Utilizing Supercritical CO2 for Bee Brood Oil Extraction and Analysis of Its Chemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemicals and Reagents
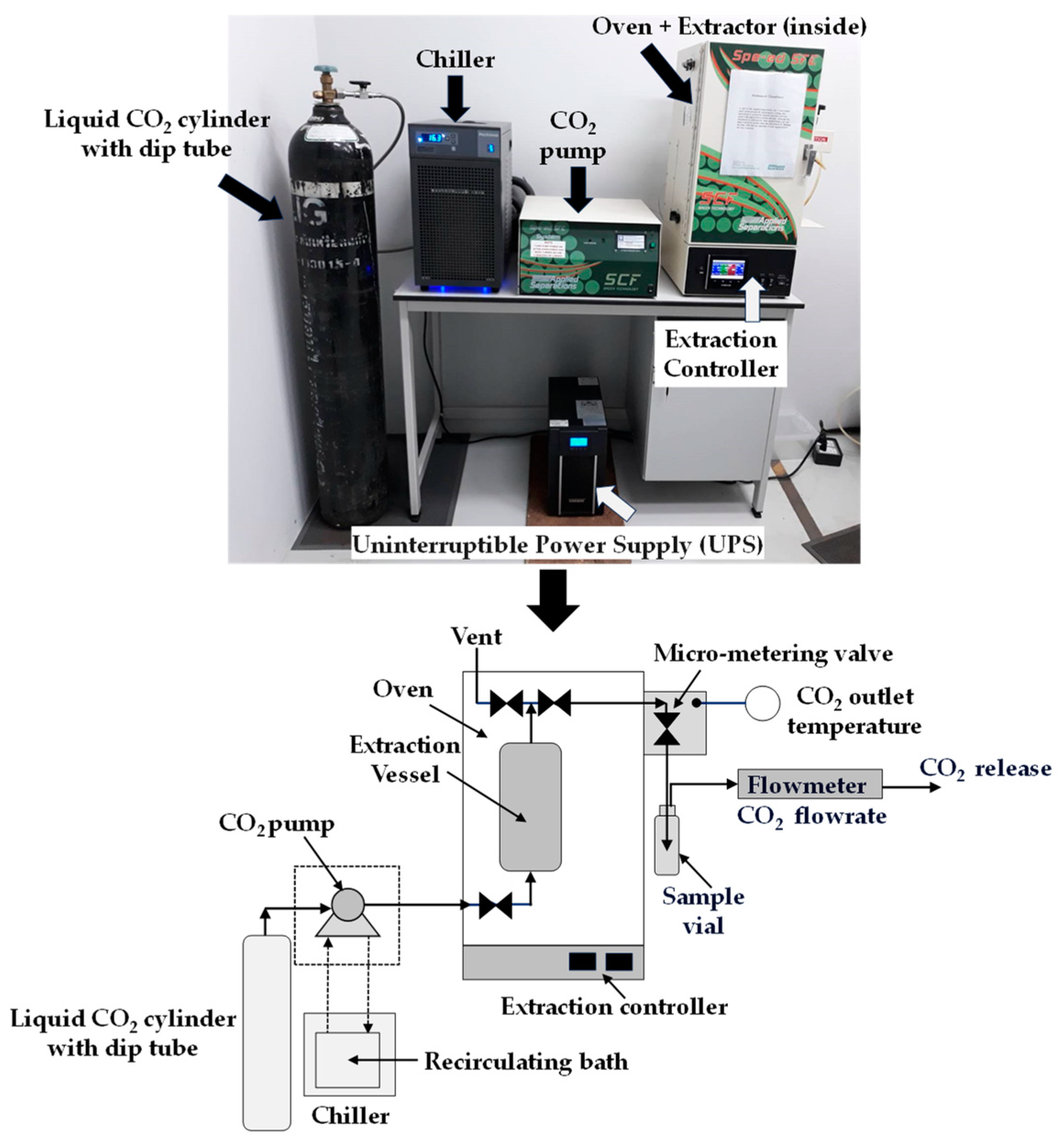
2.3. Supercritical CO2 Extraction
2.4. Chemical Properties of Bee Brood and Extracted Oil
2.5. Fatty Acid Composition Analysis
2.6. Determination of Total Phenolic Compound Content
2.7. Identification and Quantification of Phenolic Compounds Using HPLC
2.8. Determination of Total Flavonoid Content
2.9. Determination of Total Carotenoid Content
2.10. Amino Acid Analysis
2.11. Determination of Free Radical Scavenging (ABTS) Assay
2.12. Determination of Free Radical Scavenging (DPPH) Assay
2.13. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.14. Determination of Stability
2.15. Statistical Analysis
3. Results
3.1. Proximate Composition of Bee Brood
3.2. Oil Extraction Using Supercritical CO2
3.3. Acid, Iodine, Saponification, and Peroxide Values of Extracted Oil from Dried Bee Brood
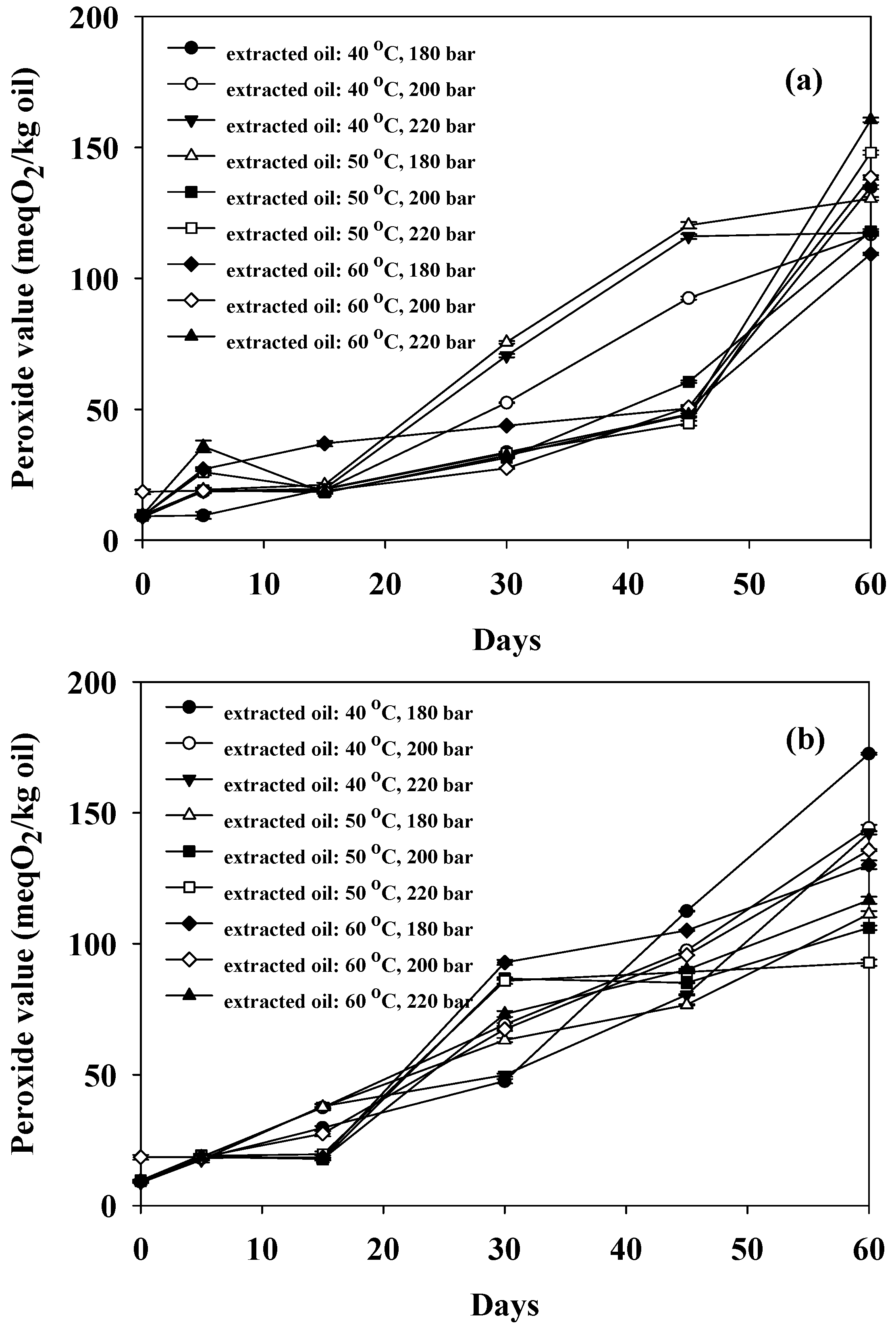
3.4. Stability of Extracted Oil
3.5. Total Phenolic Compound Content, Total Flavonoid Content, and Antioxidant Activities
3.6. Identification and Quantification of Phenolic Compounds
3.7. Fatty Acid Composition of Extracted Oil
3.8. Amino Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camazine, S. Self-organizing pattern formation on the combs of honey bee colonies. Behav. Ecol. Sociobiol. 1991, 28, 61–76. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Correia, P.M.R.; Anjos, O.; Coelho, C.; Costa, C.A. Honey bee (Apis mellifera L.) broods: Composition, technology, and gastronomic applicability. Foods 2022, 11, 2750. [Google Scholar] [CrossRef] [PubMed]
- Narumi, S. Honeybee brood as a nutritional food. Honeybee Sci. 2004, 25, 119–124. [Google Scholar]
- Abd Al-Fattah, M.A.; Sorour, M.A.; El-Din, E.O.N. Chemical composition of honeybee (Apis mellifera L.) brood. J. Agric. Sci. Mansoura Univ. 2009, 34, 5365–5371. [Google Scholar] [CrossRef]
- Finke, M.D. Nutrient composition of bee brood and its potential as human food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar] [CrossRef]
- Mishyna, M.; Keppler, J.K.; Chen, J. Techno-functional properties of edible insect proteins and effects of processing. Curr. Opin. Colloid Interface Sci. 2021, 56, 101508. [Google Scholar] [CrossRef]
- Zhao, X.; Vazquez-Gutierrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes—Extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable resources from insects: Exploitation, properties, and refining of fat obtained by cold-pressing from Hermetia illucens (Black Soldier Fly) larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1800376. [Google Scholar] [CrossRef]
- Saviane, A.; Tassoni, L.; Naviglio, D.; Lupi, D.; Savoldelli, S.; Bianchi, G.; Cortellino, G.; Bondioli, P.; Folegatti, L.; Casartelli, M.; et al. Mechanical processing of Hermetia illucens larvae and Bombyx mori pupae produces oils with antimicrobial activity. Animals 2021, 11, 783. [Google Scholar] [CrossRef]
- Ishak, S.; Kamari, A.; Yusoff, S.N.M.; Halim, A.L.A. Optimisation of biodiesel production of black soldier fly larvae rearing on restaurant kitchen waste. J. Phys. Conf. Ser. 2018, 1097, 012052. [Google Scholar] [CrossRef]
- Hao, M.J.; Elias, N.H.; Aminuddin, M.H.; Zainalabidin, N. Microwave-assisted extraction of black soldier fly larvae (BSFL) lipid. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012057. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, T.S.; Ha, Y.J.; Gal, S.W.; Noh, C.W.; Kim, I.S.; Lee, J.H.; Yoo, J.H. Removal of fat from crushed black soldier fly larvae by carbon dioxide supercritical extraction. J. Anim. Feed Sci. 2019, 8, 83–88. [Google Scholar] [CrossRef]
- Purschke, B.; Stegmann, T.; Schreiner, M.; Jäger, H. Pilot scale supercritical CO2 extraction of edible insect oil from Tenebrio molitor L. larvae-Influence of extraction conditions on kinetics, defatting performance and compositional properties. Eur. J. Lipid Sci. Technol. 2017, 119, 1600134. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.M.C.S.; Gamarra, F.M.C.; Paviani, L.C.; Gonçalves, L.A.G.; Cabral, F.A. Solubility of triacylglyerols in supercritical carbon dioxide. J. Supercrit. Fluids 2007, 43, 25–31. [Google Scholar] [CrossRef]
- Sarker, M.Z.I.; Ferdosh, S.; Manap, M.Y.A.; Rahman, N.N.N.A.; Kadir, M.O.A. Applications of supercritical fluid extraction (SFE) of palm oil and oil from natural sources. Molecules 2012, 17, 1764–1794. [Google Scholar] [CrossRef]
- Muangrat, R.; Pannasai, S. Exploring the potential of black soldier fly larvae oil: Supercritical CO2 extraction, physicochemical analysis, antioxidant properties, shelf life, and keratinocyte growth inhibition. J. Agric. Food Res. 2024, 15, 101008. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; American Oil Chemists’ Society: Champaign, IL, USA, 2004. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; American Oil Chemists’ Society: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; American Oil Chemists’ Society: Champaign, IL, USA, 2003. [Google Scholar]
- Kim, D.; Oh, I. The characteristic of insect oil for a potential component of oleogel and its application as a solid fat replacer in cookies. Gels 2022, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Fratini, F.; Cacchiarelli, C.; Martinis, V.; Tuccinardi, T.; Paci, G.; Dal Bosco, A.; Mancini, S. Chemical composition, fatty acid profile, antioxidant content, and microbiological loads of lesser mealworm, mealworm, and superworm larvae. Ital. J. Anim. Sci. 2024, 23, 125–137. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mancinelli, A.C.; Vaudo, G.; Cavallo, M.; Castellini, C.; Mattioli, S. Indexing of fatty acids in poultry meat for its characterization in healthy human nutrition: A Comprehensive application of the scientific literature and new proposals. Nutrients 2022, 14, 3110. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Houshia, O.J.; Qutit, A.; Zaid, O.; Shqair, H.; Zaid, M. Determination of total polyphenolic antioxidants contents in West-Bank olive oil. J. Nat. Sci. 2014, 4, 71–79. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total favonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Chinarak, K.; Chaijan, M.; Panpipat, W. Farm-raised sago palm weevil (Rhynchophorus ferrugineus) larvae: Potential and challenges for promising source of nutrients. J. Food Compos. Anal. 2020, 92, 103542. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Moerman, R.; Rasmont, P.; Lognay, G.; Wathelet, B.; Wattiez, R.; Michez, D. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS ONE 2014, 9, e86209. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of pasma (FRAP) as a measure of antioxidant power: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef]
- Przybylski, R.; Wu, J.; Eskin, N.A.M. A rapid method for determining the oxidative stability of oils suitable for breeder size samples. J. Am. Oil Chem. Soc. 2013, 90, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Cruz, V.A.; Ferreira, N.J.; Cornelio-Santiago, H.P.; Santos, G.M.T.; Oliveira, A.L. Oil extraction from black soldier fly (Hermetia illucens L.) larvae meal by dynamic and intermittent processes of supercritical CO2—Global yield, oil characterization, and solvent consumption. J. Supercrit. Fluids 2023, 195, 105861. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.M.; Silva, D.; Simoes, P.C. Supercritical fluid extraction of lipids from spent coffee grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish byproducts: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Food and Agriculture Organization/World Health Organization (FAO/WHO). Report of the 21st Session of the Codex Alimentarius Committee on Fats and Oils; Food and Agriculture Organization/World Health Organization (FAO/WHO): Kota Kinabalu, Malaysia, 2009. [Google Scholar]
- Sasongko, H.; Efendi, N.R.; Budihardjo, A.; Farida, Y.; Amartiwi, T.; Rahmawati, A.A.; Wicaksono, A.; Sugiyarto. Solvent and extraction methods effects on the quality of eel (Anguilla bicolor) oil. J. Phys. Conf. Ser. 2017, 795, 012021. [Google Scholar] [CrossRef]
- Uddin, S.M.; Chun, B.S. Measurement of stability and astaxanthin content of squid viscera oil extracted by supercritical carbon dioxide and organic solvent. J. Int. Food Res. 2010, 19, 633–740. [Google Scholar]
- Rahman, N.; Hashem, S.; Akther, S.; Jothi, J.S. Impact of various extraction methods on fatty acid profile, physicochemical properties, and nutritional quality index of Pangus fish oil. Food Sci. Nutr. 2023, 11, 4688–4699. [Google Scholar] [CrossRef] [PubMed]
- Zaidul, I.S.M.; Norulaini, N.A.N.; Omar, A.K.M.; Smith, R.L., Jr. Supercritical carbon dioxide (SC-CO2) extraction of palm kernel oil from palm kernel. J. Food Eng. 2007, 73, 1007–1014. [Google Scholar] [CrossRef]
- Ivanova, M.; Hanganu, A.; Dumitriu, R.; Mihaela Tociu, M.; Ivanov, G.; Stavarache, C.; Popescu, L.; Ghendov-Mosanu, A.; Sturza, R.; Deleanu, C.; et al. Saponification value of fats and oils as determined from 1H-NMR data: The case of dairy fats. Foods 2022, 11, 1466. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Md Saleh, N. Parameter effects and optimisation in supercritical fluid extraction of phenolic compounds from Labisia pumila. Separations 2022, 9, 385. [Google Scholar] [CrossRef]
- Wang, T.; Hicks, K.B.; Moreau, R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J. Am. Oil Chem. Soc. 2002, 79, 1201–1206. [Google Scholar] [CrossRef]
- Ibrahim, N.; Zakaria, A.J.; Ismail, Z.; Mohd, K.S. Antibacterial and phenolic content of propolis produced by two Malaysian stingless bees, Heterotrigona itama and Geniotrigona thoracica. Int. J. Pharmacogn. Pharm. Res. 2016, 8, 156–161. [Google Scholar]
- Pratami, D.K.; Mun’im, A.; Sundowo, A.; Sahlan, M. Phytochemical profile and antioxidant activity of propolis ethanolic extract from Tetragonula bee. Pharmacogn. J. 2018, 10, 128–135. [Google Scholar] [CrossRef]
- Fikri, A.M.; Sulaeman, A.; Marliyati, S.A.; Fahrudin, M. Antioxidant activity and total phenolic content of stingless bee propolis from Indonesia. J. Apic. Sci. 2019, 63, 139–147. [Google Scholar] [CrossRef]
- Xiong, K.; Chen, Y. Supercritical carbon dioxide extraction of essential oil from tangerine peel: Experimental optimization and kinetics modelling. Chem. Eng. Res. Des. 2020, 164, 412–423. [Google Scholar] [CrossRef]
- Krešić, G.; Vulić, A.; Bačun, L.D.; Lešić, T.; Želježić, D.; Pleadin, J. Nutritive composition and lipid quality indices of commercially available filleted fish. Food Health Dis. Sci. Prof. J. Nut. Diet. 2019, 8, 67–73. [Google Scholar]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of dietary incorporation of linseed alone or together with tomato-red pepper mix on laying hens’ egg yolk fatty acids profile and health lipid indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Wondołowska-Grabowska, A. Quality Evaluation indices for soybean oil in relation to cultivar, application of N fertiliser and seed inoculation with Bradyrhizobium japonicum. Foods 2022, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Fernandes, C.E.; da Silva Vasconcelos, M.A.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; de Melo Filho, A.B. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Chee, F.L.; Iqbal, S.; Ismail, M. Effects of supercritical fluid extraction conditions on yield of protein from defatted rice bran. J. Chem. Soc. Pak. 2013, 35, 192–197. [Google Scholar]
| Sample | Moisture Content (%) | Protein (%) | Lipid (%) | Ash (%) | Crude Fiber (%) | Carbohydrate (%) |
|---|---|---|---|---|---|---|
| Bee brood | 71.59 ± 0.52 a | 10.22 ± 0.18 b | 1.57 ± 0.38 b | 2.30 ± 0.04 b | 12.17 ± 0.71 a | 2.15 ± 0.97 b |
| Tray-dried bee brood | 2.27 ± 0.05 b | 41.72 ± 0.71 a | 34.64 ± 0.48 a | 3.25 ± 0.06 a | 3.37 ± 0.08 b | 15.88 ± 0.60 a |
| Extraction Conditions | %Crude Oil | Acid Value (mg KOH/g Oil) | Iodine Value (g I2/100 g Oil) | Saponification Value (mg KOH/g Oil) | Peroxide Value (meqO2/kg Oil) | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (Bar) | |||||
| 40 | 180 | 6.14 ± 0.82 c | 49.32 ± 0.76 a | 39.34 ± 0.64 a | 292.40 ± 1.10 a | 9.14 ± 0.46 ab |
| 40 | 200 | 7.46 ± 1.57 abc | 33.72 ± 0.82 c | 30.54 ± 0.44 b | 277.40 ± 0.51 b | 9.27 ± 0.52 ab |
| 40 | 220 | 8.67 ± 1.35 a | 37.89 ± 0.51 b | 21.22 ± 0.65 d | 188.60 ± 1.70 d | 8.79 ± 0.17 b |
| 50 | 180 | 4.21 ± 0.60 d | 38.43 ± 0.65 b | 25.26 ± 0.39 c | 209.57 ± 1.19 c | 9.27 ± 0.48 ab |
| 50 | 200 | 6.86 ± 1.39 bc | 33.23 ± 0.49 c | 20.97 ± 0.40 d | 171.48 ± 1.36 e | 9.60 ± 0.30 a |
| 50 | 220 | 8.05 ± 0.69 ab | 27.58 ± 0.90 e | 16.04 ± 0.88 e | 157.62 ± 0.91 f | 9.34 ± 0.16 ab |
| 60 | 180 | 3.45 ± 0.43 d | 29.70 ± 0.94 d | 11.89 ± 0.64 f | 130.66 ± 0.79 g | 9.48 ± 0.04 ab |
| 60 | 200 | 4.21 ± 0.56 d | 27.59 ± 0.90 e | 10.83 ± 0.81 g | 115.46 ± 1.38 h | 9.82 ± 0.26 a |
| 60 | 220 | 6.22 ± 1.26 c | 25.46 ± 0.65 f | 7.55 ± 0.19 h | 98.91 ± 1.24 i | 9.63 ± 0.20 a |
| Extraction Conditions | Moisture Content (%) | Protein (%) | Lipid (%) | Crude Oil (%) | Ash (%) | Carbohydrate (%) | |
|---|---|---|---|---|---|---|---|
| Temeprature (°C) | Pressure (Bar) | ||||||
| 40 | 180 | 2.22 ± 0.04 c | 43.83 ± 0.16 ab | 31.00 ± 0.99 bc | 2.95 ± 0.38 ab | 0.26 ± 0.08 d | 19.75 ± 1.14 ab |
| 40 | 200 | 2.14 ± 0.05 c | 43.78 ± 1.27 ab | 30.49 ± 1.66 bc | 2.66 ± 0.26 bc | 0.97 ± 0.10 bc | 19.97 ± 3.15 a |
| 40 | 220 | 2.36 ± 0.15 c | 45.29 ± 0.72 a | 30.22 ± 1.46 c | 2.58 ± 0.35 bc | 0.96 ± 0.49 bc | 18.59 ± 1.28 ab |
| 50 | 180 | 2.03 ± 0.14 c | 42.70 ± 0.55 b | 32.74 ± 1.41 abc | 2.73 ± 0.01 bc | 1.09 ± 0.08 b | 18.71 ± 1.85 ab |
| 50 | 200 | 2.02 ± 0.26 c | 43.40 ± 0.53 b | 31.73 ± 0.43 bc | 2.55 ± 0.07 bc | 0.71 ± 0.07 c | 19.59 ± 0.38 ab |
| 50 | 220 | 6.84 ± 0.21 b | 43.16 ± 0.70 b | 31.10 ± 1.83 bc | 2.50 ± 0.02 c | 2.23 ± 0.06 a | 14.18 ± 1.79 c |
| 60 | 180 | 7.57 ± 0.32 a | 42.33 ± 0.76 b | 34.44 ± 0.45 a | 2.62 ± 0.05 bc | 2.12 ± 0.19 a | 10.92 ± 0.28 d |
| 60 | 200 | 7.49 ± 0.26 a | 42.43 ± 1.56 b | 34.18 ± 1.65 a | 2.79 ± 0.15 abc | 2.27 ± 0.06 a | 10.83 ± 0.51 d |
| 60 | 220 | 1.62 ± 0.16 d | 43.17 ± 1.29 b | 32.79 ± 1.10 ab | 3.12 ± 0.33 a | 2.26 ± 0.02 a | 17.05 ± 0.84 b |
| Extraction Conditions | DPPH (μmol TE/g Oil) | ABTS (μmol TE/g Oil) ns | FRAP (μmol TE/g Oil) | TPC (μg GAE/g Oil) | TFC (μg QE/g Oil) | TCC (μg βE/g Oil) | |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (Bar) | ||||||
| 40 | 180 | 2.78 ± 0.12 b | 1.32 ± 0.20 | 1.33 ± 0.08 d | 56.13 ± 3.13 c | 3.08 ± 0.22 d | 1.52 ± 0.07 f |
| 40 | 200 | 2.34 ± 0.16 c | 1.31 ± 0.13 | 1.41 ± 0.18 d | 62.24 ± 3.08 abc | 10.45 ± 0.30 a | 2.37 ± 0.08 c |
| 40 | 220 | 2.19 ± 0.26 cd | 1.35 ± 0.27 | 1.60 ± 0.25 d | 62.63 ± 1.26 abc | 3.62 ± 0.23 c | 7.32 ± 0.07 a |
| 50 | 180 | 2.07 ± 0.07 d | 1.25 ± 0.20 | 2.54 ± 0.10 c | 63.74 ± 1.17 ab | 3.21 ± 0.30 d | 2.57 ± 0.15 b |
| 50 | 200 | 1.18 ± 0.09 f | 1.38 ± 0.19 | 2.72 ± 0.08 bc | 66.14 ± 5.25 ab | 0.81 ± 0.09 f | 1.74 ± 0.16 e |
| 50 | 220 | 3.06 ± 0.04 a | 1.39 ± 0.08 | 2.87 ± 0.10 ab | 61.04 ± 2.45 bc | 0.21 ± 0.05 g | 2.59 ± 0.11 b |
| 60 | 180 | 2.34 ± 0.15 c | 1.60 ± 0.16 | 3.19 ± 0.38 a | 66.47 ± 2.15 ab | 10.53 ± 0.20 a | 1.05 ± 0.08 g |
| 60 | 200 | 1.40 ± 0.03 ef | 1.53 ± 0.05 | 2.91 ± 0.30 ab | 67.05 ± 6.70 ab | 9.30 ± 0.19 b | 1.95 ± 0.07 d |
| 60 | 220 | 1.51 ± 0.09 e | 1.51 ± 0.06 | 2.98 ± 0.16 ab | 69.65 ± 0.62 a | 1.37 ± 0.22 e | 1.74 ± 0.16 e |
| Qualities of Extracted Oil | Extraction Temperatures | ||
|---|---|---|---|
| 40 °C | 50 °C | 60 °C | |
| % Crude oil ns | 21.41 ± 1.07 | 21.50 ± 2.38 | 25.37 ± 1.52 |
| Acid value (mg KOH/g) | 32.51 ± 0.31 a | 20.97 ± 0.61 b | 10.75 ± 0.23 c |
| Iodine value (g I2/100 g) | 23.06 ± 0.68 a | 12.52 ± 0.65 b | 10.34 ± 0.35 c |
| Saponification value (mg KOH/g) ns | 190.52 ± 2.12 | 181.79 ± 8.05 | 180.89 ± 2.48 |
| Peroxide value (meqO2/kg) | 7.28 ± 0.55 b | 8.95 ± 1.98 ab | 11.62 ± 0.21 a |
| TPC (µg GAE/g oil) | 67.87 ± 1.12 b | 71.58 ± 2.02 a | 71.05 ± 0.88 a |
| TFC (µg QE/g oil) ns | 1.04 ± 0.19 | 0.85 ± 0.00 | 0.91 ± 0.11 |
| DPPH (µmol TE/g oil) | 2.11 ± 0.09 ab | 2.25 ± 0.07 a | 1.97 ± 0.05 b |
| ABTS (µmol TE/g oil) ns | 0.41 ± 0.03 | 0.46 ± 0.05 | 0.41 ± 0.04 |
| FRAP (µmol TE/g oil) | 1.31 ± 0.02 b | 1.44 ± 0.09 a | 1.40 ± 0.11 ab |
| Defatted tray-dried bee brood meal | |||
| Moisture content (%) | 6.19 ± 0.04 b | 6.45 ± 0.02 a | 6.41 ± 0.04 a |
| Protein (%) | 50.03 ± 1.01 b | 50.36 ± 0.58 b | 52.98 ± 0.90 a |
| Lipid (%) | 11.29 ± 0.26 a | 10.59 ± 0.15 a | 6.05 ± 0.60 b |
| Crude fiber (%) ns | 2.70 ± 0.43 | 2.53 ± 0.27 | 2.54 ± 0.15 |
| Ash (%) ns | 2.79 ± 0.12 | 2.86 ± 0.03 | 2.97 ± 0.22 |
| Carbohydrate (%) | 26.99 ± 0.26 b | 27.21 ± 0.91 b | 29.05 ± 1.26 a |
| Fatty Acid Composition (g/100 g) | Tray-Dried Bee Brood Oil Extracted by Using Supercritical CO2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 40 °C, 180 Bar | 40 °C, 200 Bar | 40 °C, 220 Bar | 50 °C, 180 Bar | 50 °C, 200 Bar | 50 °C, 220 Bar | 60 °C, 180 Bar | 60 °C, 200 Bar | 60 °C, 220 Bar | |
| Butyric acid (C4:0) | ND | 0.06 ± 0.00 cd | 0.05 ± 0.00 d | 0.09 ± 0.00 b | 0.16 ± 0.02 a | 0.07 ± 0.01 c | ND | ND | ND |
| Caproic acid (C6:0) | ND | 0.01 ± 0.00 | ND | ND | ND | ND | ND | ND | ND |
| Caprylic acid (C8:0) | ND | 0.01 ± 0.00 | ND | ND | ND | ND | ND | ND | ND |
| Capric acid (C10:0) | ND | 0.03 ± 0.00 | ND | ND | ND | 0.03 ± 0.00 | 0.04 ± 0.01 | ND | ND |
| Lauric acid (C12:0) | 0.26 ± 0.00 d | 0.33 ± 0.01 bcd | 0.33 ± 0.02 abcd | 0.33 ± 0.01 bcd | 0.30 ± 0.05 de | 0.37 ± 0.01 ab | 0.36 ± 0.03 abc | 0.38 ± 0.04 a | 0.32 ± 0.01 cd |
| Myristic acid (C14:0) ns | 3.17 ± 0.06 | 3.50 ± 0.02 | 3.77 ± 0.01 | 3.66 ± 0.01 | 3.49 ± 0.07 | 3.82 ± 0.03 | 3.30 ± 0.03 | 3.62 ± 0.07 | 3.83 ± 0.03 |
| Myristoleic acid (C14:1) | ND | 0.03 ± 0.00 | ND | ND | ND | ND | ND | ND | ND |
| Pentadecylic acid (C15:0) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Pentadecenoic acid (cis-10) (C15:1) | 0.05 ± 0.00 | ND | ND | ND | ND | ND | ND | ND | ND |
| Palmitic acid (C16:0) | 36.33 ± 0.44 e | 38.55 ± 0.02 c | 42.91 ± 0.13 b | 42.58 ± 0.03 b | 37.43 ± 0.10 d | 44.91 ± 0.89 a | 37.57 ± 0.16 d | 43.02 ± 0.09 b | 44.52 ± 0.06 a |
| Palmitoleic acid (C16:1) | 0.37 ± 0.03 c | 0.53 ± 0.01 bc | 0.52 ± 0.01 bc | 0.53 ± 0.03 bc | 0.47 ± 0.01 b | 0.52 ± 0.01 bc | 0.50 ± 0.06 bc | 0.56 ± 0.01 a | 0.53 ± 0.02 bc |
| Margaric acid (C17:0) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heptadecenoic acid(cis-10) (C17:1) | ND | 0.12 ± 0.01 c | 0.08 ± 0.00 e | 0.10 ± 0.00 d | 0.15 ± 0.02 b | 0.06 ± 0.00 e | 0.25 ± 0.01 a | 0.12 ± 0.00 c | 0.04 ± 0.01 f |
| Stearic acid (C18:0) | 5.75 ± 0.08 d | 6.07 ± 0.04 c | 6.73 ± 0.01 a | 6.30 ± 0.01 b | 5.64 ± 0.02 d | 6.82 ± 0.16 a | 5.09 ± 0.03 e | 5.95 ± 0.08 c | 6.73 ± 0.03 a |
| Elaidic acid (C18:1 n9t) | 0.40 ± 0.12 a | 0.17 ± 0.02 c | 0.14 ± 0.00 cd | 0.17 ± 0.01 c | 0.17 ± 0.02 c | 0.08 ± 0.03 de | 0.32 ± 0.05 b | 0.13 ± 0.00 cd | 0.05 ± 0.01 e |
| Oleic acid (C18:1 n9c) | 29.90 ± 0.60 g | 32.55 ± 0.02 e | 34.36 ± 0.08 c | 34.78 ± 0.04 c | 31.74 ± 0.11 f | 35.98 ± 0.81 b | 29.55 ± 0.11 g | 33.66 ± 0.00 d | 37.32 ± 0.04 a |
| Linoleic acid (C18:2 n6c) | 11.15 ± 0.15 a | 7.59 ± 0.01 c | 4.50 ± 0.10 f | 5.36 ± 0.03 e | 9.04 ± 0.05 b | 3.75 ± 0.06 g | 11.23 ± 0.04 a | 5.69 ± 0.06 d | 2.23 ± 0.02 h |
| Eicosadienoic acid (C20:0) | ND | 0.27 ± 0.00 a | 0.12 ± 0.01 cd | 0.11 ± 0.00 cd | 0.10 ± 0.01 d | 0.12 ± 0.02 cd | 0.18 ± 0.04 b | 0.14 ± 0.04 c | 0.13 ± 0.01 cd |
| γ-Linolenic acid (C18:3 n6) ns | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Gondoic acid (C20:1) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| α-Linolenic acid (C18:3 n3) | 0.57 ± 0.07 e | 0.61 ± 0.04 de | 0.52 ± 0.01 e | 0.72 ± 0.01 c | 0.85 ± 0.10 b | 0.53 ± 0.05 e | 1.34 ± 0.05 a | 0.76 ± 0.06 c | 0.68 ± 0.01 cd |
| Eicosadienoic acid (C20:2) | 6.46 ± 0.12 a | 4.89 ± 0.03 c | 2.68 ± 0.08 d | 2.52 ± 0.02 e | 4.90 ± 0.04 c | 1.93 ± 0.17 f | 5.26 ± 0.11 b | 2.67 ± 0.01 de | 1.08 ± 0.03 g |
| Behenic acid (C22:0) | 0.25 ± 0.07 a | 0.24 ± 0.00 a | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.07 ± 0.02 bc | 0.02 ± 0.00 c | 0.09 ± 0.02 b | ND | 0.21 ± 0.01 a |
| Dihomo-γ-linolenic acid (C20:3 n6) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Eicosatrienoic acid (C20:3 n3) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Tricosylic acid (C23:0) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Arachidonic acid (C20:4 n6) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Docosadienoic acid (C22:2) | 4.69 ± 0.77 a | 3.70 ± 0.02 b | 2.62 ± 0.03 d | 1.54 ± 0.02 e | 3.02 ± 0.00 cd | 1.26 ± 0.17 e | 3.37 ± 0.01 bc | 1.61 ± 0.02 e | 0.56 ± 0.01 f |
| Eicosapentaenoic acid (C20:5 n3) | 0.76 ± 0.01 g | 0.75 ± 0.02 g | 0.59 ± 0.01 h | 1.21 ± 0.03 e | 2.48 ± 0.03 a | 1.02 ± 0.05 f | 1.54 ± 0.06 d | 1.68 ± 0.02 c | 1.75 ± 0.02 b |
| Nervonic acid (C24:1n9) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| SFAs | 45.77 ± 0.50 g | 49.07 ± 0.02 e | 53.99 ± 0.13 c | 53.11 ± 0.02 d | 47.18 ± 0.21 f | 54.87 ± 1.15 b | 46.63 ± 0.14 f | 53.11 ± 0.11 d | 55.76 ± 0.03 a |
| MUFAs | 30.33 ± 0.55 g | 33.39 ± 0.02 e | 35.11 ± 0.10 c | 35.54 ± 0.05 c | 32.53 ± 0.14 f | 36.64 ± 0.84 b | 30.63 ± 0.03 g | 34.48 ± 0.01 d | 37.94 ± 0.02 a |
| PUFAs | 23.63 ± 0.94 a | 17.54 ± 0.02 d | 10.91 ± 0.22 f | 11.35 ± 0.07 f | 20.29 ± 0.11 c | 8.49 ± 0.32 g | 22.74 ± 0.14 b | 12.41 ± 0.10 e | 6.30 ± 0.05 h |
| PUFAs/SFAs | 0.52 ± 0.03 a | 0.36 ± 0.00 d | 0.20 ± 0.00 f | 0.21 ± 0.00 f | 0.43 ± 0.00 c | 0.15 ± 0.01 g | 0.49 ± 0.00 b | 0.23 ± 0.00 e | 0.11 ± 0.00 h |
| n-6/n-3 | 8.42 ± 0.56 a | 5.58 ± 0.07 b | 4.05 ± 0.03 c | 2.78 ± 0.02 d | 2.72 ± 0.11 de | 2.41 ± 0.04 ef | 3.90 ± 0.01 c | 2.33 ± 0.05 f | 0.92 ± 0.00 g |
| IA | 1.15 ± 0.01 c | 1.25 ± 0.00 bc | 1.43 ± 0.00 a | 1.34 ± 0.00 ab | 1.15 ± 0.01 c | 1.33 ± 0.22 ab | 1.14 ± 0.01 c | 1.36 ± 0.01 ab | 1.41 ± 0.00 b |
| IT | 1.82 ± 0.01 e | 1.94 ± 0.01 cd | 2.28 ± 0.01 a | 1.98 ± 0.01 c | 1.50 ± 0.02 f | 2.15 ± 0.10 b | 1.54 ± 0.01 f | 1.89 ± 0.02 d | 1.94 ± 0.00 cd |
| H/H ratio | 1.35 ± 0.03 a | 1.18 ± 0.00 c | 0.96 ± 0.01 de | 0.99 ± 0.00 d | 1.26 ± 0.01 b | 0.93 ± 0.05 ef | 1.27 ± 0.01 b | 0.98 ± 0.00 d | 0.90 ± 0.00 f |
| Fatty Acid Composition (g/100 g) | Oil Obtained from Tray-Dried Bee Brood, Extracted Using Supercritical CO2 | ||
|---|---|---|---|
| 40 °C, 600 Bar, 1 h | 50 °C, 600 Bar, 1 h | 60 °C, 600 Bar, 1 h | |
| Butyric acid (C4:0) | ND | ND | ND |
| Caproic acid (C6:0) | ND | ND | ND |
| Caprylic acid (C8:0) | ND | ND | ND |
| Capric acid (C10:0) | ND | ND | ND |
| Lauric acid (C12:0) | 0.17 ± 0.01 | ND | 0.27 ± 0.02 |
| Myristic acid (C14:0) | 2.88 ± 0.02 c | 3.18 ± 0.03 b | 3.45 ± 0.07 a |
| Myristoleic acid (C14:1) | ND | ND | ND |
| Pentadecylic acid (C15:0) | ND | ND | ND |
| Pentadecenoic acid (cis-10) (C15:1) | ND | ND | ND |
| Palmitic acid (C16:0) | 44.09 ± 0.15 a | 42.63 ± 0.14 b | 40.43 ± 0.08 c |
| Palmitoleic acid (C16:1) ns | 0.41 ± 0.01 | 0.44 ± 0.06 | 0.45 ± 0.01 |
| Margaric acid (C17:0) | ND | ND | ND |
| Heptadecenoic acid(cis-10) (C17:1) | 0.02 ± 0.00 | 0.07 ± 0.05 | ND |
| Stearic acid (C18:0) | 10.01 ± 0.03 a | 8.60 ± 0.09 b | 7.17 ± 0.15 c |
| Elaidic acid (C18:1 n9t) | 0.01 ± 0.01 | 0.54 ± 0.11 | ND |
| Oleic acid (C18:1 n9c) | 38.54 ± 0.09 a | 36.35 ± 0.15 b | 34.40 ± 0.15 c |
| Linoleic acid (C18:2 n6c) | 1.29 ± 0.03 c | 2.99 ± 0.02 b | 5.75 ± 0.03 a |
| Eicosadienoic acid (C20:0) | 0.23 ± 0.04 | 0.22 ± 0.04 | ND |
| γ-Linolenic acid (C18:3 n6) | ND | ND | ND |
| Gondoic acid (C20:1) | ND | ND | ND |
| α-Linolenic acid (C18:3 n3) | 0.34 ± 0.05 | ND | 0.55 ± 0.05 |
| Eicosadienoic acid (C20:2) | 0.96 ± 0.03 c | 2.11 ± 0.06 b | 3.61 ± 0.01 a |
| Behenic acid (C22:0) | 0.08 ± 0.02 b | 0.15 ± 0.03 a | 0.19 ± 0.02 a |
| Dihomo-γ-linolenic acid (C20:3 n6) | ND | ND | ND |
| Eicosatrienoic acid (C20:3 n3) | ND | ND | ND |
| Tricosylic acid (C23:0) | ND | ND | ND |
| Arachidonic acid (C20:4 n6) | ND | ND | ND |
| Docosadienoic acid (C22:2) | 0.68 ± 0.08 c | 1.60 ± 0.06 b | 2.26 ± 0.02 a |
| Eicosapentaenoic acid (C20:5 n3) | 0.28 ± 0.01 c | 0.90 ± 0.04 b | 1.47 ± 0.02 a |
| Nervonic acid (C24:1n9) | ND | ND | ND |
| SFAs | 57.46 ± 0.14 a | 54.78 ± 0.20 b | 51.51 ± 0.07 c |
| MUFAs | 38.99 ± 0.09 a | 37.38 ± 0.10 b | 34.85 ± 0.16 c |
| PUFAs | 3.54 ± 0.18 c | 7.59 ± 0.18 b | 13.64 ± 018 a |
| PUFAs/SFAs | 0.06 ± 0.00 c | 0.14 ± 0.00 b | 0.26 ± 0.00 a |
| n-6/n-3 | 2.11 ± 0.15 c | 3.33 ± 0.14 a | 2.85 ± 0.07 b |
| IA | 1.36 ± 0.01 a | 1.34 ± 0.00 b | 1.28 ± 0.01 c |
| IT | 2.54 ± 0.03 a | 2.35 ± 0.01 b | 1.91 ± 0.00 c |
| H/H ratio | 0.89 ± 0.01 c | 0.96 ± 0.01 b | 1.09 ± 0.00 a |
| Amino Acid Profile (mg/100 g) | Tray-Dried Bee Brood Sample | Defatted Tray-Dried Bee Brood Meal after Supercritical CO2 Extraction at 50 °C, 220 Bar, 1.5 h | Defatted Tray-Dried Bee Brood Meal after Supercritical CO2 Extraction at 50 °C, 600 Bar, 1 h |
|---|---|---|---|
| Alanine | 2471.13 ± 1.57 a | 2397.86 ± 16.38 b | 1559.52 ± 7.56 c |
| Arginine | 2879.96 ± 104.09 a | 2839.02 ± 44.96 a | 1760.73 ± 36.34 b |
| Aspartic acid | 5557.26 ± 14.59 a | 5449.11 ± 19.74 b | 3070.62 ± 35.76 c |
| L-Cystine | 166.87 ± 0.47 b | 180.71 ± 0.68 b | 1406.06 ± 312.71 a |
| Glutamic acid | 8143.65 ± 83.23 a | 7996.52 ± 62.42 a | 4747.52 ± 203.39 b |
| Glycine | 2055.04 ± 3.18 a | 1990.11 ± 10.61 b | 1293.27 ± 0.25 c |
| Histidine | 1093.49 ± 6.03 a | 1077.59 ± 10.97 a | 670.29 ± 2.63 b |
| Hydroxylysine | ND | ND | 25.02 ± 0.50 |
| Hydroxyproline | ND | ND | 5299.62 ± 125.18 |
| Isoleucine | 2318.10 ± 0.46 a | 2211.53 ± 15.77 b | 1391.06 ± 9.28 c |
| Leucine | 4219.74 ± 0.93 a | 4070.86 ± 29.68 b | 2421.40 ± 17.62 c |
| Lysine | 3651.46 ± 5.69 a | 3590.79 ± 30.49 a | 1973.57 ± 17.57 c |
| Methionine | 947.86 ± 0.53 a | 832.96 ± 4.74 b | 587.63 ± 8.49 c |
| Phenylalanine | 2015.32 ± 4.67 a | 1936.03 ± 24.53 a | 1239.75 ± 42.05 b |
| Proline | 3925.65 ± 24.02 a | 3751.22 ± 27.27 a | 1999.52 ± 109.50 c |
| Serine | 2567.61 ± 4.83 a | 2500.62 ± 20.81 b | 1440.52 ± 16.72 c |
| Threonine | 2342.49 ± 10.11 a | 2298.12 ± 18.95 a | 1284.71 ± 31.17 b |
| Tyrosine | 2769.48 ± 17.93 a | 2610.07 ± 26.88 b | 1658.07 ± 7.94 c |
| Valine | 2692.69 ± 19.05 | 2558.26 ± 16.16 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiriyacharee, P.; Chalermchat, Y.; Siriwoharn, T.; Jirarattanarangsri, W.; Tangjaidee, P.; Chaipoot, S.; Phongphisutthinant, R.; Pandith, H.; Muangrat, R. Utilizing Supercritical CO2 for Bee Brood Oil Extraction and Analysis of Its Chemical Properties. Foods 2024, 13, 2486. https://doi.org/10.3390/foods13162486
Wiriyacharee P, Chalermchat Y, Siriwoharn T, Jirarattanarangsri W, Tangjaidee P, Chaipoot S, Phongphisutthinant R, Pandith H, Muangrat R. Utilizing Supercritical CO2 for Bee Brood Oil Extraction and Analysis of Its Chemical Properties. Foods. 2024; 13(16):2486. https://doi.org/10.3390/foods13162486
Chicago/Turabian StyleWiriyacharee, Pairote, Yongyut Chalermchat, Thanyaporn Siriwoharn, Wachira Jirarattanarangsri, Pipat Tangjaidee, Supakit Chaipoot, Rewat Phongphisutthinant, Hataichanok Pandith, and Rattana Muangrat. 2024. "Utilizing Supercritical CO2 for Bee Brood Oil Extraction and Analysis of Its Chemical Properties" Foods 13, no. 16: 2486. https://doi.org/10.3390/foods13162486








