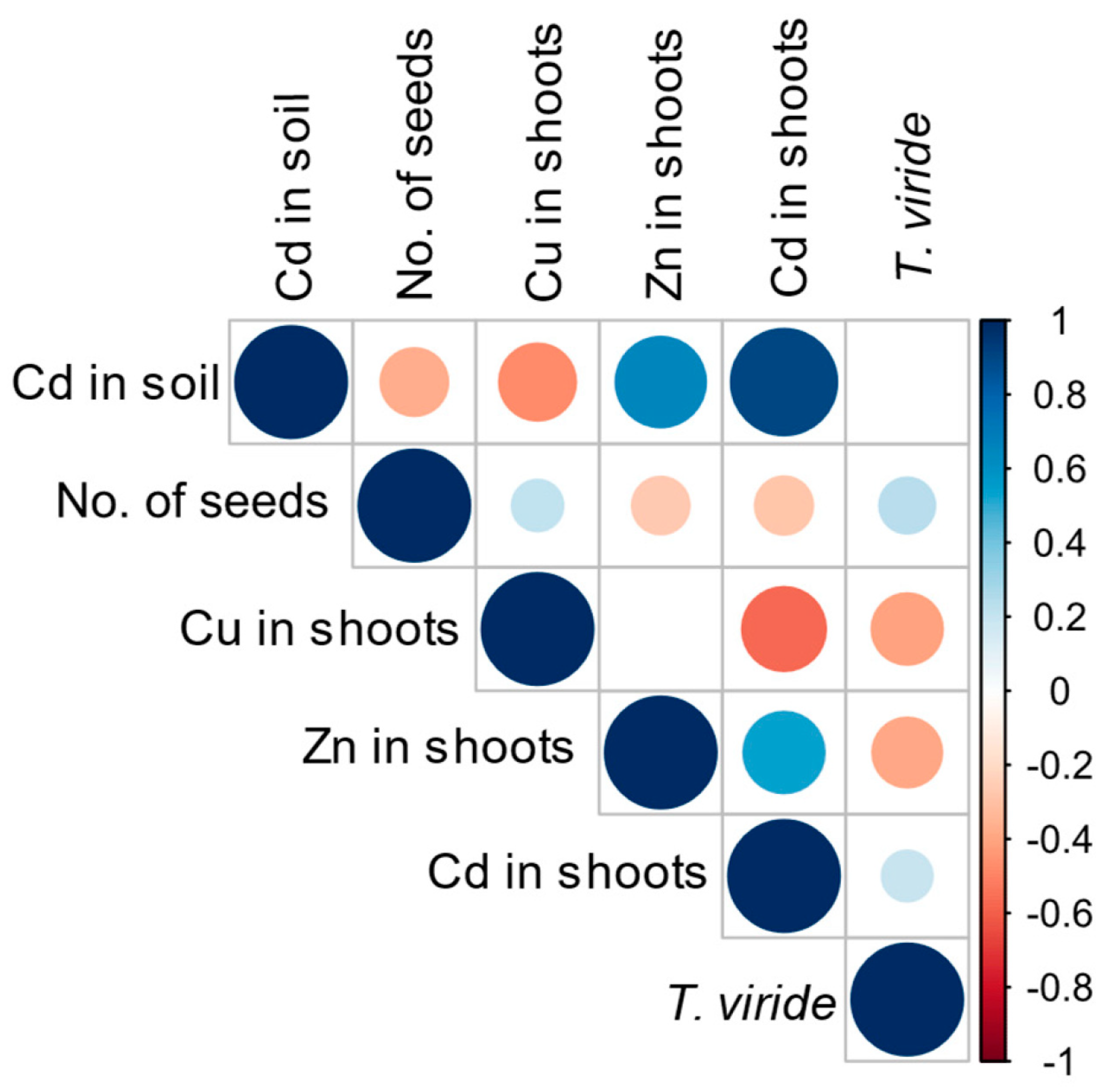
3.4. Effect of T. viride on the Growth and Yield of A. sativa Plants Grown in the Presence of Cd and on the Level of Cd Phytoextraction
3.5. Functional Analysis of A. sativa Metallothioneins (AsMT1-4) in Bacteria Cells
3.6. Expression of A. sativa AsMT1-4 in Plants Growing in Cd-Contaminated Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.L. Cadmium and Phosphorous Fertilizers: The Issues and the Science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef]
- Konieczna, W.; Mierek-Adamska, A.; Chojnacka, N.; Antoszewski, M.; Szydłowska-Czerniak, A.; Dąbrowska, G.B. Characterization of the Metallothionein Gene Family in Avena sativa L. and the Gene Expression During Seed Germination and Heavy Metal Stress. Antioxidants 2023, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Rehman, M.Z.; Maqbool, A. A Critical Review on the Effects of Zinc at Toxic Levels of Cadmium in Plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef] [PubMed]
- Stafford, A.; Jeyakumar, P.; Hedley, M.; Anderson, C. Influence of Soil Moisture Status on Soil Cadmium Phytoavailability and Accumulation in Plantain (Plantago lanceolata). Soil Syst. 2018, 2, 9. [Google Scholar] [CrossRef]
- Ellen, T.P.; Costa, M. Carcinogenic Inorganic Chemicals. In Comprehensive Toxicology (Second Edition); McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 139–160. ISBN 978-0-08-046884-6. [Google Scholar]
- Zulfiqar, U.; Jiang, W.; Wang, X.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium Phytotoxicity, Tolerance, and Advanced Remediation Approaches in Agricultural Soils; a Comprehensive Review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Directorate-General for Health and Food Safety. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Zimmerl, S.; Lafferty, J.; Buerstmayr, H. Assessing Diversity in Triticum durum Cultivars and Breeding Lines for High versus Low Cadmium Content in Seeds Using the CAPS Marker Usw47. Plant Breed. 2014, 133, 712–717. [Google Scholar] [CrossRef]
- Song, W.; Chen, S.; Liu, J.; Chen, L.; Song, N.; Li, N.; Liu, B. Variation of Cd Concentration in Various Rice Cultivars and Derivation of Cadmium Toxicity Thresholds for Paddy Soil by Species-Sensitivity Distribution. J. Integr. Agric. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef]
- Freisinger, E. Structural Features Specific to Plant Metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, G.; Mierek-Adamska, A.; Goc, A. Characterisation of Brassica napus L. Metallothionein Genes (BnMTs) Expression in Organs and during Seed Germination. Aust. J. Crop Sci. 2013, 7, 1324–1332. [Google Scholar]
- Blindauer, C.A.; Schmid, R. Cytosolic Metal Handling in Plants: Determinants for Zinc Specificity in Metal Transporters and Metallothioneins. Metallomics 2010, 2, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Deng, J.S.; Chen, H.J.; Lin, Y.H.; Huang, G.J. Antioxidant Activities of Two Metallothionein-like Proteins from Sweet Potato (Ipomoea batatas [L.] Lam. ‘Tainong 57’) Storage Roots and Their Synthesized Peptides. Bot. Stud. 2014, 55, 64. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, W.; Mierek-Adamska, A.; Warchoł, M.; Skrzypek, E.; Dąbrowska, G.B. The Involvement of Metallothioneins and Stress Markers in Response to Osmotic Stress in Avena sativa L. J. Agron. Crop Sci. 2023, 209, 371–389. [Google Scholar] [CrossRef]
- Konieczna, W.; Warchoł, M.; Mierek-Adamska, A.; Skrzypek, E.; Waligórski, P.; Piernik, A.; Dąbrowska, G.B. Changes in Physio-Biochemical Parameters and Expression of Metallothioneins in Avena sativa L. in Response to Drought. Sci. Rep. 2023, 13, 2486. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, G.; Baum, C.; Trejgell, A.; Hrynkiewicz, K. Impact of Arbuscular Mycorrhizal Fungi on the Growth and Expression of Gene Encoding Stress Protein - Metallothionein BnMT2 in the Non-Host Crop Brassica napus L. J. Plant Nutr. Soil Sci. 2014, 177, 459–467. [Google Scholar] [CrossRef]
- Dąbrowska, G.; Hrynkiewicz, K.; Trejgell, A. Do Arbuscular Mycorrhizal Fungi Affect Metallothionein MT2 Expression in Brassica napus L. Roots? Acta Biol. Cracoviensia Ser. Bot. 2012, 54, 34–39. [Google Scholar] [CrossRef]
- Dąbrowska, G.; Mierek-Adamska, A.; Goc, A. Plant Metallothioneins: Putative Functions Identified by Promoter Analysis in silico. Acta Biol. Cracoviensia Ser. Bot. 2012, 54, 109–120. [Google Scholar] [CrossRef]
- Gao, C.; Gao, K.; Yang, H.; Ju, T.; Zhu, J.; Tang, Z.; Zhao, L.; Chen, Q. Genome-Wide Analysis of Metallothionein Gene Family in Maize to Reveal Its Role in Development and Stress Resistance to Heavy Metal. Biol. Res. 2022, 55, 1–13. [Google Scholar] [CrossRef]
- Yu, Q.; He, L.; Huo, C.; Jiang, X.; Chen, H.; Wang, R.; Tang, M.; Dong, L.; Chen, J.; Li, Y.; et al. Genome-Wide Identification and Expression Analysis of Heavy Metal Stress–Responsive Metallothionein Family Genes in Nicotiana tabacum. Plant Mol. Biol. Rep. 2020, 39, 443–454. [Google Scholar] [CrossRef]
- Mirzahossini, Z.; Shabani, L.; Sabzalian, M.R.; Sharifi-Tehrani, M. ABC Transporter and Metallothionein Expression Affected by Ni and Epichloe Endophyte Infection in Tall Fescue. Ecotoxicol. Environ. Saf. 2015, 120, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Neufeld, J.D. Life in a World Without Microbes. PLoS Biol. 2014, 12, e1002020. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, G.B.; Garstecka, Z.; Trejgell, A.; Dąbrowski, H.P.; Konieczna, W.; Szyp-Borowska, I. The Impact of Forest Fungi on Promoting Growth and Development of Brassica napus L. Agronomy 2021, 11, 2475. [Google Scholar] [CrossRef]
- Kacprzak, M.J.; Rosikon, K.; Fijalkowski, K.; Grobelak, A. The Effect of Trichoderma on Heavy Metal Mobility and Uptake by Miscanthus giganteus, Salix sp., Phalaris arundinacea, and Panicum virgatum. Appl. Environ. Soil Sci. 2014, 2014, 506142. [Google Scholar] [CrossRef]
- Chacón, M.R.; Rodríguez-Galán, O.; Benítez, T.; Sousa, S.; Rey, M.; Llobell, A.; Delgado-Jarana, J. Microscopic and Transcriptome Analyses of Early Colonization of Tomato Roots by Trichoderma harzianum. Int. Microbiol. 2007, 10, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Turkan, S.; Mierek-Adamska, A.; Kulasek, M.; Konieczna, W.B.; Dąbrowska, G.B. New Seed Coating Containing Trichoderma viride with Anti-Pathogenic Properties. PeerJ 2023, 11, e15392. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma Secondary Metabolites Active on Plants and Fungal Pathogens. Open Mycol. J. 2014, 8, 127–139. [Google Scholar] [CrossRef]
- Chagas, L.F.B.; De Castro, H.G.; Colonia, B.S.O.; De Carvalho Filho, M.R.; Miller, L.D.O.; Chagas, A.F.J. Efficiency of Trichoderma spp. as a Growth Promoter of Cowpea (Vigna unguiculata) and Analysis of Phosphate Solubilization and Indole Acetic Acid Synthesis. Braz. J. Bot. 2016, 39, 437–445. [Google Scholar] [CrossRef]
- Aishwarya, S.; Viswanath, H.S.; Singh, A.; Singh, R. Biosolubilization of Different Nutrients by Trichoderma spp. and Their Mechanisms Involved: A Review. Int. J. Adv. Agric. Sci. Technol. 2020, 7, 34–39. [Google Scholar]
- Li, R.-X.; Cai, F.; Pang, G.; Shen, Q.-R.; Li, R.; Chen, W. Solubilisation of Phosphate and Micronutrients by Trichoderma harzianum and Its Relationship with the Promotion of Tomato Plant Growth. PLoS ONE 2015, 10, e0130081. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jiang, M.; Zeng, Z.; Du, A.; Tan, H.; Liu, Y. Trichoderma atroviride F6 Improves Phytoextraction Efficiency of Mustard (Brassica juncea (L.) Coss. Var. foliosa Bailey) in Cd, Ni Contaminated Soils. Chemosphere 2008, 71, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Kim, H. Myco-Phytoremediation of Arsenic- and Lead-Contaminated Soils by Helianthus annuus and Wood Rot Fungi, Trichoderma sp. Isolated from Decayed Wood. Ecotoxicol. Environ. Saf. 2018, 151, 279–284. [Google Scholar] [CrossRef] [PubMed]
- López Errasquín, E.; Vázquez, C. Tolerance and Uptake of Heavy Metals by Trichoderma atroviride Isolated from Sludge. Chemosphere 2003, 50, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Wang, L.; Jiang, X.; Wu, B.; Li, M. Functions of the C2H2 Transcription Factor Gene Thmea1 in Trichoderma harzianum under Copper Stress Based on Transcriptome Analysis. Biomed. Res. Int. 2018, 2018, 8149682. [Google Scholar] [CrossRef]
- Tansengco, M.; Tejano, J.; Coronado, F.; Gacho, C.; Barcelo, J. Heavy Metal Tolerance and Removal Capacity of Trichoderma Species Isolated from Mine Tailings in Itogon, Benguet. Environ. Nat. Resour. 2018, 16, 39–57. [Google Scholar] [CrossRef]
- Pehlivan, N.; Gedik, K.; Eltem, R.; Terzi, E. Dynamic Interactions of Trichoderma harzianum TS 143 from an Old Mining Site in Turkey for Potent Metal(Oid)s Phytoextraction and Bioenergy Crop Farming. J. Hazard. Mater. 2021, 403, 123609. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Mandal, A.; Thakur, J.; Manna, M.C.; Rao, A.S. Exploring Bioaccumulation Efficacy of Trichoderma viride: An Alternative Bioremediation of Cadmium and Lead. Natl. Acad. Sci. Lett. 2012, 35, 299–302. [Google Scholar] [CrossRef]
- Yaghoubian, Y.; Siadat, S.A.; Moradi Telavat, M.R.; Pirdashti, H.; Yaghoubian, I. Bio-Removal of Cadmium from Aqueous Solutions by Filamentous Fungi: Trichoderma Spp. and Piriformospora Indica. Environ. Sci. Pollut. Res. 2019, 26, 7863–7872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Barrera, V.; Cipollone, J.; Bedoya, C.; Kohan, L.; Toledo, A.; Sisterna, M. New Isolates of Trichoderma spp. as Biocontrol and Plant Growth–Promoting Agents in the Pathosystem Pyrenophora teres Barley in Argentina. Biol. Control 2020, 141, 104152. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.; Li, M.; Lewis, J.; Irby, C. Role of Mycorrhizal Infection in the Growth and Reproduction of Wild vs. Cultivated Plants. Oecologia 1988, 77, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Gul-Zaffar, G.Z.; Dar, A.D.; Mehfuza Habib, M.H. Review on Oat (Avena sativa L.) as a Dual-Purpose Crop. Sci. Res. Essays 2014, 9, 52–59. [Google Scholar] [CrossRef]
- Kim, I.-S.; Hwang, C.-W.; Yang, W.-S.; Kim, C.-H. Multiple Antioxidative and Bioactive Molecules of Oats (Avena sativa L.) in Human Health. Antioxidants 2021, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, W.; Khosla, B.K.; Köpke, U.; Stülpnagel, R.; Böhm, W.; Baeumer, K. Tillage Effects on Root Development, Water Uptake and Growth of Oats. Soil Tillage Res. 1980, 1, 19–34. [Google Scholar] [CrossRef]
- Hoad, S.; Russell, G.; Lucas, M.; Bingham, I. The Management of Wheat, Barley, and Oat Root Systems. Adv. Agron. 2001, 74, 193–246. [Google Scholar] [CrossRef]
- Khan, T.A.; Nadeem, F.; Gao, Y.; Yang, Y.; Wang, X.; Zeng, Z.; Hu, Y. A Larger Root System in Oat (Avena nuda L.) Is Coupled with Enhanced Biomass Accumulation and Hormonal Alterations under Low Nitrogen. Appl. Ecol. Environ. Res. 2019, 17, 4631–4653. [Google Scholar] [CrossRef]
- Bjerre, G.K.; Schierup, H.-H. Uptake of Six Heavy Metals by Oat as Influenced by Soil Type and Additions of Cadmium, Lead, Zinc and Copper. Plant Soil 1985, 88, 57–69. [Google Scholar] [CrossRef]
- Tuma, J.; Skalicky, M.; Tumova, L.; Flidr, J. Influence of Cadmium Dose and Form on the Yield of Oat (Avena sativa L.) and the Metal Distribution in the Plant. J. Elem. 2014, 19, 795–810. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning a Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; ISBN 978-85-7811-079-6. [Google Scholar]
- Yoshida, N.; Ikeda, R.; Okuno, T. Identification and Characterization of Heavy Metal-Resistant Unicellular Alga Isolated from Soil and Its Potential for Phytoremediation. Bioresour. Technol. 2006, 97, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Ranal, M.A.; de Santana, D.G. How and Why to Measure the Germination Process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Y.; Chai, T. Characterization of a Novel Plant Promoter Specifically Induced by Heavy Metal and Identification of the Promoter Regions Conferring Heavy Metal Responsiveness. Plant Physiol. 2007, 143, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.W.; Searle, P.F.; Palmiter, R.D. Identification of Multiple Metal Regulatory Elements in Mouse Metallothionein-I Promoter by Assaying Synthetic Sequences. Nature 1985, 317, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-J.J.; Wang, Y.; Yu, S.-S.S.; Liu, J.-Y.Y. Characterization of a Novel Rice Metallothionein Gene Promoter: Its Tissue Specificity and Heavy Metal Responsiveness. J. Integr. Plant Biol. 2010, 52, 914–924. [Google Scholar] [CrossRef]
- Quinn, J.M.; Merchant, S. Two Copper-Responsive Elements Associated with the Chlamydomonas Cyc6 Gene Function as Targets for Transcriptional Activators. Plant Cell 1995, 7, 623–638. [Google Scholar] [PubMed]
- Yang, Z.; Wang, K.; Aziz, U.; Zhao, C.; Zhang, M. Evaluation of duplicated reference genes for quantitative real-time PCR analysis in genome unknown hexaploid oat (Avena sativa L.). Plant Methods 2020, 16, 138. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2020. [Google Scholar]
- Zbieralski, K.; Staszewski, J.; Konczak, J.; Lazarewicz, N.; Nowicka-Kazmierczak, M.; Wawrzycka, D.; Maciaszczyk-Dziubinska, E. Multilevel Regulation of Membrane Proteins in Response to Metal and Metalloid Stress: A Lesson from Yeast. Int. J. Mol. Sci. 2024, 25, 4450. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium Sources, Toxicity, Resistance and Removal by Microorganisms—A Potential Strategy for Cadmium Eradication. J. Saudi Chem. Soc. 2022, 26, 101569. [Google Scholar] [CrossRef]
- Guo, L.; Li, Z.; Xu, J. Effects of Cadmium Stress on Bacterial and Fungal Communities in the Whitefly Bemisia tabaci. Int. J. Mol. Sci. 2023, 24, 13588. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The Biochemistry of Environmental Heavy Metal Uptake by Plants: Implications for the Food Chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil Contamination with Cadmium, Consequences and Remediation Using Organic Amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Ishikawa, S.; Murakami, M.; Abe, K.; Maejima, Y.; Makino, T. Heavy Metal Contamination of Agricultural Soil and Countermeasures in Japan. Paddy Water Environ. 2010, 8, 247–257. [Google Scholar] [CrossRef]
- Mierek-Adamska, A.; Dąbrowska, G.B.; Goc, A. Genetically modified plants and strategies of soil remediation from haevy metals. Adv. Cell Biol. 2009, 36, 649–662. [Google Scholar]
- Dąbrowska, G.; Hrynkiewicz, K.; Trejgell, A.; Baum, C. The Effect of Plant-Growth-Promoting Rhizobacteria on the Phytoextraction of Cd and Zn by Brassica napus L. Int. J. Phytoremediat. 2017, 19, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Ullah, I.; Waqas, M.; Shahzad, R.; Hong, S.J.; Park, G.S.; Jung, B.K.; Lee, I.J.; Shin, J.H. Plant Growth-Promoting Potential of Endophytic Fungi Isolated from Solanum nigrum Leaves. World J. Microbiol. Biotechnol. 2015, 31, 1461–1466. [Google Scholar] [CrossRef]
- Znajewska, Z.; Dąbrowska, G.B.; Hrynkiewicz, K.; Janczak, K. Biodegradation of Polycaprolactone by Trichoderma viride Fungi. Przem. Chem. 2018, 97, 1676–1679. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Garstecka, Z.; Olewnik-Kruszkowska, E.; Szczepańska, G.; Ostrowski, M.; Mierek-Adamska, A. Comparative Study of Structural Changes of Polylactide and Poly(Ethylene Terephthalate) in the Presence of Trichoderma viride. Int. J. Mol. Sci. 2021, 22, 3491. [Google Scholar] [CrossRef]
- Garstecka, Z.; Antoszewski, M.; Mierek-Adamska, A.; Krauklis, D.; Niedojadło, K.; Kaliska, B.; Hrynkiewicz, K.; Dąbrowska, G.B. Trichoderma viride Colonizes the Roots of Brassica napus L., Alters the Expression of Stress-Responsive Genes, and Increases the Yield of Canola under Field Conditions during Drought. Int. J. Mol. Sci. 2023, 24, 15349. [Google Scholar] [CrossRef]
- Znajewska, Z.; Narbutt, O.; Dąbrowska, G.B.; Narbutt, O. Trichoderma viride Strains Stimulating the Growth and Development of Winter Rapeseed (Brassica napus L.). Prog. Plant Prot. 2018, 58, 264–269. [Google Scholar] [CrossRef]
- Antoszewski, M.; Mierek-Adamska, A.; Dąbrowska, G.B. The Importance of Microorganisms for Sustainable Agriculture—A Review. Metabolites 2022, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Balcázar-López, E.; Méndez-Lorenzo, L.H.; Batista-García, R.A.; Esquivel-Naranjo, U.; Ayala, M.; Kumar, V.V.; Savary, O.; Cabana, H.; Herrera-Estrella, A.; Folch-Mallol, J.L. Xenobiotic Compounds Degradation by Heterologous Expression of a Trametes sanguineus Laccase in Trichoderma atroviride. PLoS ONE 2016, 11, e0147997. [Google Scholar] [CrossRef] [PubMed]
- Oshiquiri, L.H.; dos Santos, K.R.A.; Ferreira Junior, S.A.; Steindorff, A.S.; Barbosa Filho, J.R.; Mota, T.M.; Ulhoa, C.J.; Georg, R.C. Trichoderma harzianum Transcriptome in Response to Cadmium Exposure. Fungal Genet. Biol. 2020, 134, 103281. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A Potential Bioremediator for Environmental Clean Up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Nongmaithem, N.; Roy, A.; Bhattacharya, P.M. Screening of Trichoderma Isolates for Their Potential of Biosorption of Nickel and Cadmium. Braz. J. Microbiol. 2016, 47, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S.; Aishah, S.N.; Azad, S.A.; Shafawati, S.N.; Naher, L. Tolerance and Biosorption Capacity of Zn2+, Pb2+, Ni3+ and Cu2+ by Filamentous Fungi (Trichoderma harzianum, T. aureoviride and T. virens). Adv. Biosci. Biotechnol. 2013, 4, 570–583. [Google Scholar] [CrossRef]
- Sfaxi-Bousbih, A.; Chaoui, A.; El Ferjani, E. Unsuitable Availability of Nutrients in Germinating Bean Embryos Exposed to Copper Excess. Biol. Trace Elem. Res. 2010, 135, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, S.V.; Prasad, M.N.V. Cadmium Stress Affects Seed Germination and Seedling Growth in Sorghum bicolor (L.) Moench by Changing the Activities of Hydrolyzing Enzymes. Plant Growth Regul. 2008, 54, 143–156. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.; Zahir, Z.; Jamil, A. Effect of Cadmium on Seed Germination and Seedling Growth of Four Wheat (Triticum aestivum L.) Cultivars. Pak. J. Bot. 2012, 44, 1569–1574. [Google Scholar]
- Huybrechts, M.; Cuypers, A.; Deckers, J.; Iven, V.; Vandionant, S.; Jozefczak, M.; Hendrix, S. Cadmium and Plant Development: An Agony from Seed to Seed. Int. J. Mol. Sci. 2019, 20, 3971. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.E.A.; Agathokleous, E.; Nogueira, M.L.; Brunetto, G.; Brown, P.H.; Azevedo, R.A. Neutral-to-Positive Cadmium Effects on Germination and Seedling Vigor, with and without Seed Priming. J. Hazard. Mater. 2023, 448, 130813. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Joint Research Centre (European Commission); Ballabio, C.; Jones, A.; Montanarella, L.; Toth, G. Cadmium in the Soils of the EU: Analysis of LUCAS Soils Data for the Review of Fertilizer Directive; Publications Office of the European Union: Luxembourg, 2023; ISBN 978-92-79-66931-6. [Google Scholar]
- Siddique, A.B.; Rahman, M.M.; Islam, M.R.; Mondal, D.; Naidu, R. Response of Iron and Cadmium on Yield and Yield Components of Rice and Translocation in Grain: Health Risk Estimation. Front. Environ. Sci. 2021, 9, 716770. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Kochian, L.V. Phytoextraction of Zinc by Oat Avena sativa, Barley Hordeum vulgare, and Indian Mustard Brassica juncea. Environ. Sci. Technol. 1998, 32, 802–806. [Google Scholar] [CrossRef]
- Marchel, M.; Kaniuczak, J.; Hajduk, E.; Właśniewski, S. Response of Oat (Avena sativa) to the Addition Cadmium to Soil Inoculation with the Genus Trichoderma Fungi. J. Elem. 2018, 23, 471–482. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Che Mohd Zain, C.R.; Wan Yusoff, W.M. Physiological and Growth Response of Rice Plants (Oryza sativa L.) to Trichoderma spp. Inoculants. AMB Express 2014, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, A.; Singh, B.R.; Singh, H.B. Trichoderma harzianum Elicits Induced Resistance in Sunflower Challenged by Rhizoctonia solani. J. Appl. Microbiol. 2014, 116, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Akladious, S.A.; Abbas, S.M. Application of Trichoderma harzianum T22 as a Biofertilizer Potential in Maize Growth. J. Plant Nutr. 2014, 37, 30–49. [Google Scholar] [CrossRef]
- Xun, F.; Xie, B.; Liu, S.; Guo, C. Effect of Plant Growth-Promoting Bacteria (PGPR) and Arbuscular Mycorrhizal Fungi (AMF) Inoculation on Oats in Saline-Alkali Soil Contaminated by Petroleum to Enhance Phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 598–608. [Google Scholar] [CrossRef]
- Illescas, M.; Morán-Diez, M.E.; Martínez de Alba, Á.E.; Hermosa, R.; Monte, E. Effect of Trichoderma Asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions. Int. J. Mol. Sci. 2022, 23, 6782. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.S.; Rai, J.P.N. Phytoextraction of Soil Cadmium and Zinc by Microbes-Inoculated Indian Mustard (Brassica juncea). J. Plant Interact. 2009, 4, 279–287. [Google Scholar] [CrossRef]
- Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Eswaramoorthy, R.; Iqbal, R.K.; Danish, S. Metal-Tolerant and Siderophore Producing Pseudomonas Fluorescence and Trichoderma spp. Improved the Growth, Biochemical Features and Yield Attributes of Chickpea by Lowering Cd Uptake. Sci. Rep. 2023, 13, 4471. [Google Scholar] [CrossRef] [PubMed]
- Taghavi Ghasemkheili, F.; Ekelund, F.; Johansen, J.L.; Pirdashti, H.; Ghadirnezhad Shiade, S.R.; Fathi, A.; Kjøller, R. Ameliorative Effects of Trichoderma harzianum and Rhizosphere Soil Microbes on Cadmium Biosorption of Barley (Hordeum vulgare L.) in Cd-Polluted Soil. J. Plant Nutr. Soil Sci. 2022, 22, 527–539. [Google Scholar] [CrossRef]
- Niu, L.; Li, C.; Wang, W.; Zhang, J.; Scali, M.; Li, W.; Liu, H.; Tai, F.; Hu, X.; Wu, X. Cadmium Tolerance and Hyperaccumulation in Plants—A Proteomic Perspective of Phytoremediation. Ecotoxicol. Environ. Saf. 2023, 256, 114882. [Google Scholar] [CrossRef] [PubMed]
- Haag-Kerwer, A.; Schäfer, H.J.; Heiss, S.; Walter, C.; Rausch, T. Cadmium Exposure in Brassica juncea Causes a Decline in Transpiration Rate and Leaf Expansion without Effect on Photosynthesis. J. Exp. Bot. 1999, 50, 1827–1835. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, R.-Y.; Xu, X.-H.; Xie, X.-J.; Chambe, E.A. Effects of cadmium pollution in soil on growth and cadmium uptake of wheat. J. Agric. Resour. Environ. 2019, 36, 522–527. [Google Scholar] [CrossRef]
- Jian, M.; Zhang, D.; Wang, X.; Wei, S.; Zhao, Y.; Ding, Q.; Han, Y.; Ma, L. Differential Expression Pattern of the Proteome in Response to Cadmium Stress Based on Proteomics Analysis of Wheat Roots. BMC Genom. 2020, 21, 343. [Google Scholar] [CrossRef] [PubMed]
- Tanhuanpää, P.; Kalendar, R.; Schulman, A.H.; Kiviharju, E. A Major Gene for Grain Cadmium Accumulation in Oat (Avena sativa L.). Genome 2007, 50, 588–594. [Google Scholar] [CrossRef]
- Gutiérrez-Ginés, M.J.; Pastor, J.; Hernández, A.J. Effect of Heavy Metals from Mine Soils on Avena sativa L. and Education Strategies. Fresenius Environ. Bull. 2010, 19, 2083–2086. [Google Scholar]
- Fan, S.K.; Fang, X.Z.; Guan, M.Y.; Ye, Y.Q.; Lin, X.Y.; Du, S.T.; Jin, C.W. Exogenous Abscisic Acid Application Decreases Cadmium Accumulation in Arabidopsis Plants, Which Is Associated with the Inhibition of IRT1-Mediated Cadmium Uptake. Front. Plant Sci. 2014, 5, 721. [Google Scholar] [CrossRef]
- Tan, L.; Qu, M.; Zhu, Y.; Peng, C.; Wang, J.; Gao, D.; Chen, C. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 Function Synergistically in Zinc/Cadmium Uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef]
- Tang, L.; Dong, J.; Qu, M.; Lv, Q.; Zhang, L.; Peng, C.; Hu, Y.; Li, Y.; Ji, Z.; Mao, B.; et al. Knockout of OsNRAMP5 Enhances Rice Tolerance to Cadmium Toxicity in Response to Varying External Cadmium Concentrations via Distinct Mechanisms. Sci. Total Environ. 2022, 832, 155006. [Google Scholar] [CrossRef]
- Vasiliadou, S.; Dordas, C. Increased Concentration Of Soil Cadmium Affects On Plant Growth, Dry Matter Accumulation, Cd, And Zn Uptake Of Different Tobacco Cultivars (Nicotiana tabacum L.). Int. J. Phytoremediat. 2009, 11, 115–130. [Google Scholar] [CrossRef]
- Murtaza, G.; Javed, W.; Hussain, A.; Qadir, M.; Aslam, M. Soil-Applied Zinc and Copper Suppress Cadmium Uptake and Improve the Performance of Cereals and Legumes. Int. J. Phytoremediat. 2017, 19, 199–206. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Wang, M.; Chen, W.; Dai, Y. Limestone Dosage Response of Cadmium Phytoavailability Minimization in Rice: A Trade-off Relationship between Soil pH and Amorphous Manganese Content. J. Hazard. Mater. 2021, 403, 123664. [Google Scholar] [CrossRef]
- Palusińska, M.; Barabasz, A.; Kozak, K.; Papierniak, A.; Maślińska, K.; Antosiewicz, D.M. Zn/Cd Status-Dependent Accumulation of Zn and Cd in Root Parts in Tobacco Is Accompanied by Specific Expression of ZIP Genes. BMC Plant Biol. 2020, 20, 37. [Google Scholar] [CrossRef]
- Ziller, A.; Fraissinet-Tachet, L. Metallothionein Diversity and Distribution in the Tree of Life: A Multifunctional Protein. Metallomics 2018, 10, 1549–1559. [Google Scholar] [CrossRef]
- Bulathge, A.W.; Villones, R.L.E.; Herbert, F.C.; Gassensmith, J.J.; Meloni, G. Comparative Cisplatin Reactivity towards Human Zn7-Metallothionein-2 and MTF-1 Zinc Fingers: Potential Implications in Anticancer Drug Resistance. Metallomics 2022, 14, mfac061. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, Y.; Yang, Y.; Peng, Y.; Li, C. Copper Metabolism in Saccharomyces cerevisiae: An Update. Biometals 2021, 34, 3–14. [Google Scholar] [CrossRef]
- Priante, E.; Pietropoli, E.; Piva, E.; Santovito, G.; Schumann, S.; Irato, P. Cadmium–Zinc Interaction in Mus musculus Fibroblasts. Int. J. Mol. Sci. 2022, 23, 12001. [Google Scholar] [CrossRef]
- Rono, J.K.; Le Wang, L.; Wu, X.C.; Cao, H.W.; Zhao, Y.N.; Khan, I.U.; Yang, Z.M. Identification of a New Function of Metallothionein-like Gene OsMT1e for Cadmium Detoxification and Potential Phytoremediation. Chemosphere 2021, 265, 129136. [Google Scholar] [CrossRef]
- Enshaei, M.; Khanafari, A.; Sepahey, A.A. Metallothionein Induction in Two Species of Pseudomonas Exposed to Cadmium and Copper Contamination. Iran. J. Environ. Health Sci. Eng. 2010, 7, 287–298. [Google Scholar]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant Cis-Acting Regulatory DNA Elements (PLACE) Database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Lü, S.; Gu, H.; Yuan, X.; Wang, X.; Wu, A.M.; Qu, L.; Liu, J.Y. The GUS Reporter-Aided Analysis of the Promoter Activities of a Rice Metallothionein Gene Reveals Different Regulatory Regions Responsible for Tissue-Specific and Inducible Expression in Transgenic Arabidopsis. Transgenic Res. 2007, 16, 177–191. [Google Scholar] [CrossRef]
- Quinn, J.M.; Kropat, J.; Merchant, S. Copper Response Element and Crr1-Dependent Ni2+-Responsive Promoter for Induced, Reversible Gene Expression in Chlamydomonas reinhardtii. Eukaryot. Cell 2003, 2, 995–1002. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Kim, S.H.; Lee, H.S.; Marinoia, E.; Song, W.Y. Characterization of Brassica rapa Metallothionein and Phytochelatin Synthase Genes Potentially Involved in Heavy Metal Detoxification. PLoS ONE 2021, 16, e0252899. [Google Scholar] [CrossRef]
- Mierek-Adamska, A.; Dąbrowska, G.B.; Blindauer, C.A. The Type 4 Metallothionein from Brassica napus Seeds Folds in a Metal-Dependent Fashion and Favours Zinc over Other Metals. Metallomics 2018, 10, 1430–1443. [Google Scholar] [CrossRef]
- Roosens, N.H.; Leplae, R.; Bernard, C.; Verbruggen, N. Variations in Plant Metallothioneins: The Heavy Metal Hyperaccumulator Thlaspi caerulescens as a Study Case. Planta 2005, 222, 716–729. [Google Scholar] [CrossRef]
- Roosens, N.H.; Bernard, C.; Leplae, R.; Verbruggen, N. Evidence for Copper Homeostasis Function of Metallothionein (MT3) in the Hyperaccumulator Thlaspi caerulescens. FEBS Lett. 2004, 577, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hassinen, V.H.; Tuomainen, M.; Peräniemi, S.; Schat, H.; Kärenlampi, S.O.; Tervahauta, A.I. Metallothioneins 2 and 3 Contribute to the Metal-Adapted Phenotype but Are Not Directly Linked to Zn Accumulation in the Metal Hyperaccumulator, Thlaspi caerulescens. J. Exp. Bot. 2009, 60, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewicz, K.; Dąbrowska, G.; Baum, C.; Niedojadło, K.; Leinweber, P. Interactive and single effects of ectomycorrhiza formation and Bacillus cereus on metallothionein MT1 expression and phytoextraction of Cd and Zn by willows. Water Air Soil Pollut. 2012, 223, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.A.; Tomas, M.; Cattillo, J.; Bofill, R.; Capdevila, M.; Atrian, S.; Andreo, C.S. The Response of the Different Soybean Metallothionein Isoforms to Cadmium Intoxication. J. Inorg. Biochem. 2012, 117, 306–315. [Google Scholar] [CrossRef]


| Primer Name | Sequence 5′→3′ | Target | Reference |
|---|---|---|---|
| AsMT1_qPCR_f AsMT1_qPCR_r | CAAACTGCAAGTGCGGGAAG TTGTTCTCATGAGCCACGCC | AsMT1 | [17] |
| AsMT2_qPCR_f AsMT2_qPCR_r | CTGCGGAGGGTGCAAGATG AACGATGGCTTGGAAGAGGG | AsMT2 | |
| AsMT3_qPCR_f AsMT3_qPCR_r | TCCACCATGTCGAACACCTG TGGCTCTTCTCGGTGTCAAC | AsMT3 | |
| AsMT4_qPCR_f | CACGTGCGGAGAGCACTG | AsMT4 | [3] |
| AsMT4_qPCR_r | ACAGGAGGCGCAGTCACAG | ||
| EIF4A_f EIF4A_r | TCTCGCAGGATACGGATGTCG TCCATCGCATTGGTCGCTCT | EIF 4A | [60] |
| T. viride Strain | Minimal Inhibitory Concentration [mM] | ||
|---|---|---|---|
| Zn | Cu | Cd | |
| T1 | 22.3 | 1.7 | 3.6 |
| T2 | 29.0 | 2.0 | 2.6 |
| T3 | 28.5 | 2.4 | 2.6 |
| T4 | 28.5 | 2.0 | 2.7 |
| T5 | 29.2 | 1.6 | 2.9 |
| T6 | 30.0 | 2.0 | 1.9 |
| T. viride T5 Spore Concentration (Spores mL−1) | G (%) | GI (days) | MGT (days) | MGR (1/days) | CVG |
|---|---|---|---|---|---|
| 0 (Control) | 93.45 ± 1.48 a | 5.10 ± 0.08 b | 1.90 ± 0.08 a | 0.55 ± 0.02 b | 54.98 ± 2.13 b |
| 102 | 94.33 ± 1.11 a | 5.16 ± 0.05 ab | 1.84 ± 0.05 ab | 0.55 ± 0.01 ab | 55.42 ± 1.28 b |
| 104 | 100.00 ± 0.20 a | 5.38 ± 0.04 ab | 1.62 ± 0.04 ab | 0.62 ± 0.01 ab | 61.76 ± 1.39 ab |
| 106 | 80.00 ± 1.67 b | 5.63 ± 0.06 a | 1.38 ± 0.06 b | 0.73 ± 0.03 a | 73.33 ± 3.14 a |
| Treatment | Spore conc. | Shoot Length (cm) | Fresh Shoot Biomass (g) | Dry Shoot Biomass (g) | Root Length (cm) | Fresh Root Biomass (g) | Dry Root Biomass (g) |
|---|---|---|---|---|---|---|---|
| Control (non-inoculated) | 0 | 5.73 ± 0.28 a | 0.068 ± 0.004 a | 0.0054 ± 0.0005 ab | 8.26 ± 0.51 a | 0.060 ± 0.003 a | 0.0052 ± 0.0002 a |
| Inoculated with T. viride T5 | 102 | 5.24 ± 0.15 ab | 0.063 ± 0.003 a | 0.0062 ± 0.0002 a | 8.18 ± 0.49 a | 0.044 ± 0.003 b | 0.0052 ± 0.0002 a |
| 104 | 4.92 ± 0.23 b | 0.059 ± 0.004 a | 0.0051 ± 0.0005 ab | 7.74 ± 0.62 a | 0.052 ± 0.004 ab | 0.0048 ± 0.0003 a | |
| 106 | 5.43 ± 0.13 ab | 0.061 ± 0.002 a | 0.0046 ± 0.0004 b | 7.69 ± 0.28 a | 0.043 ± 0.003 b | 0.0026 ± 0.0002 b |
| Cd conc. [µM] | G (%) | GI (days) | MGT (days) | MGR (1/day) | CVG | |
|---|---|---|---|---|---|---|
| Control (non-inoculated) | 0 | 93.45 ± 1.48 a | 5.10 ± 0.08 a | 1.90 ± 0.08 b | 0.55 ± 0.02 a | 54.98 ± 2.13 a |
| 25 | 88.75 ± 1.56 a | 4.88 ± 0.04 ab | 2.12 ± 0.04 ab | 0.47 ± 0.01 ab | 47.49 ± 0.74 ab | |
| 80 | 90.47 ± 1.11 a | 4.90 ± 0.05 ab | 2.10 ± 0.05 ab | 0.48 ± 0.01 ab | 48.25 ± 1.15 ab | |
| 150 | 92.38 ± 0.84 a | 4.52 ± 0.04 b | 2.48 ± 0.04 a | 0.41 ± 0.01 b | 40.71 ± 0.73 b | |
| 245 | 89.43 ± 2.15 a | 4.42 ± 0.05 b | 2.58 ± 0.05 a | 0.39 ± 0.01 b | 39.16 ± 0.81 b | |
| Inoculated with T. viride T5 | 0 | 94.33 ± 1.11 a | 5.16 ± 0.05 a | 1.84 ± 0.05 b | 0.55 ± 0.01 a | 55.42 ± 1.28 a |
| 25 | 83.81 ± 1.37 a | 4.88 ± 0.03 ab | 2.12 ± 0.03 ab | 0.47 ± 0.01 ab | 47.36 ± 0.66 ab | |
| 80 | 89.05 ± 1.39 a | 4.91 ± 0.04 ab | 2.09 ± 0.04 ab | 0.48 ± 0.01 ab | 48.25 ± 0.98 ab | |
| 150 | 87.73 ± 0.76 a | 4.88 ± 0.05 ab | 2.12 ± 0.05 ab | 0.48 ± 0.01 ab | 47.90 ± 1.25 ab | |
| 245 | 86.85 ± 1.66 a | 4.79 ± 0.09 ab | 2.21 ± 0.09 ab | 0.48 ± 0.02 ab | 47.53 ± 2.09 ab |
| Cd conc. [µM] | Shoot Length (cm) | Fresh Shoot Biomass (g) | Dry Shoot Biomass (g) | Root Length (cm) | Fresh Root Biomass (g) | Dry Root Biomass (g) | |
|---|---|---|---|---|---|---|---|
| Control (non-inoculated) | 0 | 5.73 ± 0.28 a | 0.068 ± 0.004 a | 0.0054 ± 0.0005 bc | 8.26 ± 0.51 a | 0.060 ± 0.003 a | 0.0052 ± 0.0002 b |
| 25 | 4.27 ± 0.34 b | 0.052 ± 0.003 b | 0.0060 ± 0.0004 b | 6.47 ± 0.62 b | 0.047 ± 0.005 abc | 0.0052 ± 0.0004 b | |
| 80 | 5.60 ± 0.22 a | 0.066 ± 0.003 a | 0.0073 ± 0.0003 a | 6.46 ± 0.24 b | 0.058 ± 0.003 ab | 0.0065 ± 0.0003 a | |
| 150 | 3.21 ± 0.20 c | 0.047 ± 0.003 bc | 0.0042 ± 0.0001 d | 3.27 ± 0.19 cd | 0.037 ± 0.003 cd | 0.0030 ± 0.0000 d | |
| 245 | 3.85 ± 0.18 bc | 0.047 ± 0.003 bc | 0.0060 ± 0.0004 b | 2.57 ± 0.13 d | 0.028 ± 0.002 d | 0.0037 ± 0.0003 cd | |
| Inoculated with T. viride T5 | 0 | 5.24 ± 0.15 a | 0.063 ± 0.003 a | 0.0062 ± 0.0002 ab | 8.18 ± 0.49 a | 0.044 ± 0.003 bcd | 0.0052 ± 0.0002 b |
| 25 | 3.42 ± 0.17 c | 0.045 ± 0.002 c | 0.0042 ± 0.0002 d | 4.59 ± 0.28 c | 0.032 ± 0.002 cd | 0.0037 ± 0.0001 cd | |
| 80 | 3.45 ± 0.21 c | 0.041 ± 0.003 c | 0.0044 ± 0.0001 cd | 4.32 ± 0.30 c | 0.034 ± 0.002 cd | 0.0041 ± 0.0002 c | |
| 150 | 3.28 ± 0.19 c | 0.042 ± 0.003 c | 0.0044 ± 0.0002 d | 3.75 ± 0.21 cd | 0.033 ± 0.002 cd | 0.0040 ± 0.0001 c | |
| 245 | 3.45 ± 0.22 c | 0.039 ± 0.003 c | 0.0042 ± 0.0003 d | 3.09 ± 0.20 d | 0.034 ± 0.009 cd | 0.0034 ± 0.0002 cd |
| Cd Content in the Soil [mg kg−1] | Leaves Number | Panicles Number | Seeds Number | |
|---|---|---|---|---|
| Non-inoculated | 1 | 17.9 ± 1.1 b | 3.2 ± 0.4 bc | 11.9 ± 1.4 abd |
| 5 | 17.1 ± 1.0 b | 2.7 ± 0.2 c | 10.0 ± 0.9 bd | |
| 10 | 15.4 ± 1.1 b | 1.8 ± 0.2 d | 6.5 ± 1.3 c | |
| 20 | 17.1 ± 0.7 b | 1.9 ± 0.4 cd | 5.5 ± 1.1 c | |
| Inoculated with T. viride T5 | 1 | 19.8 ± 0.8 a | 4.1 ± 0.4 b | 14.5 ± 1.3 ab |
| 5 | 17.9 ± 0.8 ab | 4.2 ± 0.3 ab | 16.0 ± 1.5 a | |
| 10 | 16.5 ± 0.7 b | 2.2 ± 0.4 cd | 7.8 ± 1.4 cd | |
| 20 | 16.7 ± 0.8 b | 2.5 ± 0.5 cd | 9.5 ± 1.9 cd |
| Treatment | Cd content in the Soil [mg kg−1] | Content in Shoots | ||
|---|---|---|---|---|
| Cd [mg kg−1] | Cu [mg kg−1] | Zn [mg kg−1] | ||
| Non-inoculated | 1 | 0.143 ± 0.01 c | 5.674 ± 0.84 a | 29.625 ± 3.31 bc |
| 5 | 0.209 ± 0.04 c | 5.751 ± 0.33 a | 49.310 ± 1.97 a | |
| 10 | 0.387 ± 0.07 bc | 4.249 ± 0.27 a | 39.665 ± 0.41 ab | |
| 20 | 0.931 ± 0.11 a | 4.048 ± 1.55 a | 47.805 ± 6.13 a | |
| Inoculated with T. viride T5 | 1 | 0.198 ± 0.03 c | 4.134 ± 0.94 a | 25.515 ± 4.16 c |
| 5 | 0.377 ± 0.07 bc | 5.145 ± 1.60 a | 36.770 ± 8.36 abc | |
| 10 | 0.577 ± 0.08 b | 3.618 ± 1.26 a | 29.555 ± 2.86 bc | |
| 20 | 1.050 ± 0.18 a | 4.104 ± 1.16 a | 47.080 ± 3.09 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczna, W.; Turkan, S.; Warchoł, M.; Skrzypek, E.; Dąbrowska, G.B.; Mierek-Adamska, A. The Contribution of Trichoderma viride and Metallothioneins in Enhancing the Seed Quality of Avena sativa L. in Cd-Contaminated Soil. Foods 2024, 13, 2469. https://doi.org/10.3390/foods13152469
Konieczna W, Turkan S, Warchoł M, Skrzypek E, Dąbrowska GB, Mierek-Adamska A. The Contribution of Trichoderma viride and Metallothioneins in Enhancing the Seed Quality of Avena sativa L. in Cd-Contaminated Soil. Foods. 2024; 13(15):2469. https://doi.org/10.3390/foods13152469
Chicago/Turabian StyleKonieczna, Wiktoria, Sena Turkan, Marzena Warchoł, Edyta Skrzypek, Grażyna B. Dąbrowska, and Agnieszka Mierek-Adamska. 2024. "The Contribution of Trichoderma viride and Metallothioneins in Enhancing the Seed Quality of Avena sativa L. in Cd-Contaminated Soil" Foods 13, no. 15: 2469. https://doi.org/10.3390/foods13152469







