Identification of Amino Acids and Polyphenolic Metabolites in Human Plasma by UHPLC-ESI-QTOF-MS/MS, after the Chronic Intake of a Functional Meal in an Elderly Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Samples
2.2. Phenolic and Amino Acid Metabolites Extraction
2.3. Metabolite Analysis by Liquid Chromatography Coupled with Mass Spectrometry in Tandem
2.4. Statistic Analysis
3. Results and Discussion
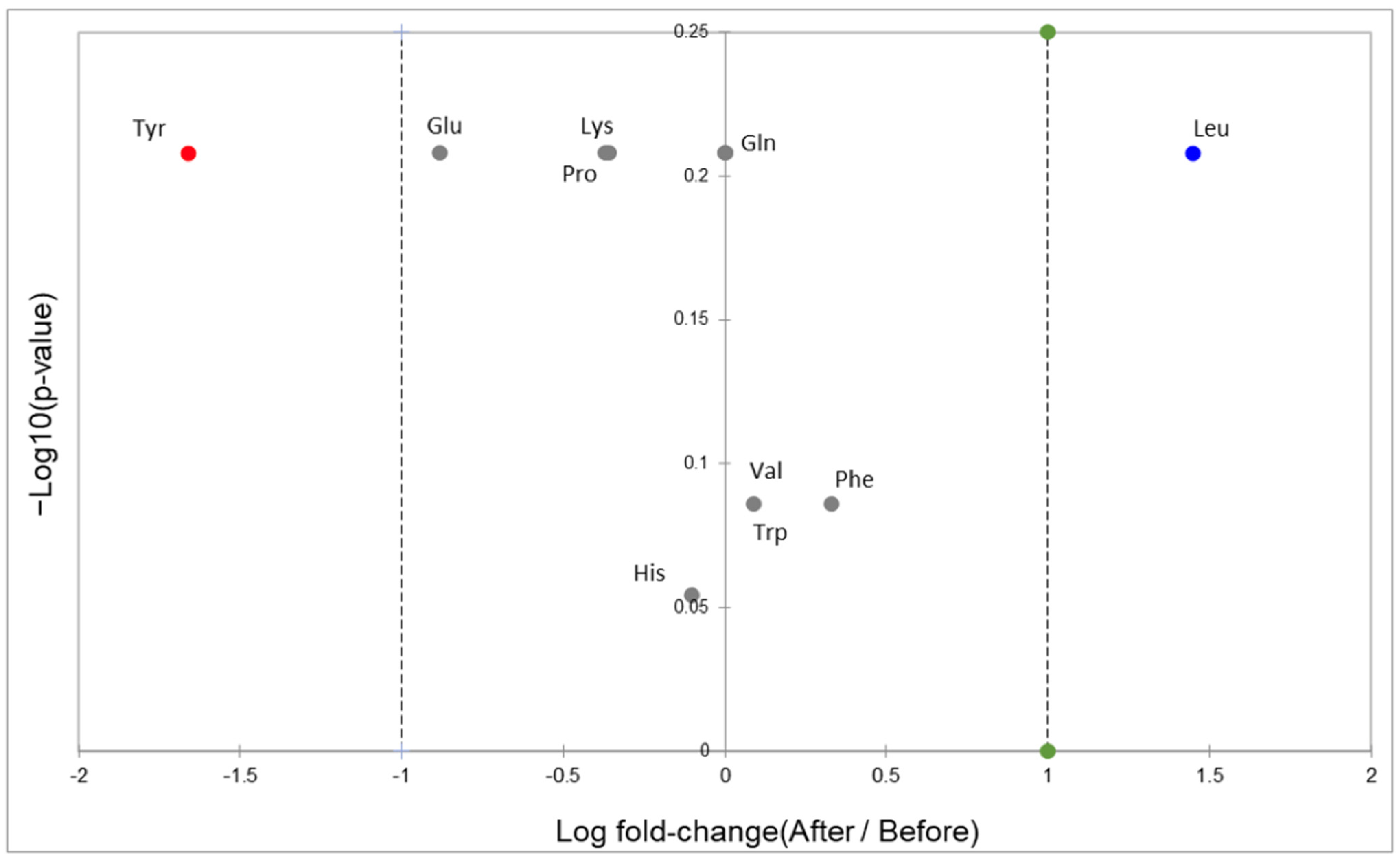
3.1. Amino Acids in Plasma
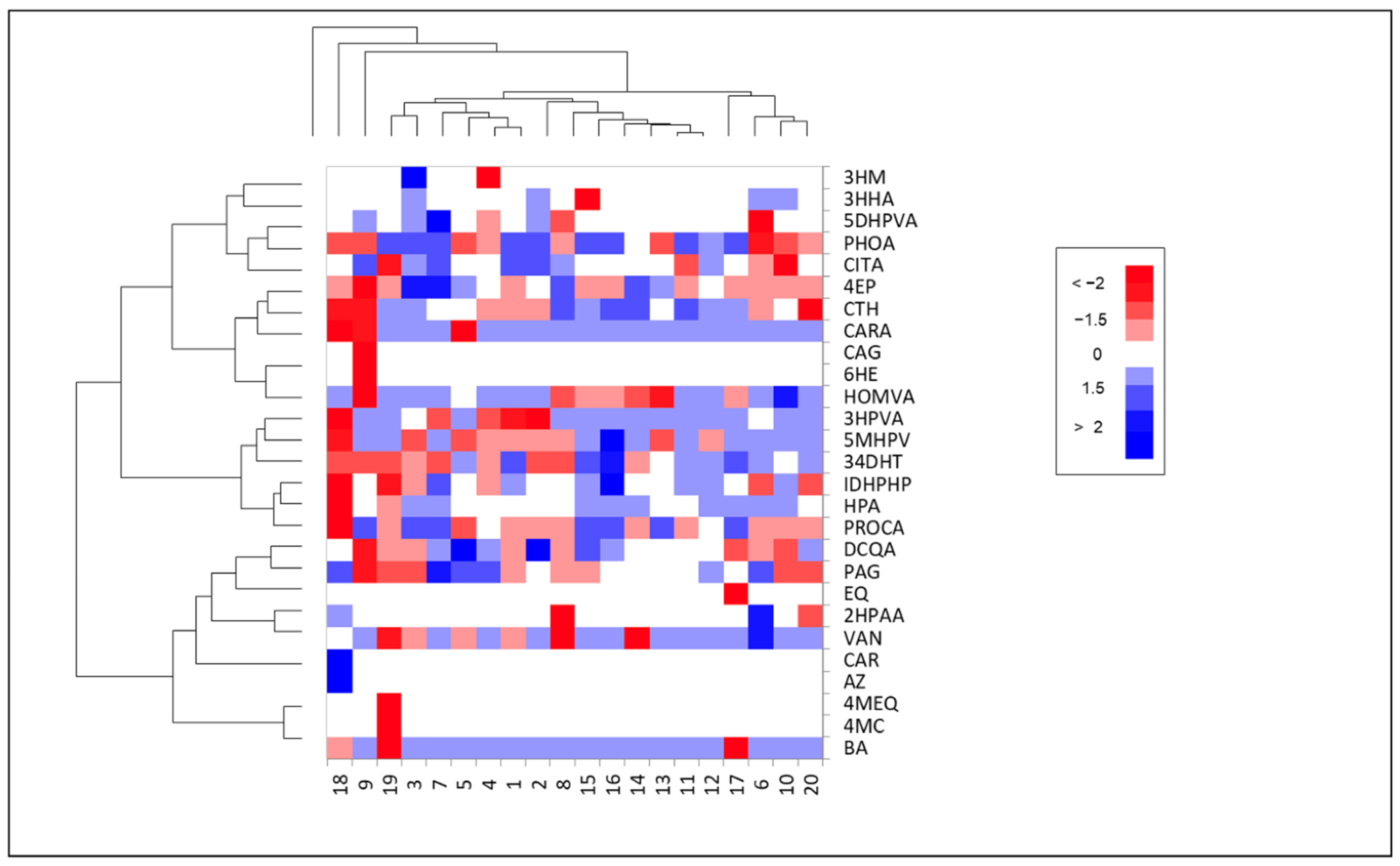
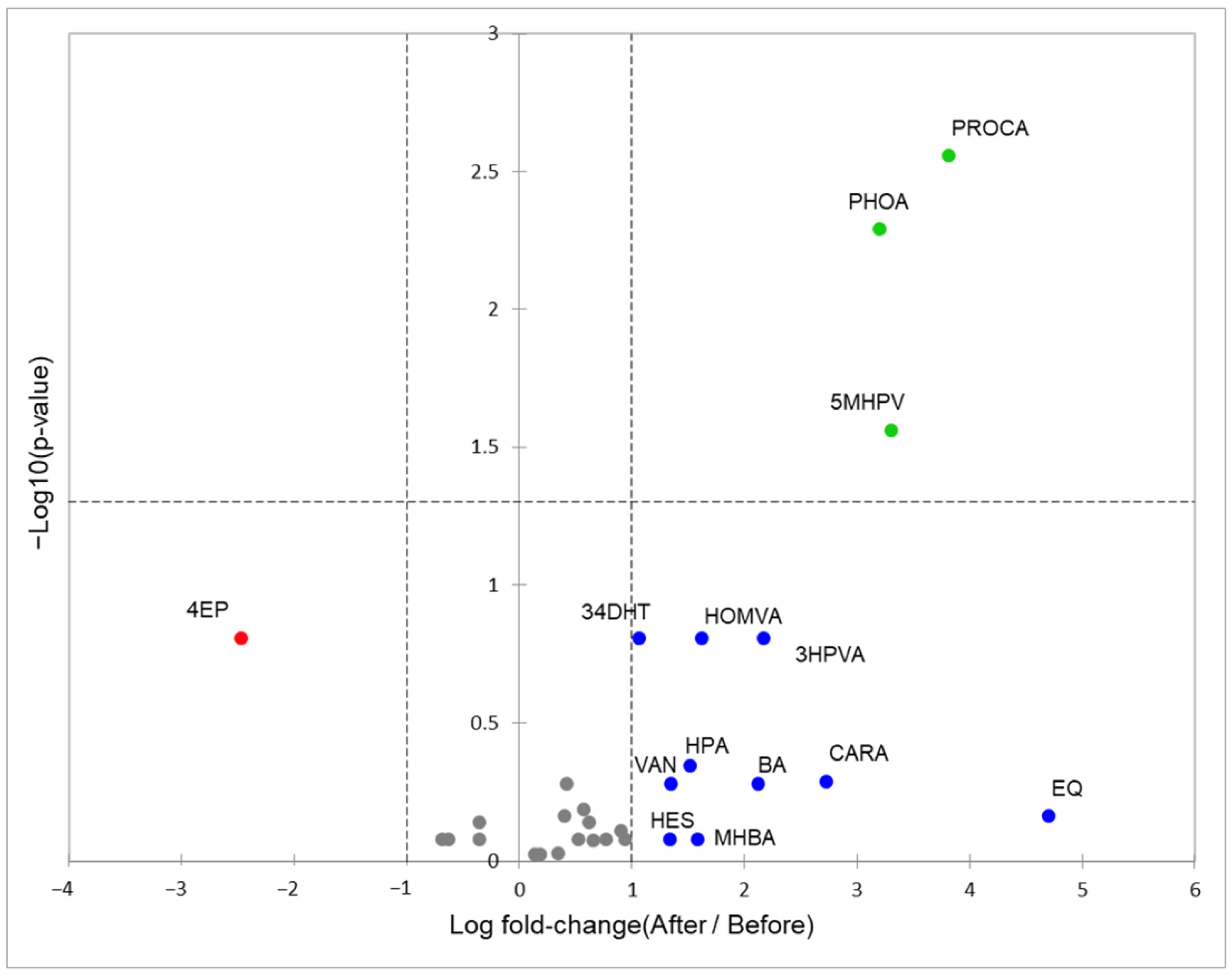
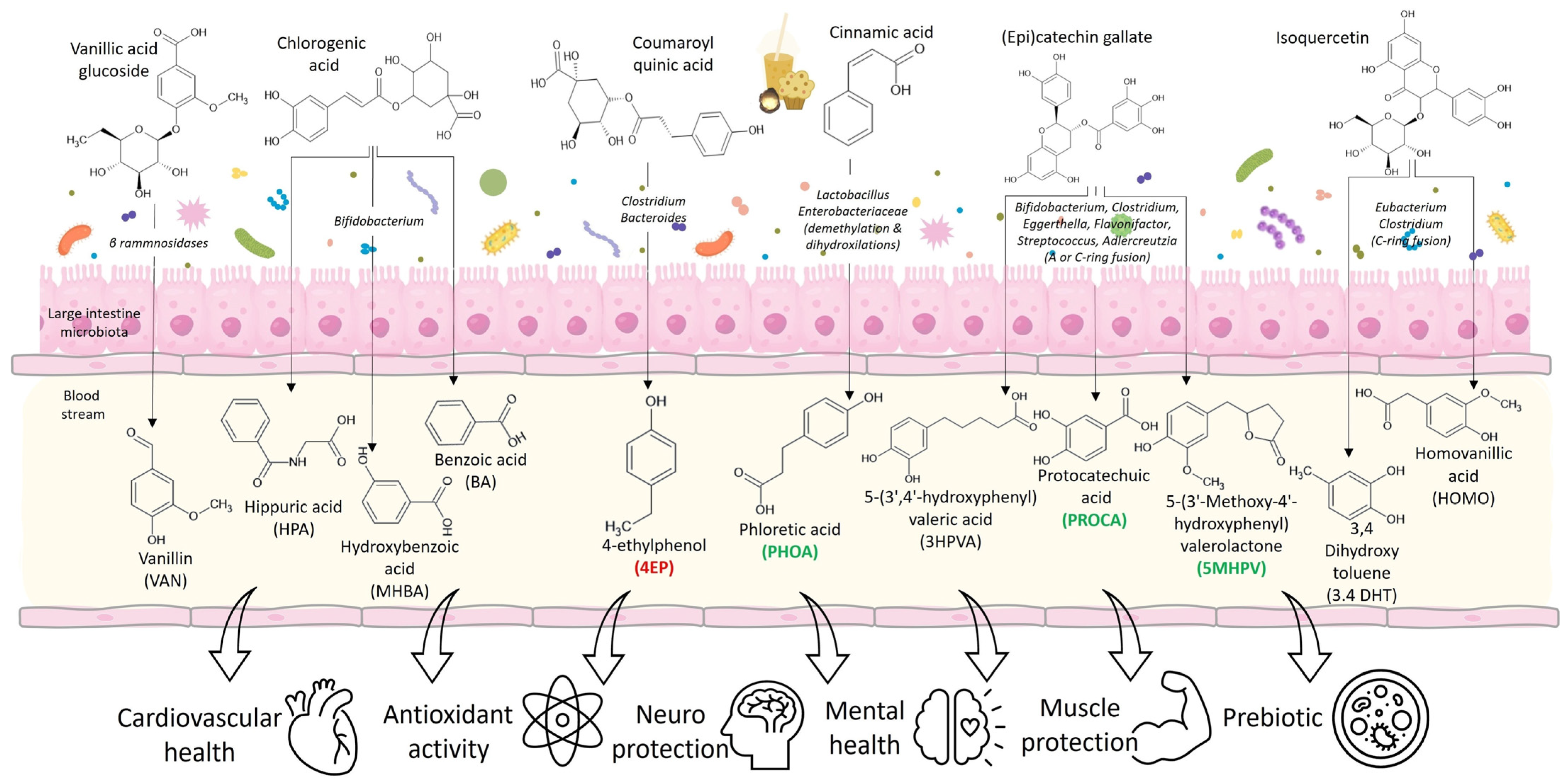
3.2. Phenolic Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients 2021, 13, 2045. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rajagukguk, Y.V.; Gramza-Michałowska, A. Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review. Foods 2021, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Padwad, Y. Perspectives of the Potential Implications of Polyphenols in Influencing the Interrelationship between Oxi-Inflammatory Stress, Cellular Senescence and Immunosenescence during Aging. Trends Food Sci. Technol. 2020, 98, 41–52. [Google Scholar] [CrossRef]
- Grochowicz, J.; Fabisiak, A.; Ekielski, A. Importance of Physical and Functional Properties of Foods Targeted to Seniors. J. Future Foods 2021, 1, 146–155. [Google Scholar] [CrossRef]
- Shang, N.; Meram, C.; Bandara, N.; Wu, J. Protein and Peptides for Elderly Health. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 112, pp. 265–308. [Google Scholar]
- Chapman, I.; Oberoi, A.; Giezenaar, C.; Soenen, S. Rational Use of Protein Supplements in the Elderly—Relevance of Gastrointestinal Mechanisms. Nutrients 2021, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Borgonjen-Van Den Berg, K.J.; Van Loon, L.J.C.; de Groot, L.C.P.G.M. Dietary Protein Intake in Dutch Elderly People: A Focus on Protein Sources. Nutrients 2015, 7, 9697–9706. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tadeo, A.; Del Hierro-Ochoa, J.C.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; de la Rosa, L.A.; Alvarez-Parrilla, E.; López-Díaz, J.A.; Vidaña-Gaytán, M.E.; González-Valles, M.N.; Larqué-Saavedra, A.; et al. Functionality of Bread and Beverage Added with Brosimum Alicastrum Sw. Seed Flour on the Nutritional and Health Status of the Elderly. Foods 2021, 10, 1764. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Small, B.J.; Rawson, K.S.; Martin, C.; Eisel, S.L.; Sanberg, C.D.; McEvoy, C.L.; Sanberg, P.R.; Shytle, R.D.; Tan, J.; Bickford, P.C. Nutraceutical Intervention Improves Older Adults’ Cognitive Functioning. Rejuvenation Res. 2014, 17, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Subiria-Cueto, R.; Larqué-Saavedra, A.; Reyes-Vega, M.L.; De La Rosa, L.A.; Santana-Contreras, L.E.; Gaytán-Martínez, M.; Vázquez-Flores, A.A.; Rodrigo-García, J.; Corral-Avitia, A.Y.; Núñez-Gastélum, J.A.; et al. Brosimum Alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla. Foods 2019, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Cereceres-Aragón, A.; Rodrigo-García, J.; Álvarez-Parrilla, E.; Rodríguez-Tadeo, A. Consumption of Phenolic Compounds in the Elderly Population. Nutr. Hosp. 2019, 36, 470–478. [Google Scholar] [PubMed]
- Martínez Ruiz, N.d.R.; Javier Torres, L.E.; Del Hierro Ochoa, J.C.; Larqué Saavedra, A. Bebida Adicionada Con Brosimum Alicastrum Sw.: Una Alternativa Para Requerimientos Dietarios Especiales. RESPYN Rev. Salud Pública Nutr. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Vazquez-Flores, A.A.; Alvarez-Parrilla, E.; Martínez-Ruiz, N.R.; de la Rosa, L.A. Semi-Targeted Ultra-High-Performance Chromatography Coupled to Mass Spectrometry Analysis of Phenolic Metabolites in Plasma of Elderly Adults. J. Vis. Exp. 2022, 182, e63164. [Google Scholar]
- Sarwar, G.; Botting, H.G.; Collins, M. A Comparison of Fasting Serum Amino Acid Profiles of Young and Elderly Subjects. J. Am. Coll. Nutr. 1991, 10, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Shi, W.; Zhang, Q. Role of Essential Amino Acids in Age-Induced Bone Loss. Int. J. Mol. Sci. 2022, 23, 11281. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan Metabolism: Mechanism-Oriented Therapy for Neurological and Psychiatric Disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef] [PubMed]
- Szwiega, S.; Pencharz, P.B.; Rafii, M.; Lebarron, M.; Chang, J.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. Dietary Leucine Requirement of Older Men and Women Is Higher than Current Recommendations. Am. J. Clin. Nutr. 2021, 113, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.M.; Cameron-Smith, D. Digestion and Postprandial Metabolism in the Elderly. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 76, pp. 79–124. [Google Scholar]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR Is a Web App for Creating, Exploring, Labeling and Sharing Volcano Plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Guerra, A.; Nouvenne, A.; Meschi, T.; Maggi, S. Disentangling the Complexity of Nutrition, Frailty and Gut Microbial Pathways during Aging: A Focus on Hippuric Acid. Nutrients 2023, 15, 1138. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tsao, R.; Wang, X.; Jiang, L.; Sun, Y.; Xiong, H. Phenolics of Yellow Pea (Pisum sativum L.) Hulls, Their Plasma and Urinary Metabolites, Organ Distribution, and in Vivo Antioxidant Activities. J. Agric. Food Chem. 2021, 69, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-Valerolactones and Phenylvaleric Acids, the Main Colonic Metabolites of Flavan-3-Ols: Synthesis, Analysis, Bioavailability, and Bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cui, T.; Xie, N.; Zhang, X.; Qian, Z.; Liu, J. Protocatechuic Acid Improves Cognitive Deficits and Attenuates Amyloid Deposits, Inflammatory Response in Aged AβPP/PS1 Double Transgenic Mice. Int. Immunopharmacol. 2014, 20, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Di Pede, G.; Mena, P.; Bresciani, L.; Achour, M.; Lamuela-Raventós, R.M.; Estruch, R.; Landberg, R.; Kulling, S.E.; Wishart, D.; Rodriguez-Mateos, A.; et al. Revisiting the Bioavailability of Flavan-3-Ols in Humans: A Systematic Review and Comprehensive Data Analysis. Mol. Asp. Med. 2023, 89, 101146. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty Seeds: Nutrients, Bioactives, Bioavailability, and Health Benefits: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, I.; Verni, M.; Verardo, V.; Gómez-Caravaca, A.M.; Rizzello, C.G. Nutritional and Functional Advantages of the Use of Fermented Black Chickpea Flour for Semolina-Pasta Fortification. Foods 2021, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Rezq, S.; Mahmoud, M.F.; El-Shazly, A.M.; El Raey, M.A.; Sobeh, M. Anti-Inflammatory, Antipyretic, and Analgesic Properties of Potamogeton Perfoliatus Extract: In Vitro and in Vivo Study. Molecules 2021, 26, 4826. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary Polyphenol Impact on Gut Health and Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Meschi, T. Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)Phenols on Skeletal Muscle in Aging. Nutrients 2023, 15, 2367. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Stanley, R.; Singh, I. The Ex Vivo Antiplatelet Activation Potential of Fruit Phenolic Metabolite Hippuric Acid. Food Funct. 2015, 6, 2679–2683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tahir, T.; Ashfaq, M.; Asghar, H.; Shahzad, M.I.; Tabassum, R.; Ashfaq, A. Medicinal Importance of Azo and Hippuric Acid Derivatives. Mini-Rev. Med. Chem. 2019, 19, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic Acid Used as Food and Feed Additives Can Regulate Gut Functions. BioMed Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, M.; D’amico, F.; Barone, M.; Conti, G.; Mengoli, M.; Brigidi, P.; Turroni, S. Polyphenol and Tannin Nutraceuticals and Their Metabolites: How the Human Gut Microbiota Influences Their Properties. Biomolecules 2022, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Bas, J.M.D.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazquez-Flores, A.A.; Muñoz-Bernal, Ó.A.; Alvarez-Parrilla, E.; Rodriguez-Tadeo, A.; Martínez-Ruiz, N.d.R.; de la Rosa, L.A. Identification of Amino Acids and Polyphenolic Metabolites in Human Plasma by UHPLC-ESI-QTOF-MS/MS, after the Chronic Intake of a Functional Meal in an Elderly Population. Foods 2024, 13, 2471. https://doi.org/10.3390/foods13162471
Vazquez-Flores AA, Muñoz-Bernal ÓA, Alvarez-Parrilla E, Rodriguez-Tadeo A, Martínez-Ruiz NdR, de la Rosa LA. Identification of Amino Acids and Polyphenolic Metabolites in Human Plasma by UHPLC-ESI-QTOF-MS/MS, after the Chronic Intake of a Functional Meal in an Elderly Population. Foods. 2024; 13(16):2471. https://doi.org/10.3390/foods13162471
Chicago/Turabian StyleVazquez-Flores, Alma A., Óscar A. Muñoz-Bernal, Emilio Alvarez-Parrilla, Alejandra Rodriguez-Tadeo, Nina del Rocío Martínez-Ruiz, and Laura A. de la Rosa. 2024. "Identification of Amino Acids and Polyphenolic Metabolites in Human Plasma by UHPLC-ESI-QTOF-MS/MS, after the Chronic Intake of a Functional Meal in an Elderly Population" Foods 13, no. 16: 2471. https://doi.org/10.3390/foods13162471
APA StyleVazquez-Flores, A. A., Muñoz-Bernal, Ó. A., Alvarez-Parrilla, E., Rodriguez-Tadeo, A., Martínez-Ruiz, N. d. R., & de la Rosa, L. A. (2024). Identification of Amino Acids and Polyphenolic Metabolites in Human Plasma by UHPLC-ESI-QTOF-MS/MS, after the Chronic Intake of a Functional Meal in an Elderly Population. Foods, 13(16), 2471. https://doi.org/10.3390/foods13162471





