Innovative Hurdle Strategies for Listeria Control on Food-Contact Surfaces: A Peroxyacetic Acid–Steam Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Activation and Cocktail Preparation of L. innocua Strains
2.2. Preparation of Food-Contact Coupons
2.3. L. innocua Biofilm Formation
2.4. Preparation of Sanitizer Solutions
2.5. Hurdle Treatment of Saturated Steam and PAA
2.6. Biofilm Detachment and Enumeration
2.7. Statistical Analysis
3. Results
3.1. Efficacy of Hurdle Treatments
3.2. Impact of Food Soils on the Efficacy of PAA–Steam Hurdle Treatments
3.3. Influence of Surface Defects on the Efficacies of PAA–Steam Hurdle Treatments
4. Discussion
4.1. The Effectiveness of PAA–Steam Hurdle against L. innocua Biofilms
4.2. The Influence of Surface Conditions on the Efficacy of PAA–Steam Hurdle Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, L.L.; Friedrich, L.M.; Strawn, L.K.; Danyluk, M.D. Prevalence of Listeria monocytogenes and indicator microorganisms in Florida cantaloupe packinghouses, 2013–2014. Food Microbiol. 2022, 104, 103970. [Google Scholar] [CrossRef]
- Prazak, A.M.; Murano, E.A.; Mercado, I.; Acuff, G.R. Prevalence of Listeria monocytogenes during production and postharvest processing of cabbage. J. Food Prot. 2002, 65, 1728–1734. [Google Scholar] [CrossRef]
- Murugesan, L.; Kucerova, Z.; Knabel, S.J.; LaBorde, L.F. Predominance and distribution of a persistent Listeria monocytogenes clone in a commercial fresh mushroom processing environment. J. Food Prot. 2015, 78, 1988–1998. [Google Scholar] [CrossRef]
- Simonetti, T.; Peter, K.; Chen, Y.; Jin, Q.; Zhang, G.; LaBorde, L.F.; Macarisin, D. Prevalence and distribution of Listeria monocytogenes in three commercial tree fruit packinghouses. Front. Microbiol. 2021, 12, 652708. [Google Scholar] [CrossRef]
- Estrada, E.M.; Hamilton, A.M.; Sullivan, G.B.; Wiedmann, M.; Critzer, F.J.; Strawn, L.K. Prevalence, persistence, and diversity of Listeria monocytogenes and Listeria species in produce packinghouses in three US States. J. Food Prot. 2020, 83, 277–286. [Google Scholar] [CrossRef]
- John, J.; Joy, W.C.; Jovana, K. Prevalence of Listeria spp. in produce handling and processing facilities in the Pacific Northwest. Food Microbiol. 2020, 90, 103468. [Google Scholar] [CrossRef]
- McCollum, J.T.; Cronquist, A.B.; Silk, B.J.; Jackson, K.A.; O’Connor, K.A.; Cosgrove, S.; Gossack, J.P.; Parachini, S.S.; Jain, N.S.; Ettestad, P.; et al. Multistate outbreak of listeriosis associated with cantaloupe. N. Engl. J. Med. 2013, 369, 944–953. [Google Scholar] [CrossRef]
- Angelo, K.M.; Conrad, A.R.; Saupe, A.; Dragoo, H.; West, N.; Sorenson, A.; Barnes, A.; Doyle, M.; Beal, J.; Jackson, K.A.; et al. Multistate outbreak of Listeria monocytogenes infections linked to whole apples used in commercially produced, prepackaged caramel apples: United States, 2014–2015. Epidemiol. Infect. 2017, 145, 848–856. [Google Scholar] [CrossRef]
- FDA. Country Fresh Expands Voluntary Recall. 2020. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/country-fresh-expands-voluntary-recall (accessed on 3 October 2020).
- Chen, Y.; Burall, L.S.; Luo, Y.; Timme, R.; Melka, D.; Muruvanda, T.; Payne, J.; Wang, C.; Kastanis, G.; Maounounen-Laasri, A.; et al. Listeria monocytogenes in stone fruits linked to a multistate outbreak: Enumeration of cells and whole-genome sequencing. Appl. Environ. Microbiol. 2016, 82, 7030–7040. [Google Scholar] [CrossRef] [PubMed]
- FDA; FRESHOUSE II, LLC. Voluntarily Recalls Select Mesh Bags and Bulk Shipments of Potatoes, Limes, Valencia Oranges and Lemons Because of Possible Health Risk. 2020. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/freshouse-ii-llc-voluntarily-recalls-select-mesh-bags-and-bulk-shipments-potatoes-limes-valencia (accessed on 9 August 2020).
- FDA; Wegmans Food Markets, Inc. Announces Recall of Select Valencia Oranges, Lemons, and Various Products Containing Fresh Lemon Because of Possible Health Risk. 2020. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/wegmans-food-markets-inc-announces-recall-select-valencia-oranges-lemons-and-various-products (accessed on 9 August 2020).
- FDA; Dole Fresh Vegetables, Inc. Announces Voluntary Recall for Certain Salads Processed at Its Springfield, OH and Soledad, CA Facilities and Containing Iceberg Lettuce Due to Possible Health Risk from Listeria monocytogenes. 2022. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/dole-fresh-vegetables-inc-announces-voluntary-recall-certain-salads-processed-its-springfield-oh-and (accessed on 7 January 2022).
- Magdovitz, B.F.; Gummalla, S.; Thippareddi, H.; Harrison, M.A. Evaluating environmental monitoring protocols for Listeria spp.; Listeria monocytogenes in frozen food manufacturing facilities. J. Food Prot. 2020, 83, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pimentel, V.M.; Regalado-González, C.; Nava-Morales, G.M.; Meas-Vong, Y.; Castañeda-Serrano, M.P.; García-Almendárez, B.E. Effect of neutral electrolyzed water as antimicrobial intervention treatment of chicken meat and on trihalomethanes formation. J. Appl. Poult. Res. 2020, 29, 622–635. [Google Scholar] [CrossRef]
- Dell’Erba, A.; Falsanisi, D.; Liberti, L.; Notarnicola, M.; Santoro, D. Disinfection by-products formation during wastewater disinfection with peracetic acid. Desalination 2007, 215, 177–186. [Google Scholar] [CrossRef]
- Korany, A.M.; Hua, Z.; Green, T.; Hanrahan, I.; El-Shinawy, S.H.; El-Kholy, A.; Hassan, G.; Zhu, M.J. Efficacy of ozonated water, chlorine, chlorine dioxide, quaternary ammonium compounds and peroxyacetic acid against Listeria monocytogenes biofilm on polystyrene surfaces. Front. Microbiol. 2018, 9, 2296. [Google Scholar] [CrossRef]
- Hua, Z.; Korany, A.M.; El-Shinawy, S.H.; Zhu, M.J. Comparative evaluation of different sanitizers against Listeria monocytogenes biofilms on major food-contact surfaces. Front. Microbiol. 2019, 10, 2462. [Google Scholar] [CrossRef]
- Dhowlaghar, N.; De Abeysundara, P.A.; Nannapaneni, R.; Schilling, M.W.; Chang, S.; Cheng, W.H.; Sharma, C.S. Growth and biofilm formation by Listeria monocytogenes in catfish mucus extract on four food contact surfaces at 22 and 10 °C and their reduction by commercial disinfectants. J. Food Prot. 2018, 81, 59–67. [Google Scholar] [CrossRef]
- Berrang, M.E.; Frank, J.F.; Meinersmann, R.J. Effect of chemical sanitizers with and without ultrasonication on Listeria monocytogenes as a biofilm within polyvinyl chloride drain pipes. J. Food Prot. 2008, 71, 66–69. [Google Scholar] [CrossRef]
- Hua, Z.; Zhu, M.-J. Unlocking the hidden threat: Impacts of surface defects on the efficacy of sanitizers against Listeria monocytogenes biofilms on food-contact surfaces in tree fruit packing facilities. J. Food Prot. 2024, 87, 100213. [Google Scholar] [CrossRef]
- Ban, G.H.; Kang, D.H. Effect of sanitizer combined with steam heating on the inactivation of foodborne pathogens in a biofilm on stainless steel. Food Microbiol. 2016, 55, 47–54. [Google Scholar] [CrossRef]
- Ban, G.H.; Park, S.H.; Kim, S.O.; Ryu, S.; Kang, D.H. Synergistic effect of steam and lactic acid against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes biofilms on polyvinyl chloride and stainless steel. Int. J. Food Microbiol. 2012, 157, 218–223. [Google Scholar] [CrossRef]
- Hua, Z.; Younce, F.; Tang, J.; Ryu, D.; Rasco, B.; Hanrahan, I.; Zhu, M.J. Efficacy of saturated steam against Listeria innocua biofilm on common food-contact surfaces. Food Control 2021, 125, 107988. [Google Scholar] [CrossRef]
- Ban, G.H.; Yoon, H.; Kang, D.H. A comparison of saturated steam and superheated steam for inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes biofilms on polyvinyl chloride and stainless steel. Food Control 2014, 40, 344–350. [Google Scholar] [CrossRef]
- Lee, S.H.; Frank, J.F. Inactivation of surface-adherent Listeria monocytogenes hypochlorite and heat. J. Food Prot. 1991, 54, 4–7. [Google Scholar] [CrossRef]
- Masuku, S.M.; Babu, D.; Martin, E.M.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Lethality of moist heat and silver dihydrogen citrate sanitizer combinations on Listeria spp. adhered to components of a deli meat slicer. Food Control 2014, 44, 227–232. [Google Scholar] [CrossRef]
- Nimanpure, S.; Hashmi, S.A.R.; Kumar, R.; Bhargaw, H.N.; Kumar, R.; Nair, P.; Naik, A. Mechanical, electrical, and thermal analysis of sisal fibril/kenaf fiber hybrid polyester composites. Polym. Compos. 2019, 40, 664–676. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.; Wei, A.; Jiang, J.; Ji, Y.; Qiang, H.; Jiang, Y.; Zhang, H.; Liu, H. Preparation and characterization of blue-emitting carbon quantum dots and their silicone rubber composites. Mater. Res. Express 2019, 6, 045310. [Google Scholar] [CrossRef]
- FDA. CFR-Code of Federal Regulations Title 21. 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=178.1010 (accessed on 29 March 2022).
- Kim, S.S.; Kim, S.H.; Park, S.H.; Kang, D.H. Inactivation of Bacillus cereus spores on stainless steel by combined superheated steam and UV-C irradiation treatment. J. Food Prot. 2020, 83, 13–16. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, D.H. Inactivation of biofilm cells of foodborne pathogens by steam pasteurization. Eur. Food Res. Technol. 2014, 238, 471–476. [Google Scholar] [CrossRef]
- Gao, B.Z.; Xu, J.Z.; Peng, J.J.; Kang, F.Y.; Du, H.D.; Li, J.; Chiang, S.W.; Xu, C.J.; Hu, N.; Ning, X.S. Experimental and theoretical studies of effective thermal conductivity of composites made of silicone rubber and Al2O3 particles. Thermochim. Acta 2015, 614, 1–8. [Google Scholar] [CrossRef]
- Idicula, M.; Boudenne, A.; Umadevi, L.; Ibos, L.; Candau, Y.; Thomas, S. Thermophysical properties of natural fibre reinforced polyester composites. Compos. Sci. Technol. 2006, 66, 2719–2725. [Google Scholar] [CrossRef]
- Miyara, A. Thermal performance investigation of several types of vertical ground heat exchangers with different operation mode. Appl. Therm. Eng. 2012, 33–34, 167–174. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, D.H. Influence of surface properties of produce and food contact surfaces on the efficacy of chlorine dioxide gas for the inactivation of foodborne pathogens. Food Control 2017, 81, 88–95. [Google Scholar] [CrossRef]
- Berzins, A.; Hellström, S.; Siliņš, I.; Korkeala, H. Contamination patterns of Listeria monocytogenes in cold-smoked pork processing. J. Food Prot. 2010, 73, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Llacsahuanga, B.; Hamilton, A.; Zaches, R.; Hanrahan, I.; Critzer, F. Prevalence of Listeria species on food contact surfaces in Washington State apple packinghouses. Appl. Environ. Microbiol. 2021, 87, e02932-20. [Google Scholar] [CrossRef] [PubMed]
- Tompkin, R.B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cheon, H.L.; Park, K.H.; Chung, M.S.; Choi, S.H.; Ryu, S.; Kang, D.H. Inactivation of biofilm cells of foodborne pathogen by aerosolized sanitizers. Int. J. Food Microbiol. 2012, 154, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Schlisselberg, D.B.; Yaron, S. The effects of stainless steel finish on Salmonella Typhimurium attachment, biofilm formation and sensitivity to chlorine. Food Microbiol. 2013, 35, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Ryu, G.J.; Park, H.Y.; Ryu, K. Resistance of Staphylococcus aureus on food contact surfaces with different surface characteristics to chemical sanitizers. J. Food Saf. 2017, 37, e12354. [Google Scholar] [CrossRef]
- Kwon, S.A.; Song, W.J.; Kang, D.H. Comparison of the effect of saturated and superheated steam on the inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes on cantaloupe and watermelon surfaces. Food Microbiol. 2018, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Aarnisalo, K.; Salo, S.; Miettinen, H.; SUIHKO, M.L.; Wirtanen, G.U.N.; Autio, T.; Lundén, J.; Korkeala, H.; Sjöberg, A.M. Bactericidal efficiencies of commercial disinfectants against on surfaces. J. Food Saf. 2000, 20, 237–250. [Google Scholar] [CrossRef]
- Ayebah, B.; Hung, Y.C.; Kim, C.; Frank, J.F. Efficacy of electrolyzed water in the inactivation of planktonic and biofilm Listeria monocytogenes in the presence of organic matter. J. Food Prot. 2006, 69, 2143–2150. [Google Scholar] [CrossRef]

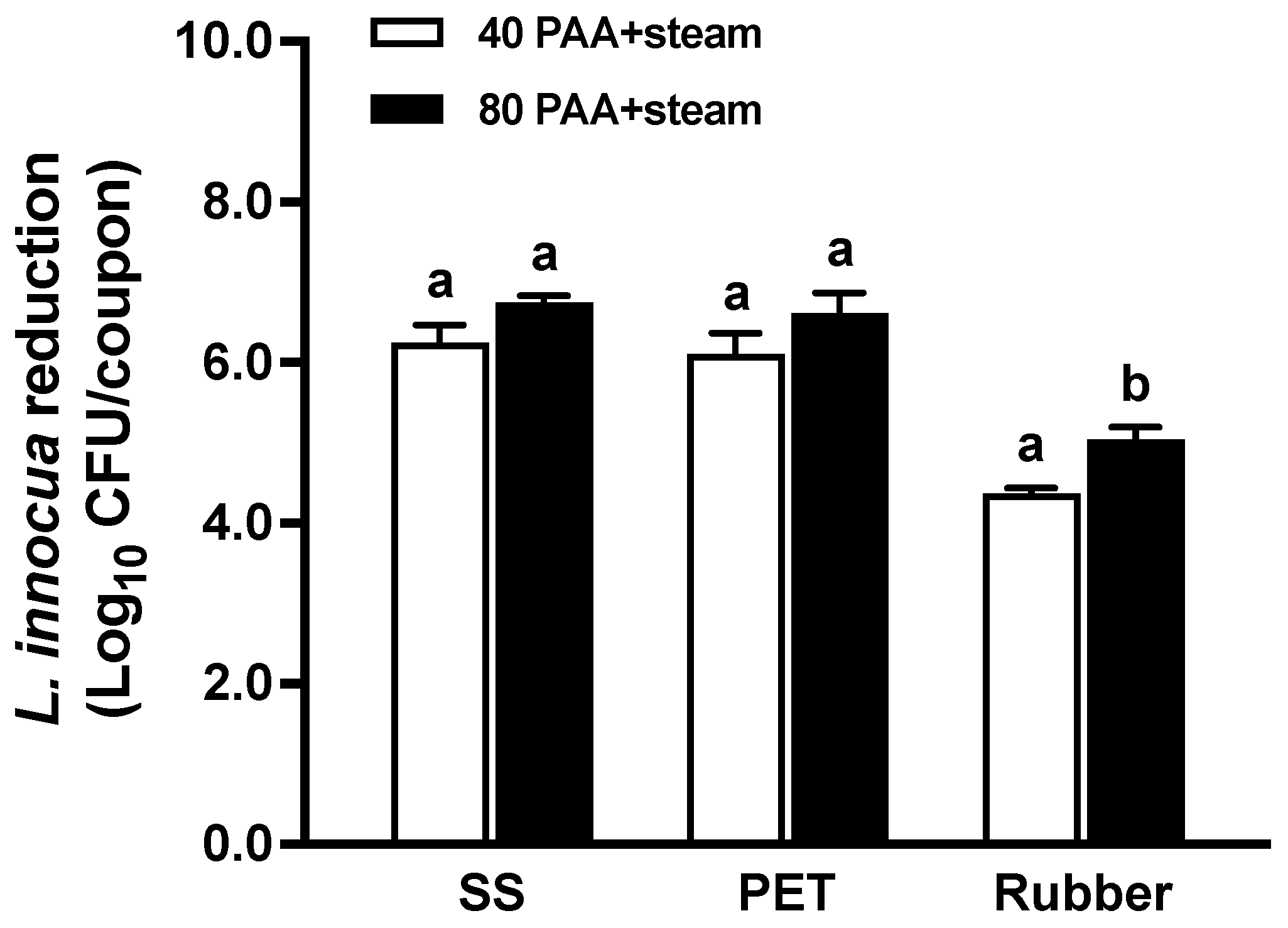
| Surface | PAA Conc. (ppm) | L. innocua Reduction in Biofilms (Log10 CFU/Coupon) | |
|---|---|---|---|
| PAA + Steam | Steam + PAA | ||
| SS | 40 | 6.25 ± 0.22 aA | 6.31 ± 0.25 aA |
| 80 | >6.53 aA | >6.53 aA | |
| PET | 40 | 6.11 ± 0.26 aA | 6.27 ± 0.30 aA |
| 80 | 6.61 ± 0.26 aA | 6.26 ± 0.28 aA | |
| Rubber | 40 | 4.37 ± 0.07 aA | 4.61 ± 0.23 aA |
| 80 | 5.04 ± 0.16 bA | 4.84 ± 0.15 aA | |
| Surface | Conditions | Initial Levels (Log10 CFU/Coupon) | L. innocua Reduction in Biofilms (Log10 CFU/Coupon) | |
|---|---|---|---|---|
| Steam | PAA + Steam | |||
| SS | Clean | 6.83 ± 0.05 | 3.34 ± 0.04 aA | 6.25 ± 0.22 aB |
| Soiled | 7.17 ± 0.08 | 3.56 ± 0.05 aA | 5.56 ± 0.18 bB | |
| PET | Clean | 7.13 ± 0.09 | 2.59 ± 0.07 aA | 6.11 ± 0.26 aB |
| Soiled | 7.32 ± 0.06 | 2.72 ± 0.07 aA | 5.76 ± 0.21 bB | |
| Rubber | Clean | 7.03 ± 0.09 | 2.65 ± 0.09 aA | 4.37 ± 0.07 aB |
| Soiled | 7.32 ± 0.08 | 2.64 ± 0.07 aA | 4.17 ± 0.04 aB | |
| Surface | Conditions | Initial Levels (Log10 CFU/Coupon) | L. innocua Reduction in Biofilms (Log10 CFU/Coupon) | |
|---|---|---|---|---|
| Steam | PAA + Steam | |||
| SS | New, clean | 6.83 ± 0.05 a | 3.34 ± 0.04 aA | >6.53 aB |
| Worn, clean | 7.22 ± 0.04 a | 2.56 ± 0.04 bA | 5.91 ± 0.27 bB | |
| Worn, soiled | 7.15 ± 0.06 a | 2.70 ± 0.12 bA | 5.08 ± 0.12 cB | |
| PET | New, clean | 7.13 ± 0.09 a | 2.59 ± 0.07 aA | 6.61± 0.26 aB |
| Worn, clean | 8.28 ± 0.07 b | 3.50 ± 0.07 bA | 5.69± 0.22 bB | |
| Worn, soiled | 8.18 ± 0.07 b | 3.33 ± 0.05 bA | 5.18 ± 0.08 cB | |
| Rubber | New, clean | 7.03 ± 0.09 a | 2.65 ± 0.09 aA | 4.37 ± 0.07 aB |
| Worn, clean | 8.00 ± 0.05 b | 3.23 ± 0.10 bA | 4.84 ± 0.04 bB | |
| Worn, soiled | 7.97 ± 0.07 b | 2.79 ± 0.13 aA | 4.49 ± 0.04 cB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Z.; Zhu, M.-J. Innovative Hurdle Strategies for Listeria Control on Food-Contact Surfaces: A Peroxyacetic Acid–Steam Approach. Foods 2024, 13, 2481. https://doi.org/10.3390/foods13162481
Hua Z, Zhu M-J. Innovative Hurdle Strategies for Listeria Control on Food-Contact Surfaces: A Peroxyacetic Acid–Steam Approach. Foods. 2024; 13(16):2481. https://doi.org/10.3390/foods13162481
Chicago/Turabian StyleHua, Zi, and Mei-Jun Zhu. 2024. "Innovative Hurdle Strategies for Listeria Control on Food-Contact Surfaces: A Peroxyacetic Acid–Steam Approach" Foods 13, no. 16: 2481. https://doi.org/10.3390/foods13162481
APA StyleHua, Z., & Zhu, M.-J. (2024). Innovative Hurdle Strategies for Listeria Control on Food-Contact Surfaces: A Peroxyacetic Acid–Steam Approach. Foods, 13(16), 2481. https://doi.org/10.3390/foods13162481





