In Vitro Antioxidant Activity of Liposomal Formulations of Sea Buckthorn and Grape Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extract Preparation
2.3. Analysis of Phenolic Compounds in Grape Pomace
2.4. Analysis of Carotenoids in Sea Buckthorn Samples
2.5. Free Radical Scavenging Method (DPPH)
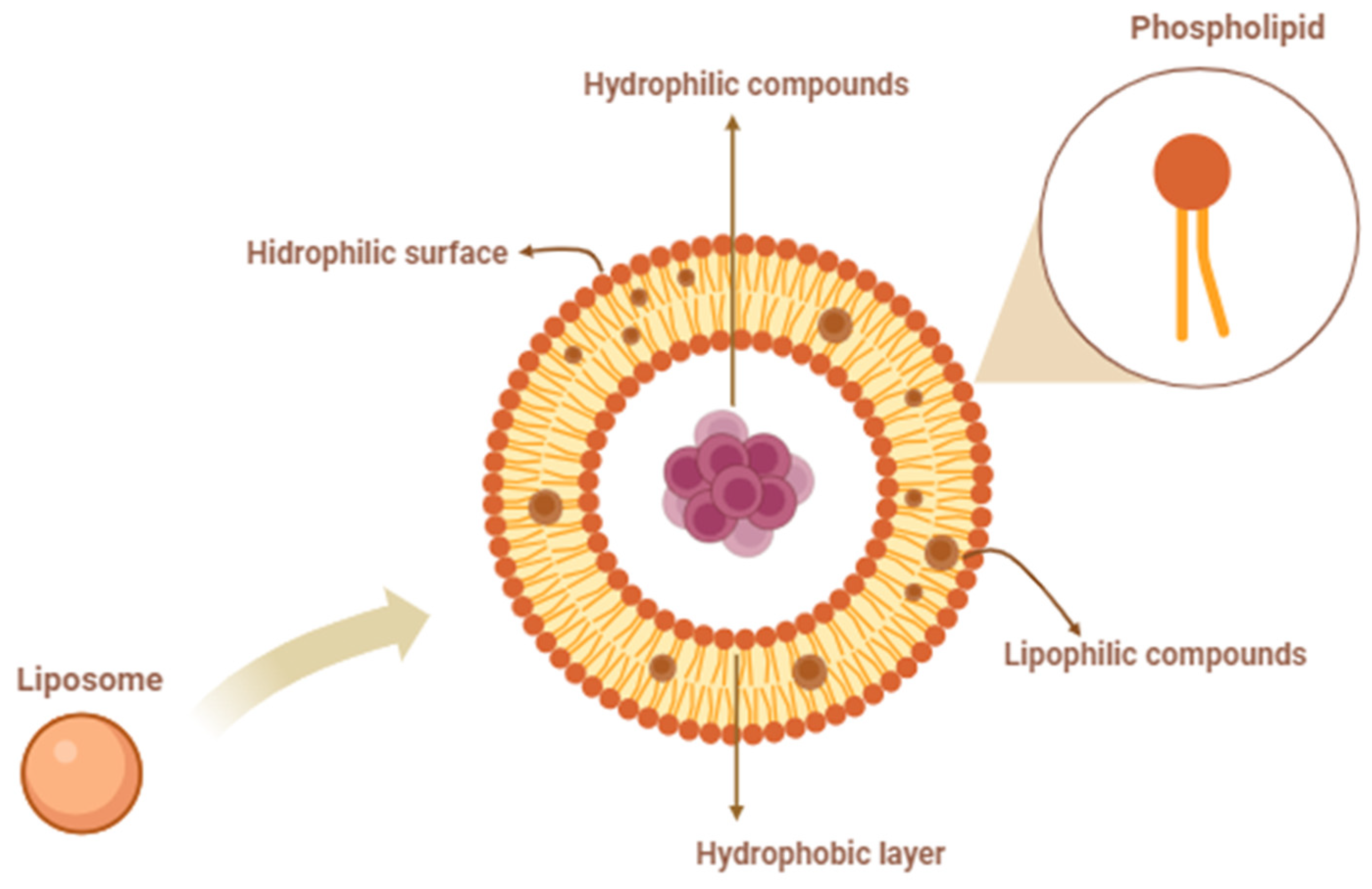
2.6. Liposome Preparation
2.7. Encapsulation Efficiency
2.8. Retention Rate
2.9. In Vitro Antioxidant Activity
2.10. Statistical Analysis
3. Results
3.1. Characterization of Extracts
3.2. Preparation and Characterization of Liposomes
3.3. In Vitro Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Mancebo-Campos, V.; Desamparados Salvador, M.; Fregapane, G. Oxidation Kinetics in Olive Oil Triacylglycerols under Accelerated Shelf-Life Testing (25–75 °C). Eur. J. Lipid Sci. Technol. 2004, 106, 369–375. [Google Scholar] [CrossRef]
- Kaur, D.; Wani, A.A.; Singh, D.P.; Sogi, D.S. Shelf Life Enhancement of Butter, Ice-Cream, and Mayonnaise by Addition of Lycopene. Int. J. Food Prop. 2011, 14, 1217–1231. [Google Scholar] [CrossRef]
- Berger, M. Can Oxidative Damage Be Treated Nutritionally? Clin. Nutr. 2005, 24, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, X.; Liu, X.; Liu, L.; Fang, Y.; Rao, R.; Ren, Y.; Yang, X.; Liu, W. Enhanced Anticancer Effect of Doxorubicin by TPGS-Coated Liposomes with Bcl-2 siRNA-Corona for Dual Suppression of Drug Resistance. Asian J. Pharm. Sci. 2020, 15, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Park, J. Food Liposomes: Structures, Components, Preparations, and Applications. Food Chem. 2024, 432, 137228. [Google Scholar] [CrossRef] [PubMed]
- Eidhin, D.N.; Burke, J.; O’Beirne, D. Oxidative Stability of Ω3-Rich Camelina Oil and Camelina Oil-Based Spread Compared with Plant and Fish Oils and Sunflower Spread. J. Food Sci. 2003, 68, 345–353. [Google Scholar] [CrossRef]
- Song, F.; Chen, J.; Zheng, A.; Tian, S. Effect of Sterols on Liposomes: Membrane Characteristics and Physicochemical Changes during Storage. LWT 2022, 164, 113558. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of Rosemary Extract by Liposomes Formulated by Mozafari Method: Physicochemical Characterization and Optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Jara-Quijada, E.; Pérez-Won, M.; Tabilo-Munizaga, G.; Lemus-Mondaca, R.; González-Cavieres, L.; Palma-Acevedo, A.; Herrera-Lavados, C. Liposomes Loaded with Green Tea Polyphenols—Optimization, Characterization, and Release Kinetics under Conventional Heating and Pulsed Electric Fields. Food Bioprocess Technol. 2024, 17, 396–408. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.-X.; Luo, Z.-G.; Tamer, T.M. Co-Encapsulation of Vitamin C and β-Carotene in Liposomes: Storage Stability, Antioxidant Activity, and In Vitro Gastrointestinal Digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Zhu, L.; Gan, Q.; Le, X. Temperature-Dependent Structure Stability and In Vitro Release of Chitosan-Coated Curcumin Liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Ruedt, C.; Weiss, J. In Vitro Release of Grape-Seed Polyphenols Encapsulated from Uncoated and Chitosan-Coated Liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef] [PubMed]
- da Silva Haas, I.C.; Toaldo, I.M.; Gomes, T.M.; Luna, A.S.; de Gois, J.S.; Bordignon-Luiz, M.T. Polyphenolic Profile, Macro- and Microelements in Bioaccessible Fractions of Grape Juice Sediment Using in Vitro Gastrointestinal Simulation. Food Biosci. 2019, 27, 66–74. [Google Scholar] [CrossRef]
- Xu, X.; Tian, M.; Deng, L.; Jiang, H.; Han, J.; Zhen, C.; Huang, L.; Liu, W. Structural Degradation and Uptake of Resveratrol-Encapsulated Liposomes Using an In Vitro Digestion Combined with Caco-2 Cell Absorption Model. Food Chem. 2023, 403, 133943. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Srinivasan, V.; Chavan, S.; Jain, U.; Tarwadi, K. Liposomes for Nanodelivery Systems in Food Products. In Nanoscience for Sustainable Agriculture; Pudake, R.N., Chauhan, N., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 627–638. ISBN 978-3-319-97851-2. [Google Scholar]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Popovici, V.; Constantinescu (Pop), C.G.; Deseatnicova, O.; Siminiuc, R.; Subotin, I.; Druta, R.; Pintea, A.; Socaciu, C.; Sturza, R. Stabilization of Sunflower Oil with Biologically Active Compounds from Berries. Molecules 2023, 28, 3596. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Niculaua, M. Rose Hips, a Valuable Source of Antioxidants to Improve Gingerbread Characteristics. Molecules 2020, 25, 5659. [Google Scholar] [CrossRef]
- Ghendov-Moşanu, A.; Sturza, R.; Opriş, O.; Lung, I.; Popescu, L.; Popovici, V.; Soran, M.-L.; Patraş, A. Effect of Lipophilic Sea Buckthorn Extract on Cream Cheese Properties. J. Food Sci. Technol. 2020, 57, 628–637. [Google Scholar] [CrossRef]
- Morar, I.M.; Stefan, R.; Dan, C.; Sestras, R.E.; Truta, P.; Medeleanu, M.; Ranga, F.; Sestras, P.; Truta, A.M.; Sestras, A.F. FT-IR and HPLC Analysis of Silver Fir (Abies alba Mill.) Bark Compounds from Different Geographical Provenances. Heliyon 2024, 10, e26820. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focşan, M.; Rugină, D.O.; Pintea, A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients 2019, 12, 76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Popovici, V. Evaluation of the Oxidative Stability of Sea Buckthorn (Hippophae Rhamnoides L.) Lipophilic Extract. J. Eng. Sci. 2018, 25, 111–115. [Google Scholar] [CrossRef]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Yazid, A.M. Comparative Study of the Oxidative and Physical Stability of Liposomal and Nanoliposomal Polyunsaturated Fatty Acids Prepared with Conventional and Mozafari Methods. Food Chem. 2012, 135, 2761–2770. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as Delivery Systems for Carotenoids: Comparative Studies of Loading Ability, Storage Stability and In Vitro Release. Food Funct. 2014, 5, 1232. [Google Scholar] [CrossRef]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The Effect of in Vitro Digestion on the Antioxidant Activity of Fruit Extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT-Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Carvajal, A.K.; Rustad, T.; Mozuraityte, R.; Storrø, I. Kinetic Studies of Lipid Oxidation Induced by Hemoglobin Measured by Consumption of Dissolved Oxygen in a Liposome Model System. J. Agric. Food Chem. 2009, 57, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. BioNanoScience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Islam Shishir, M.R.; Karim, N.; Gowd, V.; Zheng, X.; Chen, W. Liposomal Delivery of Natural Product: A Promising Approach in Health Research. Trends Food Sci. Technol. 2019, 85, 177–200. [Google Scholar] [CrossRef]
- Toh, M.-R.; Chiu, G.N.C. Liposomes as Sterile Preparations and Limitations of Sterilisation Techniques in Liposomal Manufacturing. Asian J. Pharm. Sci. 2013, 8, 88–95. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Pintea, A.; Boldianu, A.; Mereuta, I.; Caraus, V.; Cicalchin, S.; Covaci, E.; Ghendov-Mosanu; Sturza, R. Liposomal Encapsulated Extract with Biologically Active Lipophilic or Hydrophilic Substances, Which Possess Antioxidant and Immunostimulatory Activity, and Method for Obtaining It, AGEPI, Patent Application. Entry No. 2547, 17 May 2024. [Google Scholar]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional Spray-Drying and Future Trends for the Microencapsulation of Fish Oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Šeregelj, V.; Tumbas Šaponjac, V.; Lević, S.; Kalušević, A.; Ćetković, G.; Čanadanović-Brunet, J.; Nedović, V.; Stajčić, S.; Vulić, J.; Vidaković, A. Application of Encapsulated Natural Bioactive Compounds from Red Pepper Waste in Yogurt. J. Microencapsul. 2019, 36, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Oluwatuyi, M.; Kaatz, G.; Gibbons, S. Antibacterial and Resistance Modifying Activity of of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef]
- Pan, L.; Meng, H.; Li, J.; Liu, Z.; Zhang, D.; Liu, Z.; Zhao, Q.; Xu, F. Enhancement of Astaxanthin Bioaccessibility by Encapsulation in Liposomes: An In Vitro Study. Molecules 2024, 29, 1687. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research Progress on Liposomes: Application in Food, Digestion Behavior and Absorption Mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
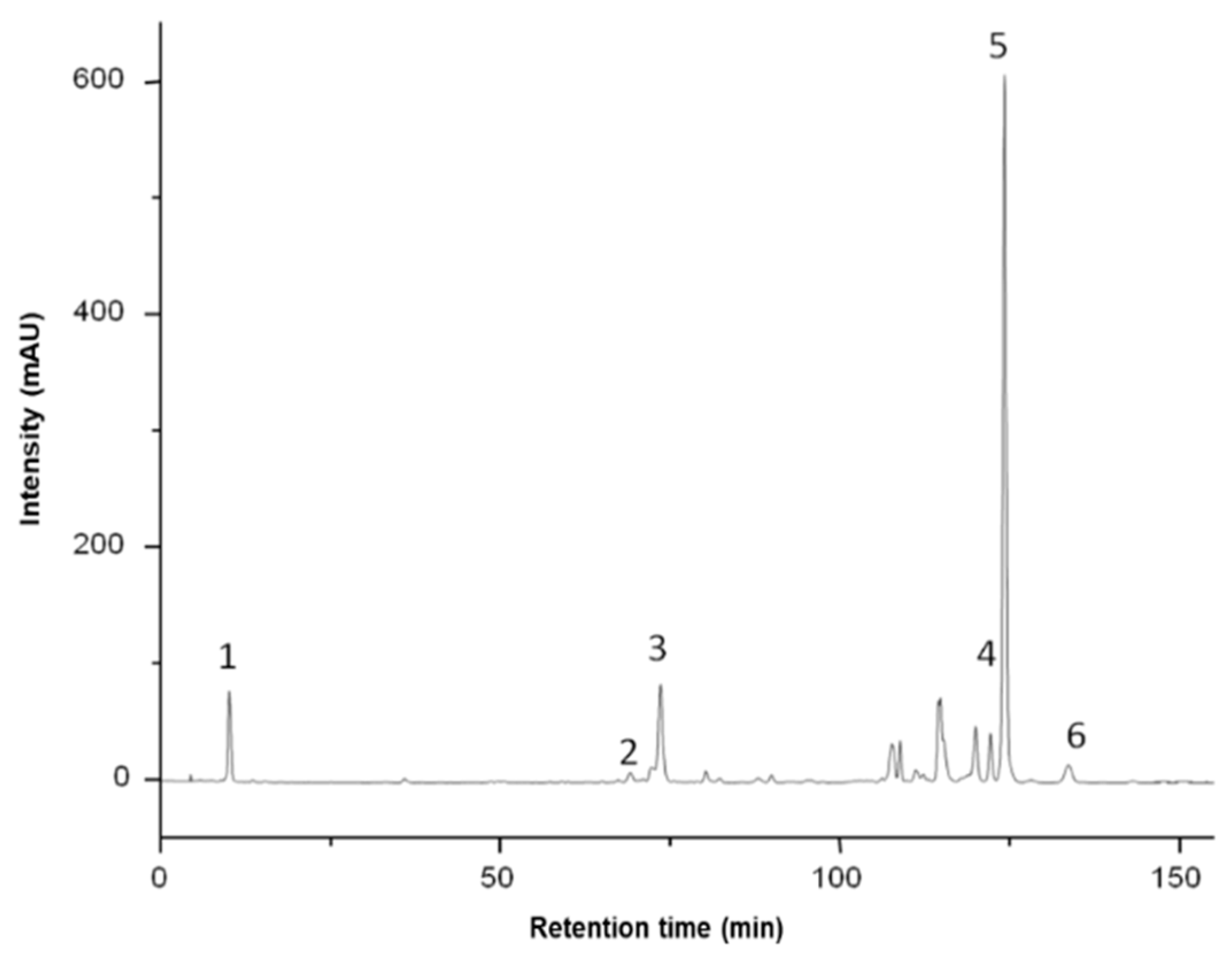

| Carotenoid Compound | Retention Time, min | Max Absorption, nm | Quantity, mg/100 g FW |
|---|---|---|---|
| Zeaxanthin | 10.01 | 426, 450, 476 | 0.72 ± 0.02 |
| Cis-β-carotene | 69.14 | 424, 446, 472 | 0.20 ± 0.01 |
| All-trans-β-carotene | 73.57 | 421, 452, 478 | 0.79 ± 0.03 |
| Lycopene | 95.55 | 448, 471, 503 | 0.14 ± 0.01 |
| β-cryptoxanthin/zeaxanthin/mutatoxanthin esters (other than the ones below) | 107.51 | 2.80 ± 0.04 | |
| 108.72 | |||
| 114.64 | |||
| 115.11 | |||
| 119.83 | |||
| Lutein dipalmitate | 121.99 | 421, 446, 474 | 0.54 ± 0.05 |
| Zeaxanthin dipalmitate | 124.06 | 427, 450, 476 | 4.53 ± 0.12 |
| Zeaxanthin palmitate-stearate | 133.44 | 427, 450, 477 | 0.47 ± 0.06 |
| Phenolic Compounds | Retention Time, min | Max Absorption, nm | Subclass | Quantity, mg/100 g DW |
|---|---|---|---|---|
| 3-Hydroxybenzoic acid | 2.99 | 270 | Hydroxybenzoic acid | 7.23 ± 0.15 |
| 2-Hydroxybenzoic acid | 3.32 | 270 | Hydroxybenzoic acid | 7.08 ± 0.21 |
| Gallic acid-gallate | 5.94 | 270 | Hydroxybenzoic acid | 9.66 ± 0.08 |
| Kaempferol-rutinoside | 10.54 | 260, 340 | Flavonol | 1.05 ± 0.01 |
| Prodelphinidin dimer B9 | 10.81 | 280 | Flavanol | 8.72 ± 0.15 |
| Procyanidin dimmer B3 | 11.34 | 280 | Flavanol | 16.66 ± 0.18 |
| Kaempferol-acetyl-glucoside | 11.42 | 270, 340 | Flavonol | 2.10 ± 0.04 |
| Peonidin-glucoside | 11.78 | 280, 518 | Anthocyanin | 0.47 ± 0.01 |
| Procyanidin dimmer B1 | 11.96 | 280 | Flavanol | 11.80 ± 0.21 |
| Malvidin-glucoside | 12.01 | 280, 528 | Anthocyanin | 0.84 ± 0.02 |
| Catechin | 12.43 | 280 | Flavanol | 20.47 ± 0.31 |
| Caffeic acid-glucoside | 12.91 | 332 | Hydroxycinnamic acid | 5.69 ± 0.05 |
| 3,4-Dicaffeoylquinic acid | 13.43 | 332 | Hydroxycinnamic acid | 4.55 ± 0.08 |
| Epicatechin | 13.72 | 280 | Flavanol | 47.67 ± 0.33 |
| Malvidin-glucoside-pyruvic acid | 13.95 | 280, 530 | Anthocyanin | 1.06 ± 0.08 |
| Dephinidin-p-coumaroyl-glucoside | 14.17 | 280, 529 | Anthocyanin | 0.94 ± 0.02 |
| Malvidin-acetyl-glucoside | 14.52 | 280, 530 | Anthocyanin | 0.98 ± 0.01 |
| Procyanidin dimmer B2 | 14.61 | 280 | Flavanol | 18.39 ± 0.19 |
| Petunidin-p-coumaroyl-glucoside | 14.77 | 280, 528 | Anthocyanin | 1.65 ± 0.03 |
| Peonidin-p-coumaroyl-glucoside | 15.17 | 280, 523 | Anthocyanin | 1.06 ± 0.01 |
| Malvidin-caffeoyl-glucoside | 15.62 | 280, 530 | Anthocyanin | 1.41 ± 0.02 |
| Quercetin-glucoside | 15.98 | 360, 250 | Flavonol | 3.24 ± 0.01 |
| Malvidin-p-coumaroyl-glucoside | 16.35 | 280, 530 | Anthocyanin | 0.90 ± 0.02 |
| Quercetin-glucuronide | 16.67 | 360, 250 | Flavonol | 3.63 ± 0.08 |
| Isorhamnetin-glucoside | 17.07 | 360, 260 | Flavonol | 2.88 ± 0.02 |
| Quercetin-acetyl-glucoside | 18.48 | 360, 250 | Flavonol | 1.07 ± 0.03 |
| Quercetin | 21.36 | 360, 250 | Flavonol | 0.86 ± 0.01 |
| Sample | Encapsulation Efficiency, % | Retention Rate after 4 Weeks of Storage, % | Encapsulated Bioactive Compound Amount, μg |
|---|---|---|---|
| CDW | 90.90 ± 0.65 d | 86.74 ± 0.18 d | 83.74 ± 0.26 d |
| CEt | 87.83 ± 0.54 b | 80.18 ± 0.67 b | 81.18 ± 0.24 c |
| PDW | 89.59 ± 0.51 c | 84.79 ± 0.32 c | 80.19 ± 0.38 b |
| PEt | 84.13 ± 0.29 a | 79.18 ± 0.21 a | 78.98 ± 0.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, V.; Boldianu, A.-B.; Pintea, A.; Caraus, V.; Ghendov-Mosanu, A.; Subotin, I.; Druta, R.; Sturza, R. In Vitro Antioxidant Activity of Liposomal Formulations of Sea Buckthorn and Grape Pomace. Foods 2024, 13, 2478. https://doi.org/10.3390/foods13162478
Popovici V, Boldianu A-B, Pintea A, Caraus V, Ghendov-Mosanu A, Subotin I, Druta R, Sturza R. In Vitro Antioxidant Activity of Liposomal Formulations of Sea Buckthorn and Grape Pomace. Foods. 2024; 13(16):2478. https://doi.org/10.3390/foods13162478
Chicago/Turabian StylePopovici, Violina, Adrian-Bogdan Boldianu, Adela Pintea, Vladimir Caraus, Aliona Ghendov-Mosanu, Iurie Subotin, Raisa Druta, and Rodica Sturza. 2024. "In Vitro Antioxidant Activity of Liposomal Formulations of Sea Buckthorn and Grape Pomace" Foods 13, no. 16: 2478. https://doi.org/10.3390/foods13162478
APA StylePopovici, V., Boldianu, A.-B., Pintea, A., Caraus, V., Ghendov-Mosanu, A., Subotin, I., Druta, R., & Sturza, R. (2024). In Vitro Antioxidant Activity of Liposomal Formulations of Sea Buckthorn and Grape Pomace. Foods, 13(16), 2478. https://doi.org/10.3390/foods13162478








