Innovative Postharvest Management for Hass Avocado at the Preclimacteric Stage: A Combined Technology with GABA and 1-MCP
Abstract
:1. Introduction
2. Materials and Methods
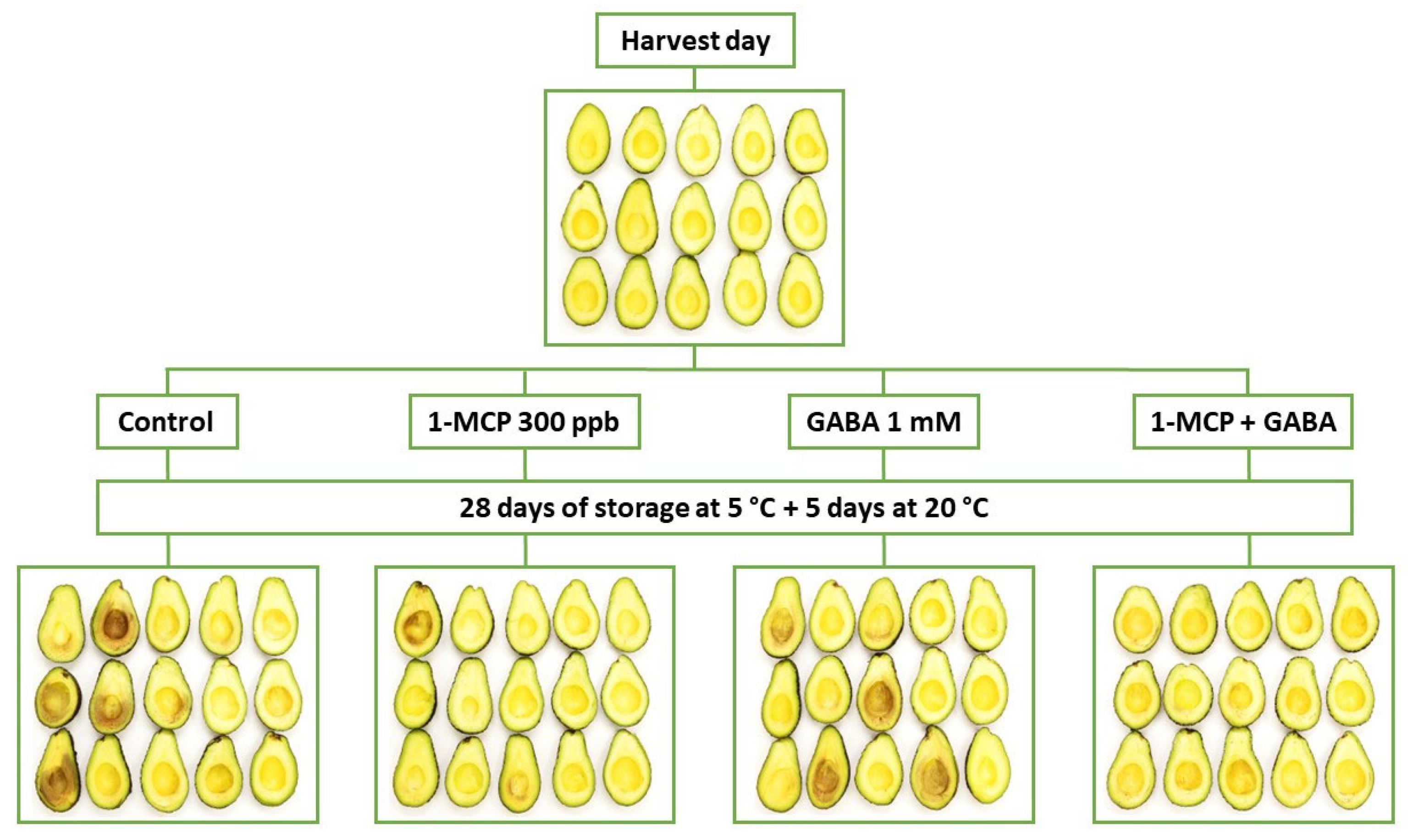
2.1. Plant Material and Postharvest Treatments
2.2. Postharvest Quality Parameters
2.3. Statistical Analysis
3. Results and Discussion
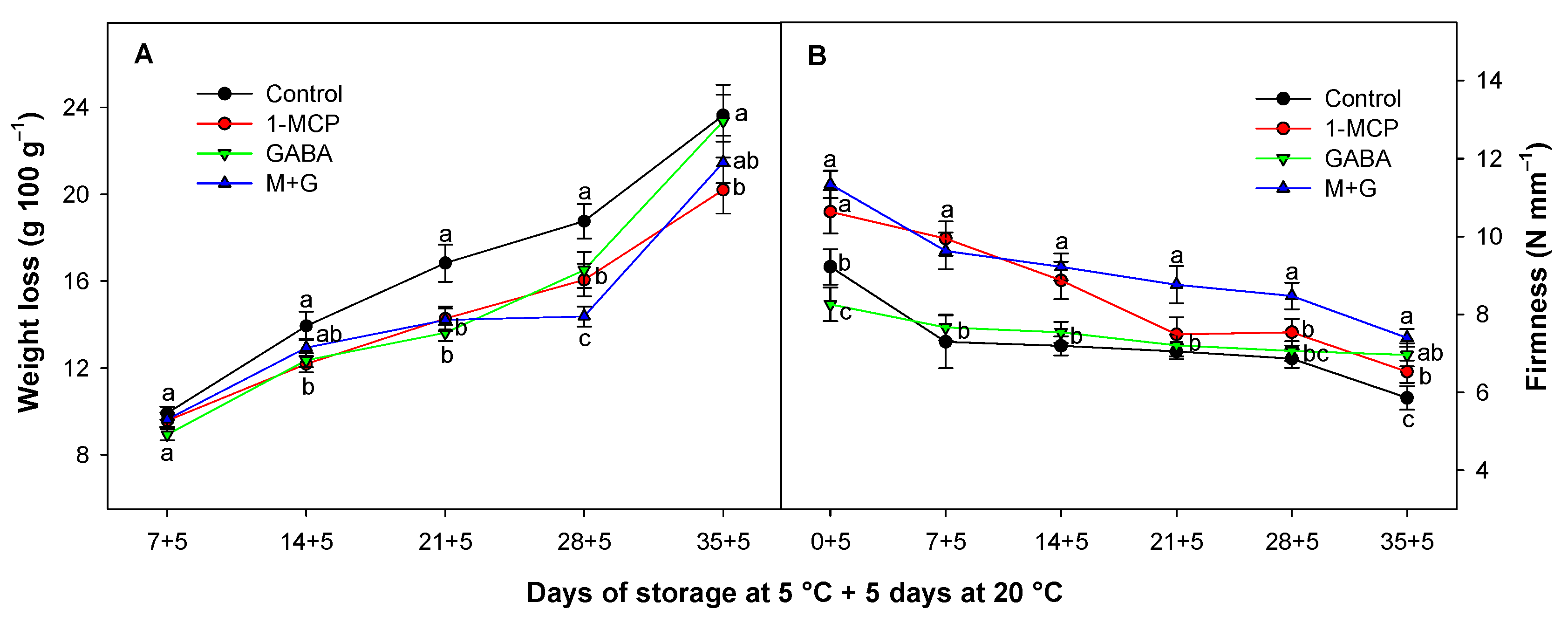
3.1. Effect of 1-MCP and GABA Treatments on Weight Loss and Firmness
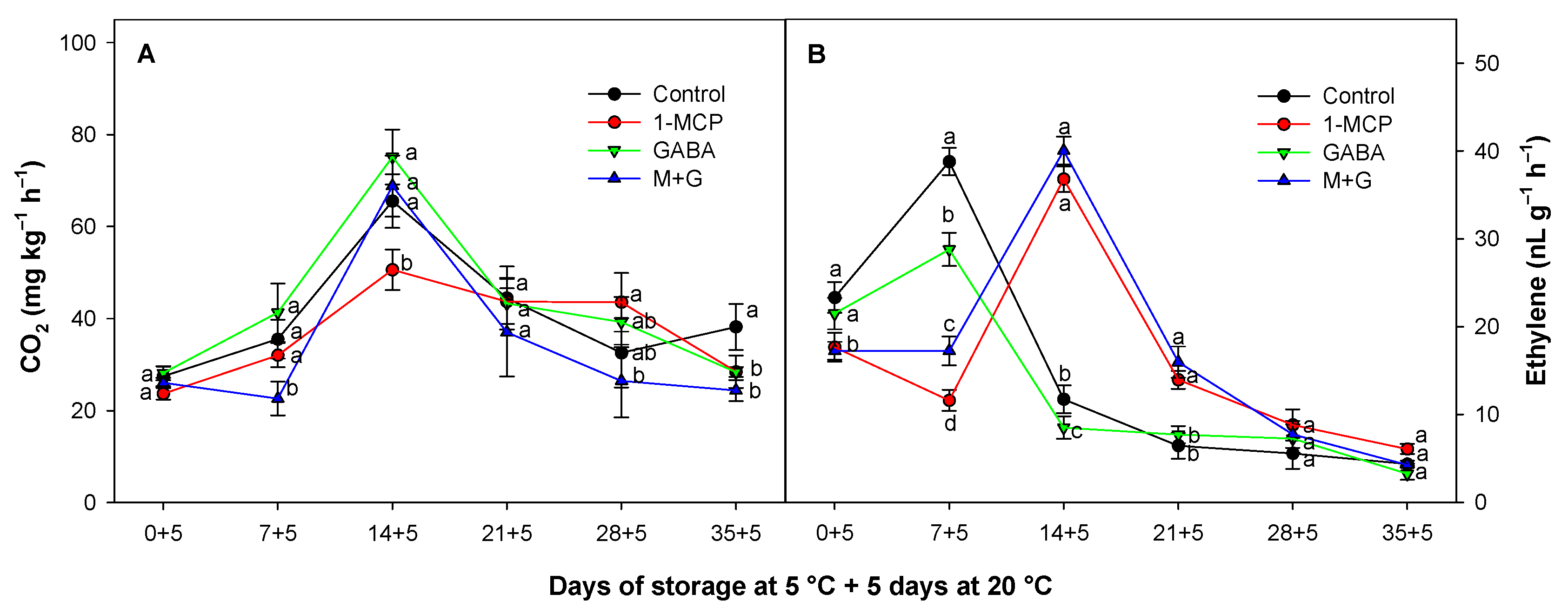
3.2. Effect of 1-MCP and GABA Treatments on Respiration Rate and Ethylene
3.3. Effect of 1-MCP and GABA Treatments on Total Soluble Solids (TSSs) and Total Acidity (TA)
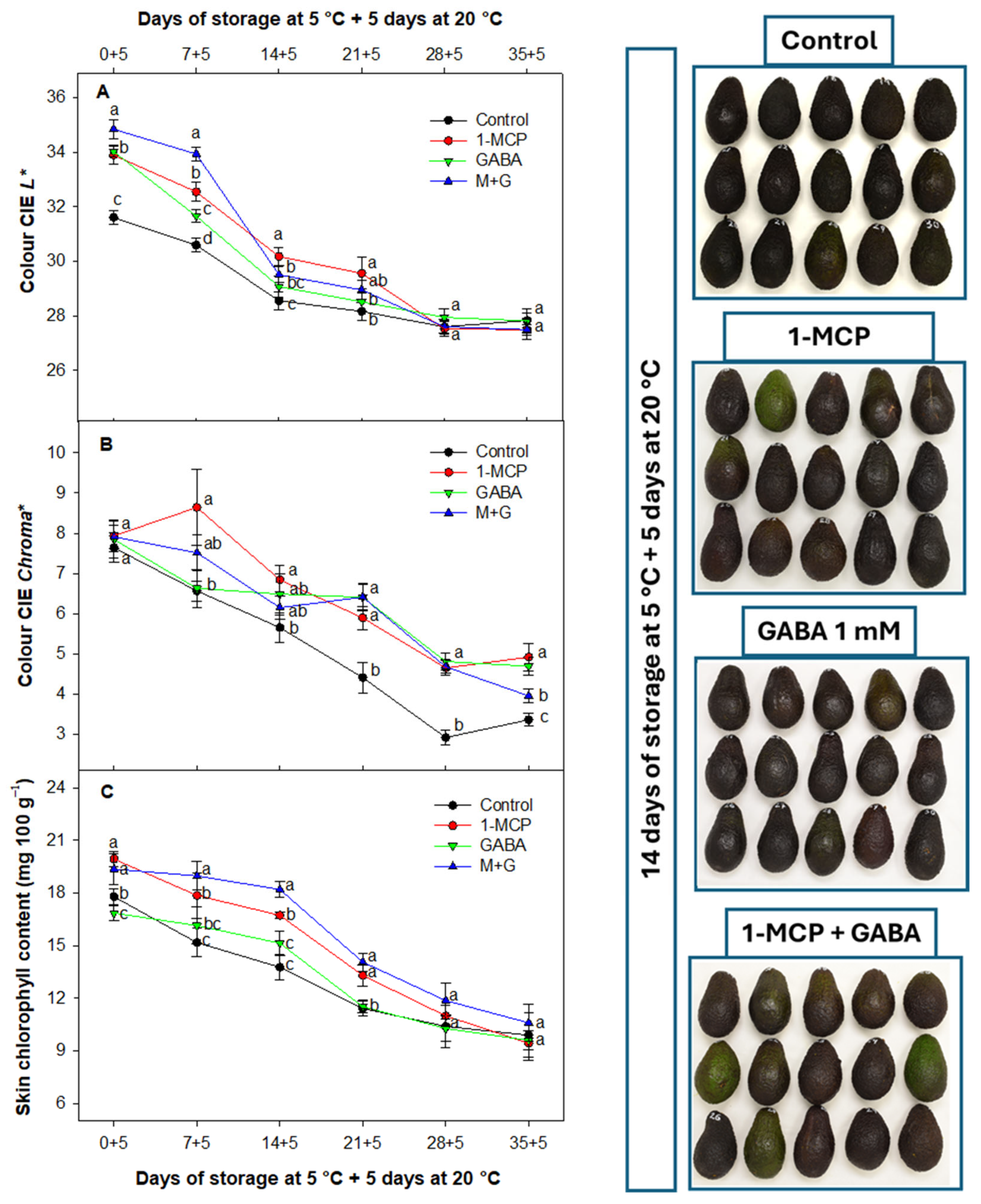
3.4. Effect of 1-MCP and GABA Treatments on Skin Color and Chlorophyll Content
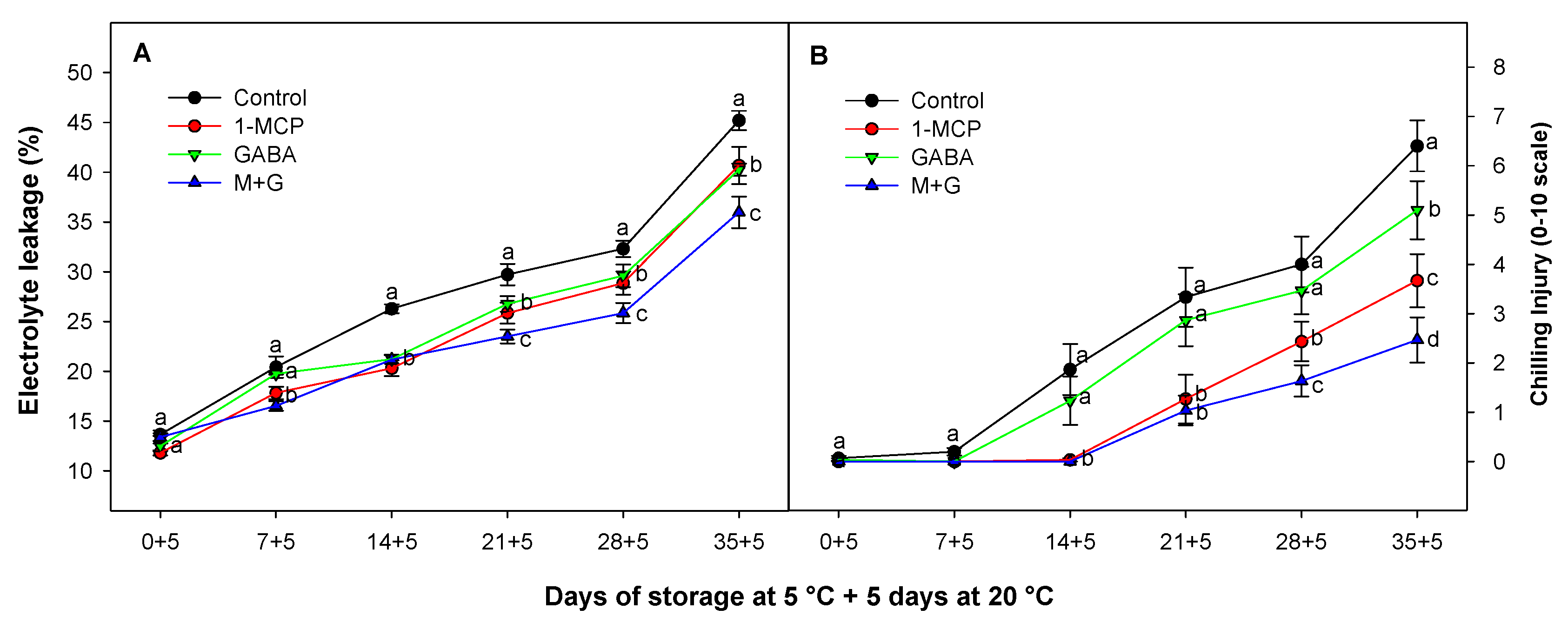
3.5. Effect of 1-MCP and GABA Treatments on Electrolyte Leakage and Chilling Injury
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Quezada, V.; Campos-Vega, R.; Loarca-Piña, G. Prediction of the physicochemical and nutraceutical characteristics of ‘Hass’ avocado seeds by correlating the physicochemical avocado fruit properties according to their ripening state. Plant Foods Hum. Nutr. 2021, 76, 311–318. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Thompson, A.K.; Korsten, L. Avocado fruit quality management during the postharvest supply chain. Food Rev. Int. 2014, 30, 169–202. [Google Scholar] [CrossRef]
- Yahia, E.M.; Fonseca, J.M.; Kitinoja, L. Chapter 2—Postharvest losses and waste. In Postharvest Technology of Perishable Horticultural Commodities; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 43–69. ISBN 9780128132760. [Google Scholar]
- Karoney, E.M.; Molelekoa, T.; Bill, M.; Siyoum, N.; Korsten, L. Global research network analysis of fresh produce postharvest technology: Innovative trends for loss reduction. Postharvest Biol. Technol. 2024, 208, 112642. [Google Scholar] [CrossRef]
- Vargas-Ortiz, M.; Rodríguez-Jimenes, G.; Salgado-Cervantes, M.; Pallet, D. Minimally Processed Avocado Through Flash Vacuum-Expansion: Its Effect in Major Physicochemical Aspects of the Puree and Stability on Storage. J. Food Process. Pres. 2017, 41, e12988. [Google Scholar] [CrossRef]
- Arpaia, M.L.; Collin, S.; Sievert, J.; Obenland, D. ‘Hass’ avocado quality as influenced by temperature and ethylene prior to and during final ripening. Postharvest Biol. Technol. 2018, 140, 76–84. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Tesfay, S.Z. A review of destructive and non-destructive methods for determining avocado fruit maturity. Food Bioprocess Technol. 2015, 8, 1995–2011. [Google Scholar] [CrossRef]
- Chirinos, R.; Ramon, K.; Mendoza, M.; Figueroa-Merma, A.; Pacheco-Ávalos, A.; Campos, D.; Pedreschi, R. Effect of Prolonged Cold Storage on the Dynamics of the Enzymatic and Non-Enzymatic Antioxidant System in the Mesocarp of Avocado (Persea americana) cv. Hass: Relationship with Oxidative Processes. Horticulturae 2022, 8, 880. [Google Scholar] [CrossRef]
- Yahia, E.M.; Woolf, A.B. Avocado (Persea americana Mill.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 125–186. ISBN 9781845697341. [Google Scholar]
- Woolf, A.B.; Arpaia, M.L.; Defilippi, B.G.; Bower, J.P. Subtropical fruits: Avocados. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M.I., Beaudry, R., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 389–397. ISBN 9780128045992. [Google Scholar]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.M.M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Kruger, F.J.; Volschenk, G.O. Ripening patterns of South African export “Hass” avocado hold-back samples from commercial 1-methylcyclopropene (SmartFreshSM) applications. In Proceedings of the VIII World Avocado Congress, Cairns, Australia, 5–9 September 2011. [Google Scholar]
- Satekge, T.K.; Magwaza, L.S. Postharvest application of 1-Methylcyclopropene (1-MCP) on climacteric fruits: Factors affecting efficacy. Int. J. Fruit Sci. 2022, 22, 595–607. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Serrano, M.; Ruiz-Aracil, M.C.; Ilea, M.I.M.; Martínez-Romero, D.; Guillén, F. A synergistic effect based on the combination of melatonin with 1-methylcyclopropene as a new strategy to increase chilling tolerance and general quality in zucchini fruit. Foods 2022, 11, 2784. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Serrano, M.; Martínez-Romero, D.; Ruiz-Aracil, M.C.; Valero, D.; Guillén, F. The Simultaneous Use of 1-Methylcyclopropene and Methyl Jasmonate Vapor as an Innovative Strategy for Reducing Chilling Injury and Maintaining Pomegranate Fruit Quality at Suboptimal Temperatures. Foods 2023, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Perato, S.M.; Martínez-Zamora, M.G.; Salazar, S.M.; Díaz-Ricci, J.C. The elicitor AsES stimulates ethylene synthesis, induce ripening and enhance protection against disease naturally produced in avocado fruit. Sci. Hortic. 2018, 240, 288–292. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; Zarei, A. 4-Aminobutyrate (GABA): A metabolite and signal with practical significance. Botany 2017, 95, 1015–1032. [Google Scholar] [CrossRef]
- Yang, A.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011, 129, 1619–1622. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Dong, W.; Jin, S.; Liu, Q.; Shi, L.; Cao, S.; Li, S.; Chen, W.; Yang, Z. Γ-Aminobutyric acid treatment induced chilling tolerance in postharvest peach fruit by upregulating ascorbic acid and glutathione contents at the molecular level. Front. Plant Sci. 2022, 13, 1059979. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, B.G.; Ejsmentewicz, T.; Covarrubias, M.P.; Gudenschwager, O.; Campos-Vargas, R. Changes in cell wall pectins and their relation to postharvest mesocarp softening of “Hass” avocados (Persea americana Mill.). Plant Physiol. Biochem. 2018, 128, 142–151. [Google Scholar] [CrossRef]
- Ramírez-Gil, J.G.; López, J.H.; Henao-Rojas, J.C. Causes of Hass Avocado Fruit Rejection in Preharvest, Harvest, and Packinghouse: Economic Losses and Associated Variables. Agronomy 2020, 10, 8. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Esti, M. Novel food colorants from tomatoes: Stability of carotenoid-containing chromoplasts under different storage conditions. LWT 2021, 140, 110725. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Changes of phytochemicals and antioxidant capacity of banana peel during the ripening process; with and without ethylene treatment. Sci. Hortic. 2019, 253, 255–262. [Google Scholar] [CrossRef]
- Hershkovitz, V.; Saguy, S.I.; Pesis, E. Postharvest application of 1-MCP to improve the quality of various avocado cultivars. Postharvest Biol. Technol. 2005, 37, 252–264. [Google Scholar] [CrossRef]
- Pachón, Y.V.; Balaguera-López, H.E.; Florez-Velasco, N. Postharvest behavior and chilling injury in avocado (Persea americana Mill) fruit cv. Hass treated with 1-methylcyclopropene, ethylene, and intermittent warming. Rev. Fac. Nac. Agron. Medellín 2022, 75, 9895–9907. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Effect of pectin-based coating on the kinetics of quality change associated with stored avocados. J. Food Process. Pres. 2008, 32, 621–643. [Google Scholar] [CrossRef]
- Palou, L.; Serrano, M.; Martínez-Romero, D.; Valero, D. New approaches for postharvest quality retention of table grapes. Fresh Prod. 2010, 4, 103–110. [Google Scholar]
- Mekontso, F.N.; Duan, W.; Cisse, E.H.M.; Chen, T.; Xu, X. Alleviation of postharvest chilling injury of carambola fruit by γ-aminobutyric acid: Physiological, biochemical, and structural characterization. Front. Nutr. 2021, 8, 752583. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, B.; Lin, H.; Lin, M.; Chen, J.; Lin, Y. Γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chem. 2022, 13, 100208. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effect of γ-aminobutyric acid on the antioxidant system and biochemical changes of mango fruit during storage. J. Food Meas. Charact. 2020, 14, 778–789. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I.K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Rao, J. Ripening delay of mid-climacteric avocado fruit in response to elevated doses of 1-methylcyclopropene and hypoxia-mediated reduction in internal ethylene concentration. Postharvest Biol. Technol. 2011, 60, 83–91. [Google Scholar] [CrossRef]
- Olivares, D.; García-Rojas, M.; Ulloa, P.A.; Riveros, A.; Pedreschi, R.; Campos-Vargas, R.; Meneses, C.; Defilippi, B.G. Response Mechanisms of “Hass” Avocado to Sequential 1–methylcyclopropene Applications at Different Maturity Stages during Cold Storage. Plants 2022, 11, 1781. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Lum, G.B.; Brikis, C.J.; Deyman, K.L.; Subedi, S.; DeEll, J.R.; Shelp, B.J.; Bozzo, G.G. Pre-storage conditioning ameliorates the negative impact of 1-methylcyclopropene on physiological injury and modifies the response of antioxidants and γ-aminobutyrate in ‘Honeycrisp’ apples exposed to controlled-atmosphere conditions. Postharvest Biol. Technol. 2016, 116, 115–128. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Olivares, D.; Alvarez, E.; Véliz, D.; García-Rojas, M.; Díaz, C.; Defilippi, B.G. Effects of 1-Methylcyclopropene and Controlled Atmosphere on Ethylene Synthesis and Quality Attributes of Avocado cvs. Edranol and Fuerte. J. Food Qual. 2020, 20, 5075218. [Google Scholar] [CrossRef]
- Fan, X.; Argenta, L.; Mattheis, J.P. Inhibition of ethylene action by 1-methylcyclopropene prolongs storage life of apricots. Postharvest Biol. Technol. 2000, 20, 135–142. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Guillén, F.; Ilea, M.I.M.; Martínez-Romero, D.; Lorente-Mento, J.M.; Valverde, J.M. Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life. Agriculture 2023, 13, 806. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Lwin, H.P.; Rudell, D.R.; Lee, J. Metabolism and cold chain performance of ‘Chuhwangbae’ Asian pears as impacted by 1-MCP treatment. Sci. Hortic. 2021, 288, 110357. [Google Scholar] [CrossRef]
- Deyman, K.L.; Brikis, C.J.; Bozzo, G.G.; Shelp, B.J. Impact of 1-methylcyclopropene and controlled atmosphere storage on polyamine and 4-aminobutyrate levels in “Empire” apple fruit. Front. Plant Sci. 2014, 5, 81066. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Nehela, Y.; Killiny, N. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta 2018, 248, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Peng, J.; Cao, S.F.; Zheng, Y.H. Managing chilling injury in fruits. Acta Hortic. 2013, 1012, 1087–1095. [Google Scholar] [CrossRef]
- Li, Z.; Huang, T.; Tang, M.; Cheng, B.; Peng, Y.; Zhang, X. iTRAQ-based proteomics reveals key role of γ-aminobutyric acid (GABA) in regulating drought tolerance in perennial creeping bentgrass (Agrostis stolonifera). Plant Physiol. Biochem. 2019, 145, 216–226. [Google Scholar] [CrossRef]
- Hershkovitz, V.; Friedman, H.; Goldschmidt, E.E.; Pesis, E. Ethylene regulation of avocado ripening differs between seeded and seedless fruit. Postharvest Biol. Technol. 2010, 56, 138–146. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Campbell-Palmer, L.; Fillmore, S.; Zhang, Z. Effect of ethylene and 1-MCP on expression of genes involved in ethylene biosynthesis and perception during ripening of apple fruit. Postharvest Biol. Technol. 2013, 78, 55–66. [Google Scholar] [CrossRef]
- Jeong, J.; Huber, D.J.; Sargent, S.A. Influence of 1-methylcyclopropene (1-MCP) on ripening and cell-wall matrix polysaccharides of avocado (Persea americana) fruit. Postharvest Biol. Technol. 2002, 25, 241–256. [Google Scholar] [CrossRef]
- Han, S.; Nan, Y.; Qu, W.; He, Y.; Ban, Q.; Lv, Y.; Rao, J. Exogenous γ-aminobutyric acid treatment that contributes to regulation of malate metabolism and ethylene synthesis in apple fruit during storage. J. Agric. Food Chem. 2018, 66, 13473–13482. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Jiang, X.; Chen, C.; Cheng, Y. Effects of γ-aminobutyric acid treatment on the quality properties and chilling tolerance of guava fruit during cold storage. Food Ferm. Indust. 2021, 47, 130–136. [Google Scholar] [CrossRef]
- Salameh, M.; Nacouzi, D.; Lahoud, G.; Riachy, I.; El Kayal, W. Evaluation of Postharvest Maturity Indices of Commercial Avocado Varieties Grown at Various Elevations Along Lebanon’s Coast. Front. Plant Sci. 2022, 13, 895964. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, Y.; Iribe-Salazar, R.; Carrazco-Escalante, M.; Gaxiola-Camacho, S.; Corrales, J.C. Quality variables of “Hass” avocado stored in modified atmosphere packaging. Agrociencia 2022, 56, 805–829. [Google Scholar] [CrossRef]
- Meyer, M.D.; Terry, L.A. Fatty acid and sugar composition of avocado, cv. Hass, in response to treatment with an ethylene scavenger or 1-methylcyclopropene to extend storage life. Food Chem. 2010, 121, 1203–1210. [Google Scholar] [CrossRef]
- Niazi, Z.; Razavi, F.; Khademi, O.; Aghdam, M.S. Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 2021, 285, 110198. [Google Scholar] [CrossRef]
- Homez-Jara, A.; Cardenas-Roa, H.; Montealegre, M.; Lim, L.T.; Corradini, M.G.; Váquiro-Herrera, H.A.; Sandoval-Aldana, A. Postharvest Treatments of Hass Avocado (Persea americana Mill.) and Estimation of Its Quality Using Hyperspectral Imaging (HSI). ACS Food Sci. Technol. 2023, 3, 932–944. [Google Scholar] [CrossRef]
- Ashton, O.B.; Wong, M.; Mcghie, T.K.; Vather, R.; Wang, Y.; Requejo-Jackman, C.; Ramankutty, P.; Woolf, A.B. Pigments in avocado tissue and oil. J. Agric. Food Chem. 2006, 54, 10151–10158. [Google Scholar] [CrossRef]
- Nunes, M.C.N. Correlations between subjective quality and physicochemical attributes of fresh fruits and vegetables. Postharvest Biol. Technol. 2015, 107, 43–54. [Google Scholar] [CrossRef]
- Al-Shoffe, Y.; Nock, J.F.; Zhang, Y.; Watkins, C.B. Pre-and post-harvest γ-aminobutyric acid application in relation to fruit quality and physiological disorder development in ‘Honeycrisp’ apples. Sci. Hortic. 2021, 289, 110431. [Google Scholar] [CrossRef]
- Saeedi, M.; Mirdehghan, S.H.; Nazoori, F.; Esmaeilizadeh, M.; Saba, M.K. Impact of calcium and γ-aminobutyric acid (GABA) on qualitative attributes and shelf life characteristics of fresh in-hull pistachio during cold storage. Postharvest Biol. Technol. 2022, 187, 111863. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Li, B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.M.; López-Rodriguez, V.; De La Plaza, J.L. An improved technique for measuring the electrical conductivity of intact fruits. LWT-Food Sci. Technol. 1994, 27, 29–33. [Google Scholar] [CrossRef]
- Chirinos, R.; Delgado-Pariona, J.; Aguilar-Galvez, A.; Figueroa-Merma, A.; Pacheco-Ávalos, A.; Campos, D.; Pedreschi, R. Postharvest Storage Differentially Modulates the Enzymatic and Non-Enzymatic Antioxidant System of the Exocarp and Mesocarp of Hass Avocado: Implications for Disorders. Plants 2023, 12, 4008. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Naderi, R.; Sarcheshmeh, M.A.A.; Babalar, M. Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 2015, 110, 70–76. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pu, J.; Liu, H.; Wang, M.; Du, Y.; Tang, X.; Luo, X.; Wang, Y.; Deng, Q. Effects of L-Cysteine and γ-Aminobutyric Acid Treatment on Postharvest Quality and Antioxidant Activity of Loquat Fruit during Storage. Int. J. Mol. Sci. 2023, 24, 10541. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Antolí, A.; Badiche-El Hilali, F.; Lorente-Mento, J.M.; Díaz-Mula, H.M.; Serrano, M.; Valero, D. Antioxidant Systems and Quality in Sweet Cherries Are Improved by Preharvest GABA Treatments Leading to Delay Postharvest Senescence. Int. J. Mol. Sci. 2024, 25, 260. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, B.; Lin, Y.; Chen, Y.; Chen, G.; Chen, J.; Wang, H.; Chen, Y.; Lin, H. Alleviation of chilling injury in cold-stored Chinese olive (Canarium album Lour.) fruit by γ-aminobutyric acid treatment in relation to ROS metabolism. Sci. Hortic. 2024, 327, 112851. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Palma, F.; Carvajal, F.; Jiménez-Muñoz, R.; Pulido, A.; Jamilena, M.; Garrido, D. Exogenous γ-aminobutyric acid treatment improves the cold tolerance of zucchini fruit during postharvest storage. Plant Physiol. Biochem. 2019, 136, 188–195. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aracil, M.C.; Valverde, J.M.; Ilea, M.I.M.; Valero, D.; Castillo, S.; Guillén, F. Innovative Postharvest Management for Hass Avocado at the Preclimacteric Stage: A Combined Technology with GABA and 1-MCP. Foods 2024, 13, 2485. https://doi.org/10.3390/foods13162485
Ruiz-Aracil MC, Valverde JM, Ilea MIM, Valero D, Castillo S, Guillén F. Innovative Postharvest Management for Hass Avocado at the Preclimacteric Stage: A Combined Technology with GABA and 1-MCP. Foods. 2024; 13(16):2485. https://doi.org/10.3390/foods13162485
Chicago/Turabian StyleRuiz-Aracil, María Celeste, Juan Miguel Valverde, Mihaela Iasmina Madalina Ilea, Daniel Valero, Salvador Castillo, and Fabián Guillén. 2024. "Innovative Postharvest Management for Hass Avocado at the Preclimacteric Stage: A Combined Technology with GABA and 1-MCP" Foods 13, no. 16: 2485. https://doi.org/10.3390/foods13162485







