Improving Structural, Physical, and Sensitive Properties of Sodium Alginate–Purple Sweet Potato Peel Extracts Indicator Films by Varying Drying Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Measurement of Anthocyanins
2.3. Preparation of the Indicator Films
2.4. Structural Characterization of Films
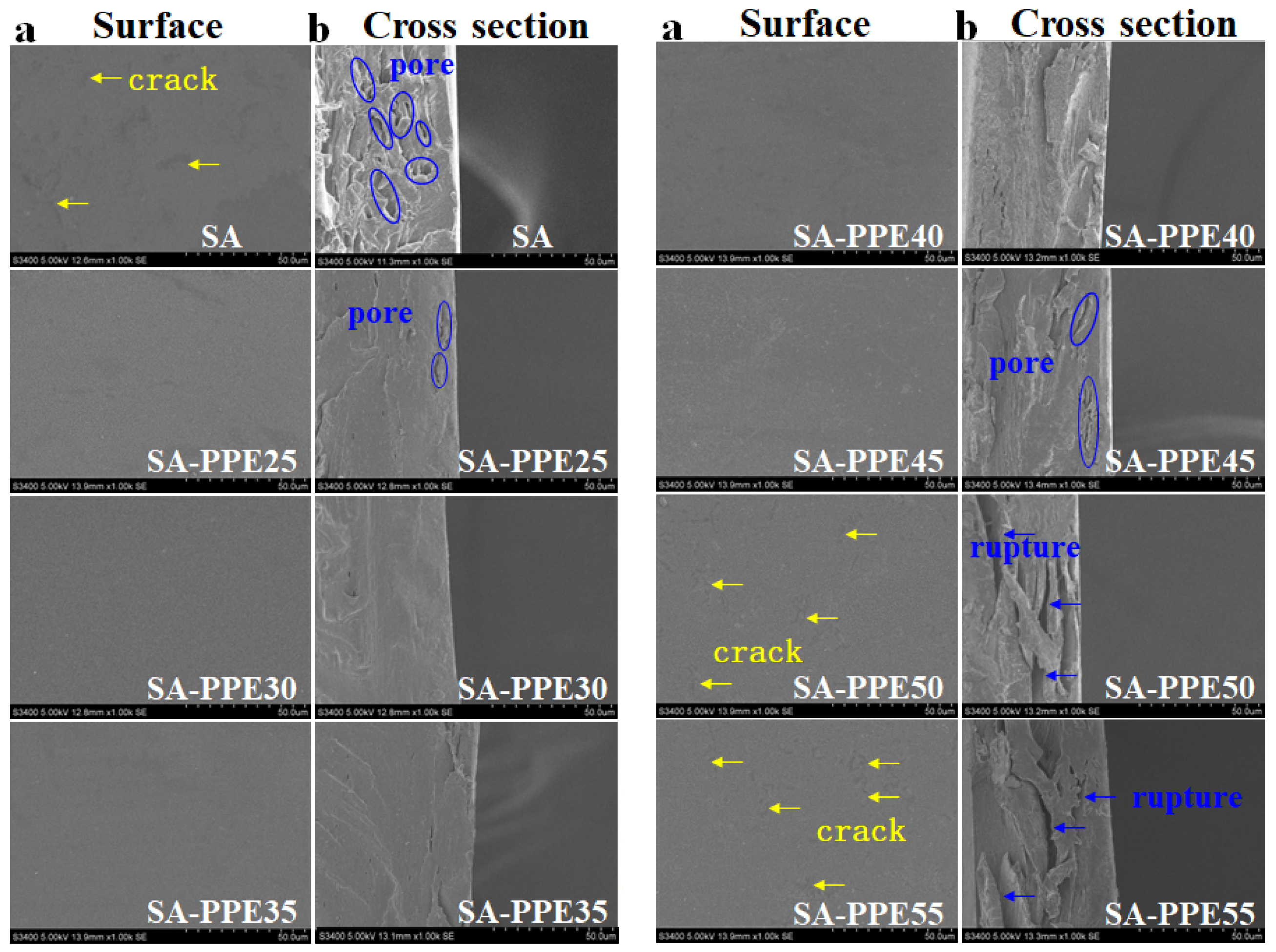
2.4.1. SEM
2.4.2. FTIR
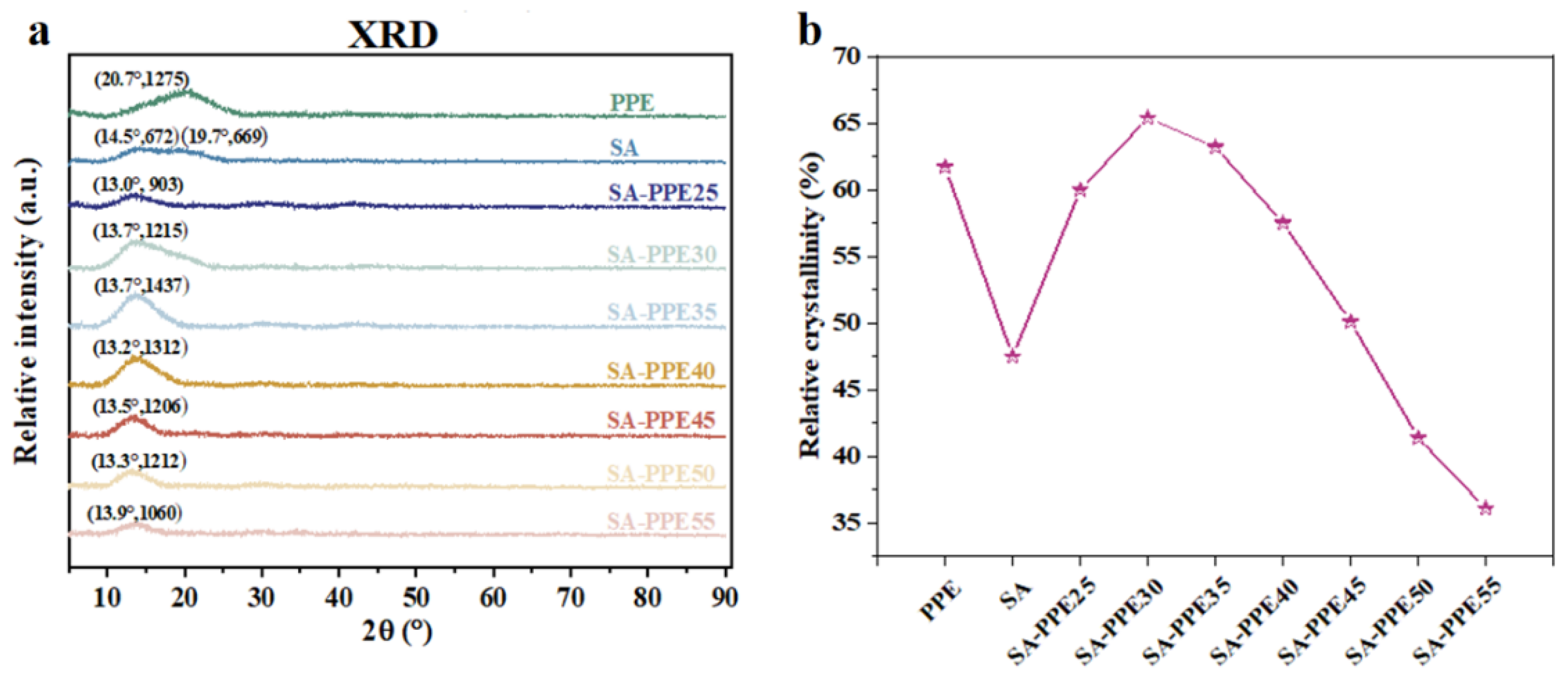
2.4.3. XRD
2.5. Physical Properties of Films
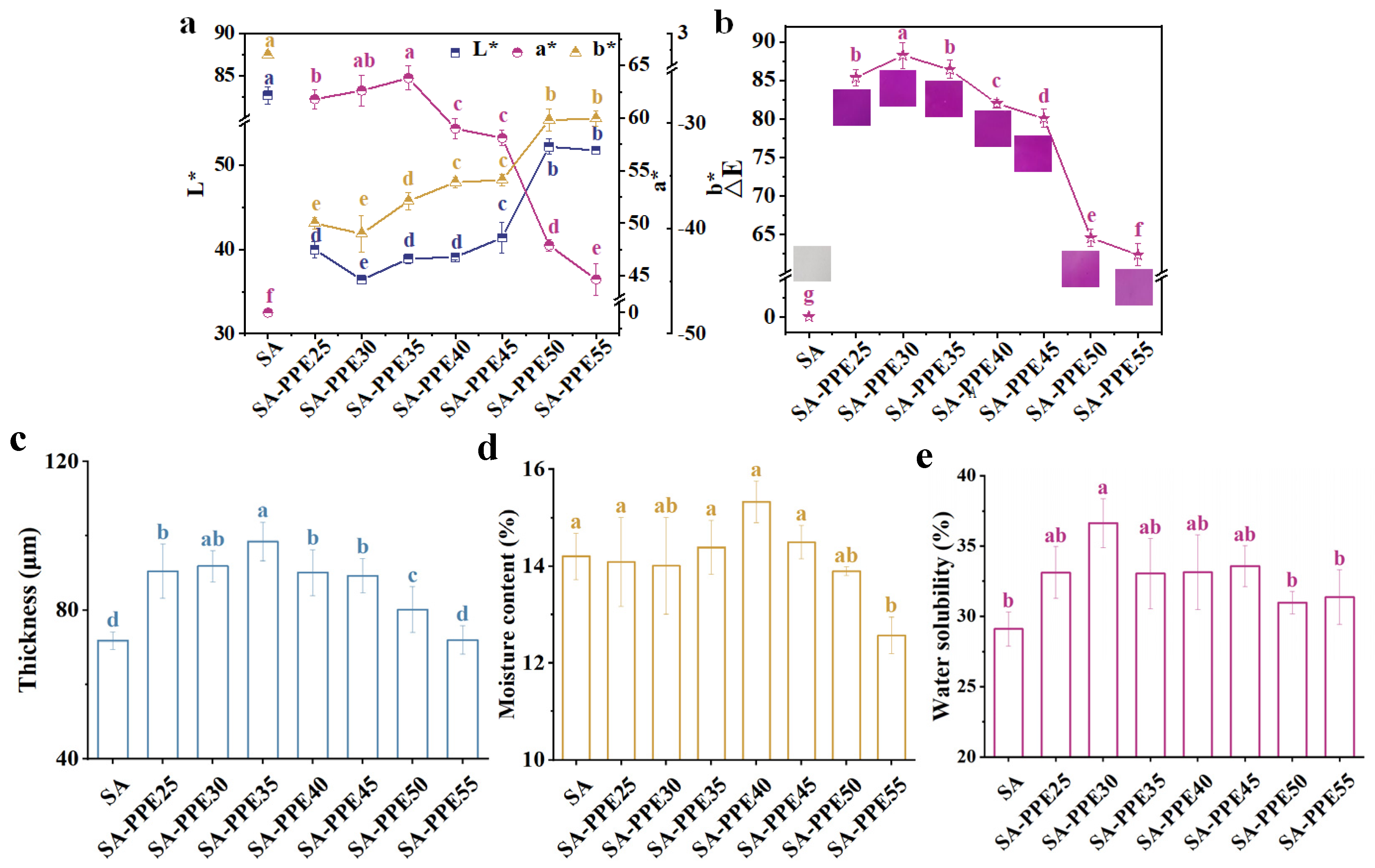
2.5.1. Color Properties
2.5.2. Thickness
2.5.3. Moisture Content and Water Solubility
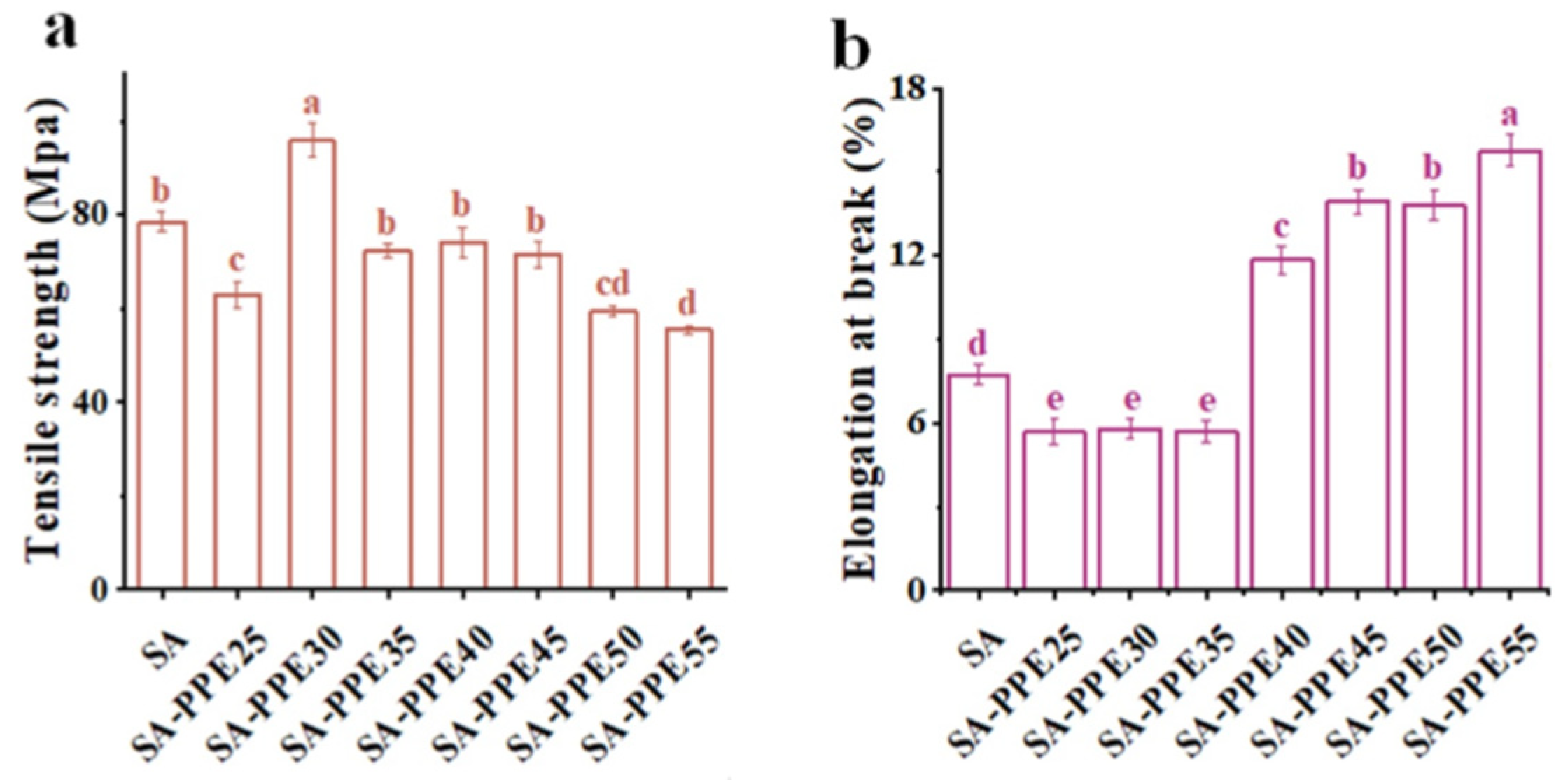
2.5.4. Mechanical Properties
2.5.5. Barrier Properties
Water-Vapor Permeability (WVP)
Light Transmission of Films
2.6. Sensitivity of Indicator Films
2.6.1. pH Sensitivity of Indicator Films
2.6.2. Color Response of Indicator Films to Volatile Ammonia
2.7. Statistical Analysis
3. Results and Discussion
3.1. Structural Characterization of Films
3.1.1. Microstructure
3.1.2. Intermolecular Interactions
3.1.3. Crystallinity
3.2. Physical Properties of Films
3.2.1. Color Properties
3.2.2. Thickness
3.2.3. Moisture Content and Water Solubility
3.2.4. Mechanical Properties
3.2.5. Barrier Properties
Water-Vapor Barrier Properties
Light-Barrier Properties
3.3. Sensitivity
3.3.1. pH Sensitivity
3.3.2. NH3 Sensitivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.J.; Sun, G.H.; Li, J.; Wang, L.J. A green strategy for maintaining intelligent response and improving antioxidant properties of κ-carrageenan-based film via cork bark extractive addition. Food Hydrocoll. 2021, 113, 106470. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhang, J.J.; Guan, Y.F.; Huang, X.W.; Arslan, M.; Shi, J.Y.; Li, Z.H.; Gong, Y.Y.; Holmes, M.; Zou, X.B. High-sensitivity bilayer nanofiber film based on polyvinyl alcohol/sodium alginate/polyvinylidene fluoride for pork spoilage visual monitoring and preservation. Food Chem. 2022, 394, 133439. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Hashim, S.B.; Tahir, H.E.; Liu, L.; Zhang, J.J.; Zhai, X.D.; Mahdi, A.A.; Awad, F.N.; Hassan, M.M.; Zou, X.B.; Shi, J.Y. Intelligent colorimetric pH sensoring packaging films based on sugarcane wax/agar integrated with butterfly pea flower extract for optical tracking of shrimp freshness. Food Chem. 2022, 373, 131514. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Roy, S.; Rhim, J.W. Gelatin/agar-based color-indicator film integrated with Clitoria ternatea flower anthocyanin and zinc oxide nanoparticles for monitoring freshness of shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Yong, H.M.; Wang, X.C.; Zhang, X.; Liu, Y.P.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Zhao, M.N.; Nuerjiang, M.; Bai, X.; Feng, J.; Kong, B.H.; Sun, F.D.; Li, Y.; Xia, X.F. Monitoring dynamic changes in chicken freshness at 4 °C and 25 °C using pH-sensitive intelligent films based on sodium alginate and purple sweet potato peel extracts. Int. J. Biol. Macromol. 2022, 216, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Júnior, L.M.; da Silva, R.G.; Anjos, C.A.R.; Vieira, R.P.; Alves, R.M.V. Effect of low concentrations of SiO2 nanoparticles on the physical and chemical properties of sodium alginate-based films. Carbohydr. Polym. 2021, 269, 118286. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhou, Y.; Chen, X.; Ma, F.; Li, P.J.; Chen, C.G. Effect of sodium alginate with three molecular weight forms on the water holding capacity of chicken breast myosin gel. Food Chem. 2018, 239, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Ran, R.; Chen, S.Y.; Su, Y.H.; Wang, L.Y.; He, S.J.; He, B.B.; Li, C.; Wang, C.X.; Liu, Y.T. Preparation of pH-colorimetric films based on soy protein isolate/ZnO nanoparticles and grape-skin red for monitoring pork freshness. Food Control 2022, 137, 108958. [Google Scholar] [CrossRef]
- Wang, X.C.; Yong, H.M.; Gao, L.; Li, L.L.; Jin, M.J.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Petropoulos, S.A.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Fernandes, A.; Leme, C.M.M.; Alexopoulos, A.; Buelga, C.S.; Ferreira, I.C.; et al. Phenolic composition and cell-based biological activities of ten coloured potato peels (Solanum tuberosum L.). Food Chem. 2021, 363, 130360. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.L.; Lonchamp, J.; Dias, M.I.; Liddle, C.; Petropoulos, S.A.; Glamočlija, J.; Alexopoulos, A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Anthocyanin-rich extracts from purple and red potatoes as natural colourants: Bioactive properties, application in a soft drink formulation and sensory analysis. Food Chem. 2021, 342, 128526. [Google Scholar] [CrossRef] [PubMed]
- Kayserilioǧlu, B.Ş.; Bakir, U.; Yilmaz, L.; Akkaş, N. Drying temperature and relative humidity effects on wheat gluten film properties. J. Agric. Food. Chem. 2003, 51, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.M.; Xiao, M.; Wang, S.S.; Peng, C.; Wu, X.H.; Jiang, F.T. Effect of drying temperature on microstructural, mechanical, and water barrier properties of konjac glucomannan/agar film produced at industrial scale. LWT Food Sci. Technol. 2023, 173, 114275. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.S.; Xua, F.F.; Ma, J.G.; Zhong, F. Tailoring physicochemical properties of chitosan films and their protective effects on meat by varying drying temperature. Carbohydr. Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Ullah, S.; Said Al, A.M.; Bekhit, A.E.A.; Karam, L.; Albratty, M.; Aldawsari, M.F.; Anwer, M.K. Combined effect of drying temperature and varied gelatin concentration on physicochemical and antioxidant properties of ginger oil incorporated chitosan based edible films. Foods 2023, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Kossyvaki, D.; Contardi, M.; Athanassiou, A.; Fragouli, D. Colorimetric indicators based on anthocyanin polymer composites: A review. Polymers 2022, 14, 4129. [Google Scholar] [CrossRef] [PubMed]
- Kossyvaki, D.; Barbetta, A.; Contardi, M.; Bustreo, M.; Dziza, K.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Highly porous curcumin-loaded polymer mats for rapid detection of volatile amines. ACS Appl. Polym. Mater. 2022, 4, 4464–4475. [Google Scholar] [CrossRef]
- Ren, S.; Giusti, M.M. Comparing the effect of whey protein preheating temperatures on the color expression and stability of anthocyanins from different sources. Food Hydrocoll. 2022, 124, 107273. [Google Scholar] [CrossRef]
- Castagnini, J.M.; Betoret, N.; Betoret, E.; Fito, P. Vacuum impregnation and air drying temperature effect on individual anthocyanins and antiradical capacity of blueberry juice included into an apple matrix. LWT Food Sci. Technol. 2015, 64, 1289–1296. [Google Scholar] [CrossRef]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2018, 90, 162–171. [Google Scholar] [CrossRef]
- Zhou, L.B.; Wang, K.Q.; Bian, L.Y.; Chang, T.T.; Zhang, C. Improving properties of curdlan/nanocellulose blended film via optimizing drying temperature. Food Hydrocoll. 2023, 137, 108421. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.W. pH-responsive color indicatorfilms based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.G.; Sousa, V.M.; Rodrigues, S.; de Brito, E.S.; Fernandes, F.A.N. Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives. Ultrason. Sonochem. 2020, 63, 104911. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of Anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Jiang, H.T.; Zhang, W.L.; Jiang, W.B. Effects of purple passion fruit peel extracts on characteristics of Pouteria campechiana seed starch films and the application in discernible detection of shrimp freshness. Food Hydrocoll. 2023, 138, 108477. [Google Scholar] [CrossRef]
- Li, Y.; Bai, X.; Zhao, M.N.; Wang, H.; Feng, J.; Xia, X.F.; Liu, Q. Sodium alginate edible coating to reduce oil absorption of French fries with maintaining overall acceptability: Based on a water replacement mechanism. Int. J. Biol. Macromol. 2023, 236, 124042. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.H.; Guo, Q.; Kong, B.H.; Liu, Q.; Xia, X.F. Inhibitory effect of chitosan coating on oil absorption in French fries based on starch structure and morphology stability. Int. J. Biol. Macromol. 2022, 219, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.Y.; Zhang, H.; Dong, M.N.; Li, L.H.; Hui, Z.S.; Wang, L. Dual-functional intelligent gelatin based packaging film for maintaining and monitoring the shrimp freshness. Food Hydrocoll. 2022, 124, 107258. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, G.Y.; Zheng, Y.; Bi, X.X.; Jia, N.; Liu, D.Y. Effects of ultrasound-assisted curing on the quality and microstructure of low-sodium beef jerky. Ultrason. Sonochem. 2023, 101, 106679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Ma, Y.L.; Liu, Y.; Zhang, J.L.; Hossen, M.A.; Sameen, D.E.; Dai, J.W.; Li, S.Q.; Qin, W. Fabrication and characterization of pH-responsive intelligent films based on carboxymethyl cellulose and gelatin/curcumin/chitosan hybrid microcapsules for pork quality monitoring. Food Hydrocoll. 2022, 124, 107224. [Google Scholar] [CrossRef]
- Cao, Y.J.; Song, Z.Y.; Ni, W.J.; Ma, Y.B.; Xin, K.Q.; Yu, Q.L.; Zhang, L. Composite nanoparticle-filled oxidized hydroxypropyl starch/carrageenan films: Robust, water-resistant, antibacterial, antioxidant and biodegradable properties. Food Hydrocoll. 2024, 155, 110224. [Google Scholar] [CrossRef]
- Yang, Y.F.; Xie, B.F.; Liu, Q.; Kong, B.H.; Wang, H. Fabrication and characterization of a novel polysaccharide based composite nanofiber films with tunable physical properties. Carbohydr. Polym. 2020, 236, 116054. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Dong, Y.; Men, H.T.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenol. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Guo, M.; Wang, H.L.; Wang, Q.; Chen, M.M.; Li, L.L.; Li, X.J.; Jiang, S.T. Intelligent double-layer fiber mats with high colorimetric response sensitivity for food freshness monitoring and preservation. Food Hydrocoll. 2020, 101, 105468. [Google Scholar] [CrossRef]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicatorfilm based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wan, Y.; Li, X.; Qian, H.; Xiao, M.; Ni, X.W.; Jiang, F.T.; Chen, S. Impact of heating and drying temperatures on the properties of konjac glucomannan/curdlan blend films. Int. J. Biol. Macromol. 2021, 167, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.K.; Yue, L.N.; Xu, L.; Qian, J.Y.; He, X.D. Variation of blending ratio and drying temperature optimize the physical properties and compatibility of HPMC/curdlan films. Carbohydr. Polym. 2022, 296, 119951. [Google Scholar] [CrossRef] [PubMed]
- Jomlapeeratikul, P.; Poomsa-Ad, N.; Wiset, L. Effect of drying temperatures and plasticizers on the properies of konjac flour film. J. Food Process Eng. 2016, 40, e12443. [Google Scholar] [CrossRef]
- Qin, Y.; Yun, D.W.; Xu, F.F.; Li, C.C.; Chen, D.; Liu, J. Impact of storage conditions on the structure and functionality of starch/polyvinyl alcohol films containing Lycium ruthenicum anthocyanins. Food Packag. Shelf Life 2021, 29, 100693. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Huang, Y.X.; Zhou, S.Y.; Zhao, G.H.; Ye, F.Y. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes: A review. Trends Food Sci. Technol. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Yong, H.M.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Li, C.; Wu, K.; Su, Y.H.; Riffat, S.B.; Ni, X.W.; Jiang, F.T. Effect of drying temperature on structural and thermomechanical properties of konjac glucomannan-zein blend films. Int. J. Biol. Macromol. 2019, 138, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Zhai, X.D.; Li, Z.H.; Zhang, J.J.; Shi, J.Y.; Zou, X.B.; Huang, X.W.; Zhang, D.; Sun, Y.; Yang, Z.K.; Holmes, M.; et al. Natural Biomaterial-Based Edible and pH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.X.; Yang, H.W.; Liu, T.T.; Zhang, Q.; Zou, Y.F.; Wang, S.Y. Fabrication, characterization and evaluation of Manihot esculenta starch based intelligent packaging films containing gum ghatti and black currant (Ribes nigrum) extract for freshness monitoring of beef meat. Food Chem. X 2024, 23, 101616. [Google Scholar] [CrossRef]
- Jara, A.H.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.V.; Silva, R.R.A.; de Oliveira, T.V.; Soares, R.R.A.; Junior, N.S.; Moraes, A.R.F.; Pires, A.C.D.S.; Soares, N.F.F. Development and characterization of intelligent cellulose acetate-based films using red cabbage extract for visual detection of volatile bases. LWT Food Sci. Technol. 2020, 132, 109780. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Kadouh, H.; Zhou, K. The antimicrobial, mechanical, physical and structural properties of chitosan-gallic acid films. LWT Food Sci. Technol. 2014, 57, 83–89. [Google Scholar] [CrossRef]
- Taherkhani, E.; Morad, M.; Tajik, H.; Molaei, R.; Ezati, P. Preparation of on-package halochromic freshness/spoilage nanocellulose label for the visual shelf life estimation of meat. Int. J. Biol. Macromol. 2020, 164, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.W.; Li, X.M.; Huang, Y.; Li, H.B.; Chen, L.Y.; Liu, X.H. Two colorimetric films based on chitin whiskers and sodium alginate/gelatin incorporated with anthocyanins for monitoring food freshness. Food Hydrocoll. 2022, 127, 107517. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Du, L.; Wang, L.J. Tara gum/polyvinyl alcohol-based colorimetric NH3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Monitoring the freshness of fish fillets by colorimetric gelatin composite film incorporated with curcumin extract. Biocatal. Agric. Biotechnol. 2023, 50, 102722. [Google Scholar] [CrossRef]



| SA-PPE25 | Color |  | |||||||||||
| L* | 43.0 ± 1.2 Ge | 53.9 ± 2.1 CDd | 56.3 ± 2.0 BCb | 49.6 ± 2.3 Fe | 57.7 ± 0.8 Bc | 52.9 ± 1.2 DEc | 40.1 ± 1.5 Hef | 51.3 ± 1.4 EFc | 36.6 ± 1.1 Ie | 34.8 ± 1.6 Ib | 51.2 ± 1.8 EFb | 65.9 ± 2.2 Ab | |
| a* | 66.2 ± 1.0 Aab | 46.5 ± 2.0 Ba | 45.8 ± 3.0 Bc | 46.9 ± 2.2 Ba | 29.6 ± 1.4 Dd | 42.0 ± 1.5 Ca | 30.3 ± 1.3 Dd | 3.00 ± 0.01 Ec | −7.10 ± 0.32 Fc | −11.4 ± 0.5 Ga | −18.1 ± 0.3 Hc | −7.70 ± 0.48 Fc | |
| b* | −5.10 ± 0.32 Bc | −12.2 ± 0.4 Cd | −12.7 ± 0.5 Cc | −15.6 ± 0.7 Dd | −15.0 ± 0.5 Dc | −23.6 ± 0.5 Fd | −32.5 ± 0.7 Hd | −38.4 ± 1.5 Id | −26.5 ± 1.4 Gc | −21.9 ± 1.4 Ed | −3.90 ± 0.32 Bd | 46.3 ± 1.6 Ad | |
| ∆E | 85.3 ± 1.0 Aa | 64.3 ± 2.5 DEa | 62.4 ± 3.5 Ec | 68.2 ± 3.0 Cb | 51.0 ± 1.5 Hc | 65.2 ± 1.8 CDEb | 71.8 ± 1.5 Bb | 59.3 ± 2.0 Fc | 65.2 ± 1.1 CDEb | 65.6 ± 1.6 CDc | 47.6 ± 1.5 Id | 54.3 ± 1.8 Gc | |
| SA-PPE30 | Color |  | |||||||||||
| L* | 46.9 ± 1.2 Dd | 57.0 ± 1.2 Bc | 51.0 ± 1.1 Cc | 57.3 ± 1.7 Bcd | 53.6 ± 2.9 Cd | 42.7 ± 2.8 Ee | 43.0 ± 2.0 Ede | 31.2 ± 1.7 Gd | 35.3 ± 1.6 Fe | 31.0 ± 0.8 Gc | 31.0 ± 1.2 Gd | 67.7 ± 1.8 Ab | |
| a* | 67.5 ± 0.5 Aa | 42.6 ± 0.8 Db | 63.0 ± 1.05 Ba | 52.2 ± 1.4 Cb | 40.8 ± 1.1 Eb | 43.3 ± 2.0 Da | 33.9 ± 0.7 Fc | 12.6 ± 0.7 Ga | −2.30 ± 0.48 Ha | −11.8 ± 0.4 Iab | −25.6 ± 1.1 Je | −2.80 ± 0.42 Ha | |
| b* | −12.8 ± 0.4 Ce | −16.1 ± 0.6 Df | −14.6 ± 0.5 Dd | −19.6 ± 0.7 Ee | −20.4 ± 0.8 Ee | −34.2 ± 1.2 Gf | −37.7 ± 1.3 He | −48.5 ± 1.4 If | −38.7 ± 0.8 Hf | −24.9 ± 1.9 Fe | −10.8 ± 0.9 Bf | 35.9 ± 1.5 Ae | |
| ∆E | 84.9 ± 0.9 Aa | 60.5 ± 1.1 Gb | 79.3 ± 1.2 Ca | 68.5 ± 2.0 Fb | 62.8 ± 1.7 Gb | 77.3 ± 3.4 Ca | 73.9 ± 2.0 Dab | 82.2 ± 2.2 Ba | 72.0 ± 1.2 DEa | 70.2 ± 0.8 EFb | 69.9 ± 1.3 EFa | 44.4 ± 1.2 Hd | |
| SA-PPE35 | Color |  | |||||||||||
| L* | 48.5 ± 1.5 Ccd | 56.8 ± 2.0 Bc | 42.3 ± 2.5 DEd | 44.9 ± 1.9 Df | 43.4 ± 2.9 De | 38.4 ± 1.5 Ff | 39.3 ± 3.6 EFf | 31.8 ± 1.4 Gd | 43.4 ± 1.08 Dd | 26.7 ± 1.34 Hd | 33.5 ± 1.7 Gd | 67.1 ± 2.0 Ab | |
| a* | 65.3 ± 0.7 Ab | 46.3 ± 2.7 Da | 51.3 ± 2.0 Cb | 56.1 ± 1.3 Ba | 47.8 ± 1.8 Da | 43.5 ± 0.5 Ea | 43.2 ± 1.9 Ea | 7.80 ± 0.42 Fb | −4.10 ± 0.32 Gb | −12.6 ± 0.5 Ib | −22.9 ± 0.7 Jd | −6.30 ± 0.48 Hb | |
| b* | −9.20 ± 0.42 Bd | −14.2 ± 0.4 Ce | −11.0 ± 0.7 Bb | −15.2 ± 0.4 Cd | −23.4 ± 0.5 Df | −27.3 ± 0.5 Ee | −26.8 ± 0.6 Ec | −44.3 ± 0.5 Ge | −33.2 ± 1.9 Fe | −25.8 ± 0.8 Ee | −9.90 ± 0.57 Be | 55.4 ± 4.4 Ac | |
| ∆E | 81.7 ± 1.4 Ab | 62.8 ± 3.2 Eab | 75.4 ± 3.0 BCb | 77.8 ± 2.0 BCa | 75.3 ± 3.0 BCa | 77.6 ± 1.2 BCa | 76.6 ± 3.0 BCa | 78.6 ± 1.2 ABb | 62.3 ± 1.7 Ec | 74.6 ± 1.2 Ca | 66.5 ± 1.6 Db | 61.4 ± 4.1 Eb | |
| SA-PPE40 | Color |  | |||||||||||
| L* | 48.9 ± 1.0 Ebc | 59.7 ± 1.7 Cb | 57.0 ± 1.7 Db | 55.9 ± 1.0 Dd | 63.8 ± 1.2 Ba | 44.1 ± 2.1 Ge | 46.0 ± 1.6 FGd | 54.6 ± 1.3 Db | 47.8 ± 2.8 EFc | 36.3 ± 1.2 Hb | 46.2 ± 1.3 FGc | 67.4 ± 2.2 Ab | |
| a* | 62.6 ± 0.8 Ac | 38.1 ± 1.3 Dc | 47.1 ± 2.3 Bc | 41.7 ± 1.3 Cd | 34.7 ± 2.3 Ec | 37.1 ± 1.4 Db | 36.1 ± 2.1 DEb | 3.00 ± 0.00 Fc | −7.60 ± 0.52 Gc | −15.5 ± 0.5 Hd | −16.2 ± 0.4 Hb | −7.80 ± 0.42 Gc | |
| b* | −2.70 ± 0.67 Cb | −12.5 ± 0.9 Dd | −12.2 ± 1.0 Dc | −15.6 ± 0.5 Ed | −16.5 ± 0.5 Ed | −20.7 ± 0.5 Fc | −27.5 ± 0.5 Gc | −38.3 ± 3.1 Id | −30.9 ± 1.6 Hd | −25.7 ± 0.5 Ge | 1.10 ± 0.32 Bc | 52.2 ± 2.8 Ac | |
| ∆E | 78.7 ± 0.9 Ac | 54.5 ± 1.9 FGc | 62.8 ± 2.7 Cc | 60.4 ± 1.5 CDc | 50.7 ± 1.8 Hc | 67.4 ± 2.5 Bb | 68.0 ± 2.4 Bc | 56.8 ± 2.7 EFc | 57.7 ± 2.9 DEFd | 66.4 ± 1.1 Bc | 51.4 ± 1.3 GHc | 58.6 ± 3.2 DEb | |
| SA-PPE45 | Color |  | |||||||||||
| L* | 48.7 ± 1.5 EFbcd | 63.8 ± 1.3 Ba | 61.1 ± 2.4 BCa | 59.4 ± 1.4 Cc | 60.5 ± 0.9 Cb | 48.3 ± 4.6 Fd | 53.4 ± 2.2 Dc | 51.8 ± 1.6 DEc | 48.4 ± 1.2 Fc | 40.1 ± 2.2 Ga | 50.0 ± 2.5 EFb | 67.9 ± 1.5 Ab | |
| a* | 60.3 ± 1.1 Ad | 37.1 ± 0.6 Cc | 38.3 ± 2.5 Cd | 41.6 ± 1.4 Bd | 31.5 ± 1.2 Dd | 29.6 ± 1.0 Ec | 27.2 ± 2.1 Fe | −4.40 ± 0.52 Gd | −11.3 ± 0.5 Id | −16.9 ± 0.7 Je | −15.4 ± 0.5 Jb | −7.50 ± 0.85 Hc | |
| b* | 3.10 ± 0.32 Ba | −9.70 ± 0.48 Ec | −6.60 ±0.52 Da | −9.80 ± 0.42 Ec | −10.4 ± 0.5 Eb | −21.4 ± 0.5 GHc | −18.5 ± 0.5 Fb | −22.8 ± 1.0 Hc | −17.5 ± 0.5 Fab | −20.2 ± 0.8 Gc | 0.90 ± 0.32 Cc | 64.8 ± 3.1 Ab | |
| ∆E | 76.9 ± 1.4 Ac | 50.5 ± 1.1 FGHd | 52.6 ± 3.1 EFd | 56.7 ± 2.0 Dd | 48.9 ± 1.4 GHc | 60.4 ± 3.8 Cc | 54.1 ± 2.7 DEd | 49.8 ± 1.7 FGHd | 51.5 ± 1.0 EFGe | 61.3 ± 2.0 Cd | 47.5 ± 2.4 Hd | 69.6 ± 2.8 Ba | |
| SA-PPE50 | Color |  | |||||||||||
| L* | 50.6 ± 1.4 Gb | 61.0 ± 3.0 CDb | 60.7 ± 0.8 CDa | 62.1 ± 2.1 Cb | 65.6 ± 1.4 Ba | 60.4 ± 2.8 CDb | 59.1 ± 2.2 DEb | 56.3 ± 2.5 EFb | 54.6 ± 1.1 Fb | 35.9 ± 2.0 Hb | 58.8 ± 1.6 DEa | 73.1 ± 0.9 Aa | |
| a* | 56.8 ± 1.0 Ae | 36.3 ± 1.6 Cc | 40.4 ± 1.6 Bd | 28.8 ± 0.6 De | 23.7 ± 1.0 Fe | 26.5 ± 2.6 Ed | 21.7 ± 1.4 Gf | −7.10 ± 0.32 Hf | −12.7 ± 0.5 Ie | −14.0 ± 0.5 Ic | −16.3 ± 0.7 Jb | −12.6 ± 0.5 Ie | |
| b* | 3.10 ± 0.32 Ba | −4.00 ± 0.00 Ca | −7.20 ± 0.42 Ea | −8.60 ± 0.52 Fb | −8.70 ± 0.48 Fa | −18.4 ± 0.5 Gb | −18.3 ± 0.7 Gb | −18.7 ± 0.7 Gb | −18.7 ± 0.8 Gb | −5.90 ± 0.32 Da | 3.00 ± 0.00 Bb | 67.0 ± 2.0 Aab | |
| ∆E | 73.0 ± 1.2 Ad | 50.9 ± 2.4 Dd | 54.5 ± 1.6 Cd | 45.6 ± 1.8 EFe | 39.8 ± 1.3 Gd | 48.5 ± 3.5 DEd | 46.9 ± 2.6 EFe | 44.2 ± 2.4 Fe | 46.8 ± 0.9 EFf | 61.2 ± 1.9 Bd | 39.6 ± 1.6 Ge | 70.4 ± 2.0 Aa | |
| SA-PPE55 | Color |  | |||||||||||
| L* | 54.6 ± 2.0 Fa | 63.6 ± 1.08 Ca | 55.0 ± 1.4 Fb | 66.7 ± 1.5 Ba | 64.0 ± 1.5 Ca | 65.8 ± 1.0 BCa | 67.5 ± 1.7 Ba | 60.7 ± 0.8 Da | 57.8 ± 1.3 Ea | 40.0 ± 1.9 Ga | 60.2 ± 2.4 Da | 73.7 ± 0.5 Aa | |
| a* | 54.7 ± 2.0 Af | 30.7 ± 2.3 Cd | 44.6 ± 1.9 Bc | 24.1 ± 1.7 Df | 20.6 ± 1.6 Ef | 16.7 ± 0.5 Fe | 15.0 ± 0.7 Fg | −5.90 ± 0.32 Ge | −12.5 ± 0.5 Ie | −17.8 ± 1.0 Jf | −11.2 ± 0.6 HIa | −10.1 ± 0.3 Hd | |
| b* | 2.90 ± 0.32 Ca | −8.30 ± 0.48 Eb | −10.7 ± 0.5 Gb | −4.10 ± 0.32 Da | −8.00 ± 0.00 Ea | −9.20 ± 0.42 Fa | −7.80 ± 0.42 Ea | −15.8 ± 0.4 Ia | −16.4 ± 0.5 Ia | −12.6 ± 0.5 Hb | 8.10 ± 0.32 Ba | 68.7 ± 1.3 Aa | |
| ∆E | 68.9 ± 2.4 Ae | 45.7 ± 2.0 Ce | 61.9 ± 2.3 Bc | 38.3 ± 2.2 EFf | 39.0 ± 2.0 Ed | 35.9 ± 1.0 FGe | 33.2 ± 1.7 Gf | 38.8 ± 0.9 Ef | 43.0 ± 1.2 Dg | 59.4 ± 1.9 Cd | 37.2 ± 2.3 EFf | 71.5 ± 1.2 Aa | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhao, M.; Xia, X.; Zhu, Y. Improving Structural, Physical, and Sensitive Properties of Sodium Alginate–Purple Sweet Potato Peel Extracts Indicator Films by Varying Drying Temperature. Foods 2024, 13, 2477. https://doi.org/10.3390/foods13162477
Li W, Zhao M, Xia X, Zhu Y. Improving Structural, Physical, and Sensitive Properties of Sodium Alginate–Purple Sweet Potato Peel Extracts Indicator Films by Varying Drying Temperature. Foods. 2024; 13(16):2477. https://doi.org/10.3390/foods13162477
Chicago/Turabian StyleLi, Wenxin, Mengna Zhao, Xiufang Xia, and Yingchun Zhu. 2024. "Improving Structural, Physical, and Sensitive Properties of Sodium Alginate–Purple Sweet Potato Peel Extracts Indicator Films by Varying Drying Temperature" Foods 13, no. 16: 2477. https://doi.org/10.3390/foods13162477







