Process-Induced Molecular-Level Protein–Carbohydrate–Polyphenol Interactions in Milk–Tea Blends: A Review
Abstract
:1. Introduction
2. Bovine (Cow) Milk
2.1. Milk Protein
2.2. Carbohydrates in Milk
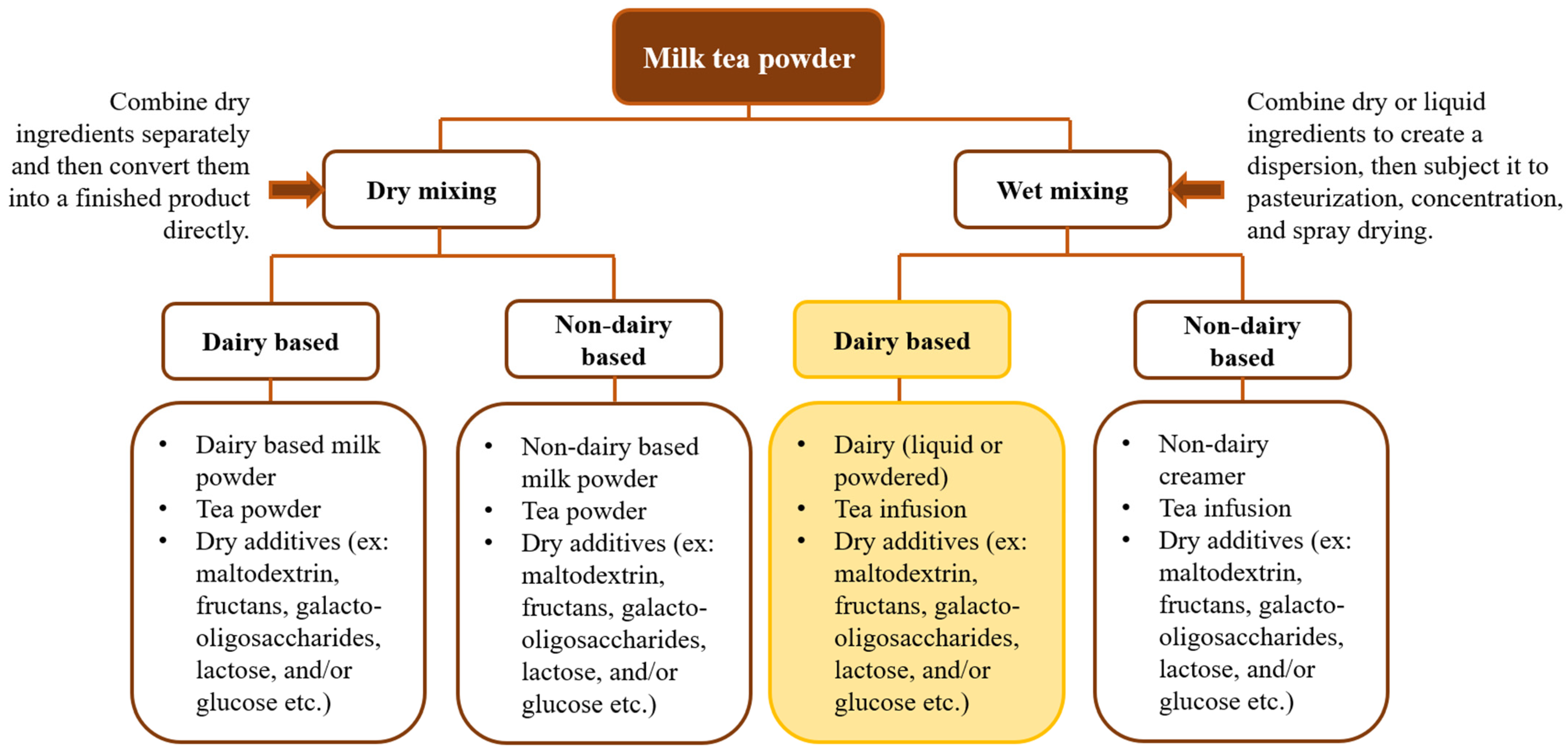
3. Milk–Tea Blends
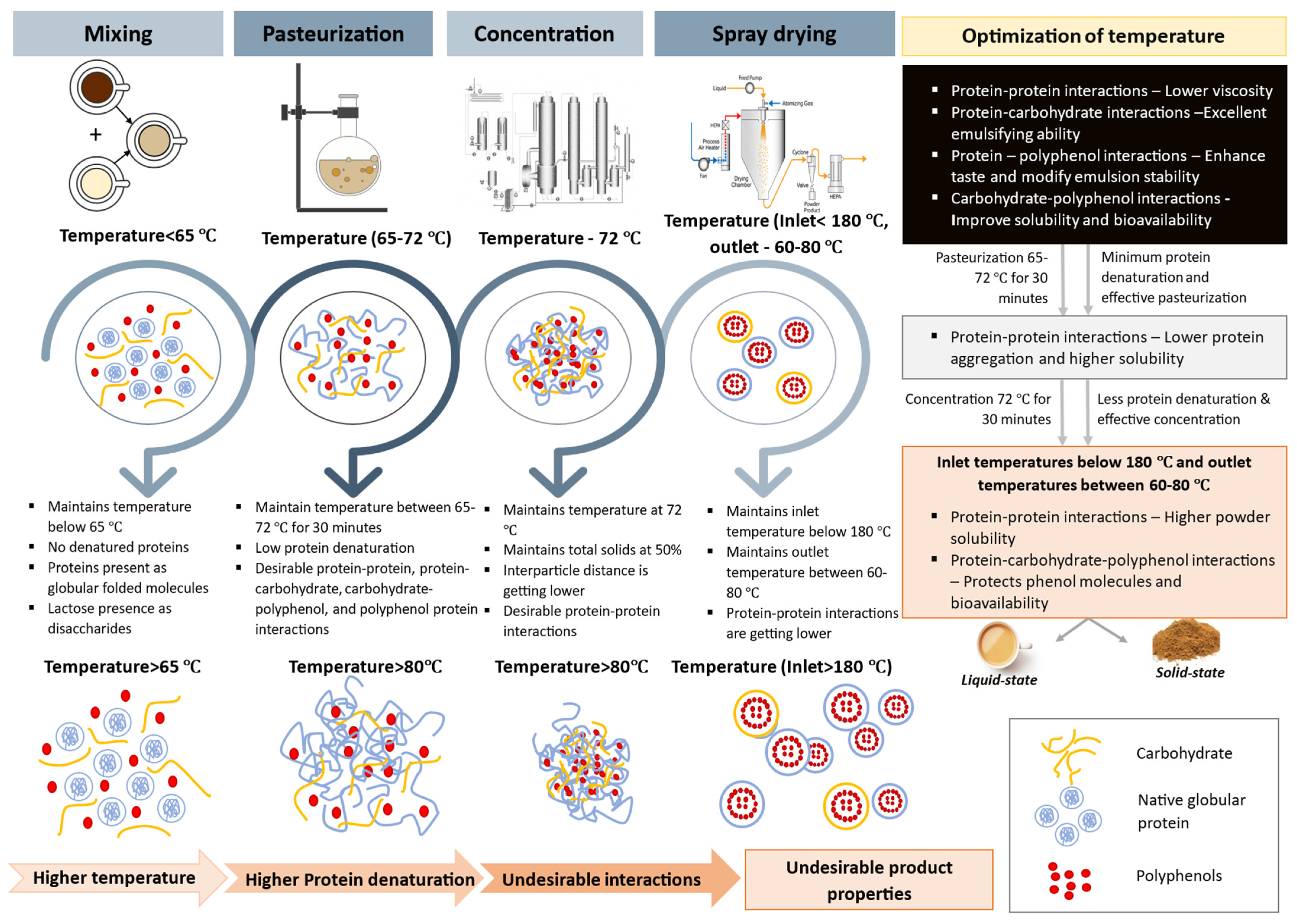
4. Interactions in Milk–Tea Blends: During Pasteurization, Concentration, and Spray Drying
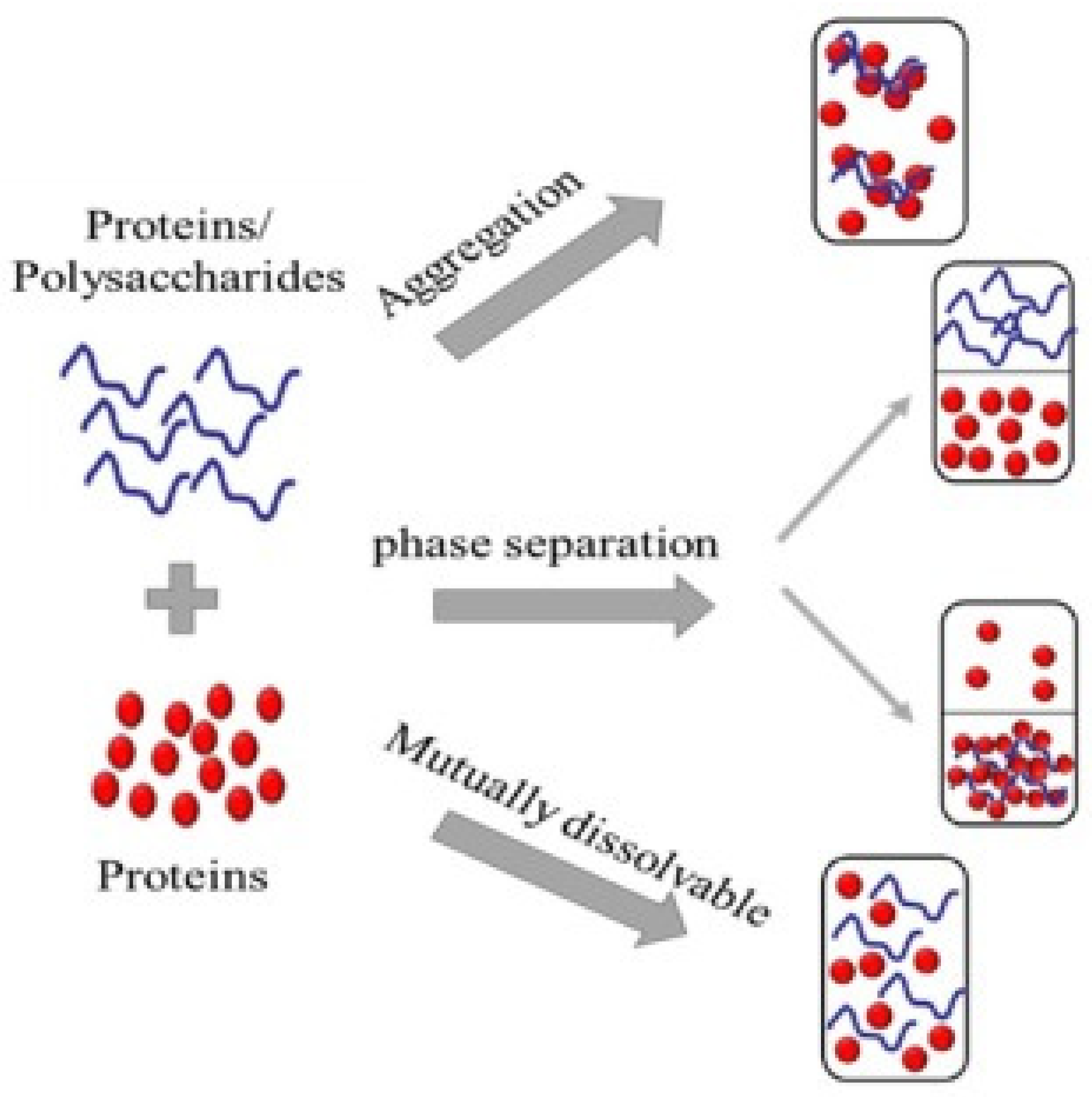
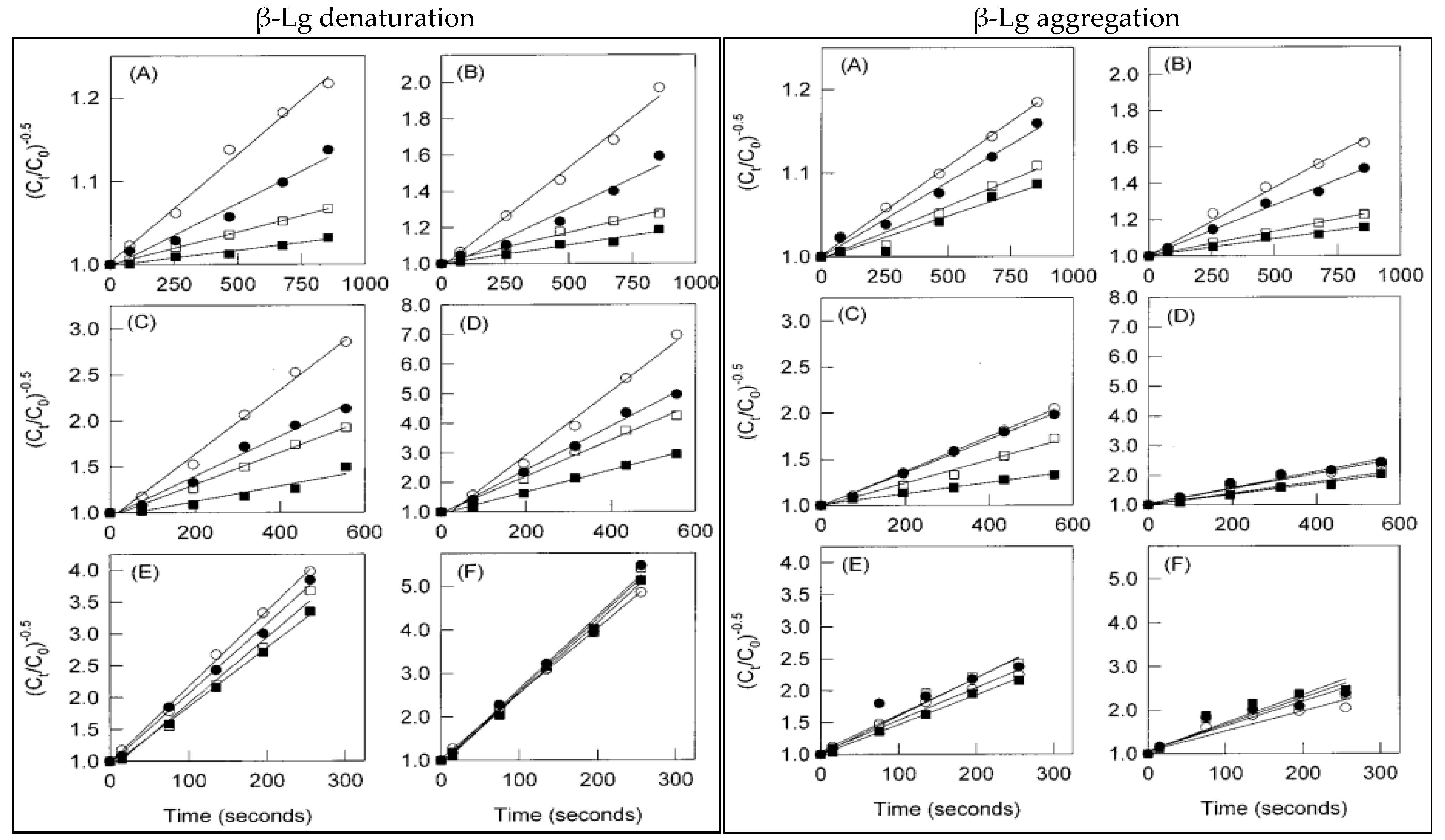
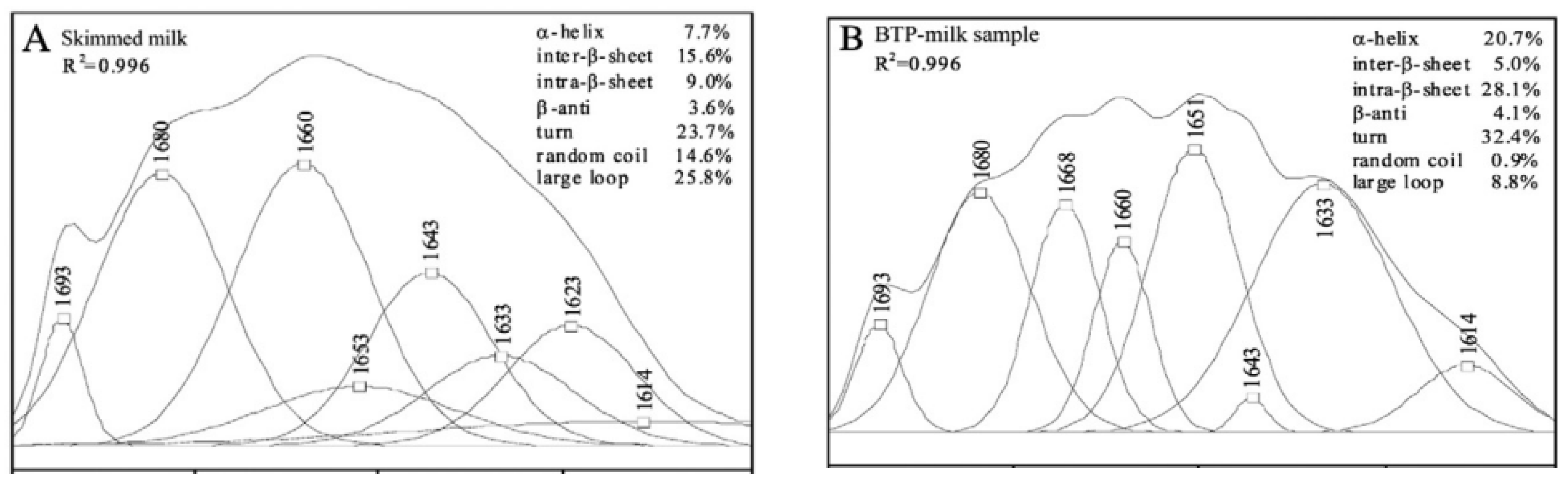
4.1. Protein–Protein Interactions
4.2. Protein–Carbohydrate Interactions
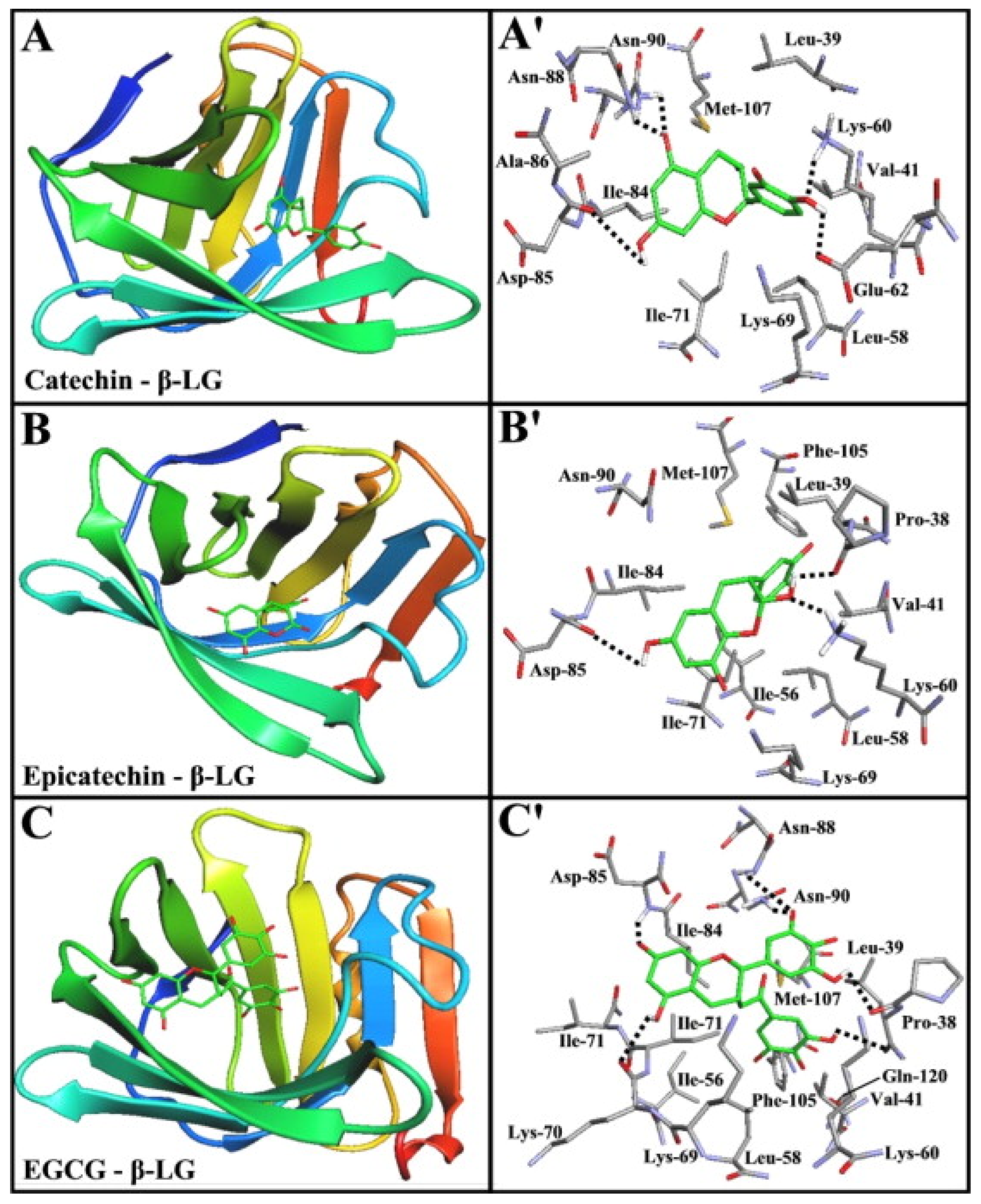
4.3. Protein–Polyphenol Interactions
4.4. Carbohydrate–Phenol Interactions
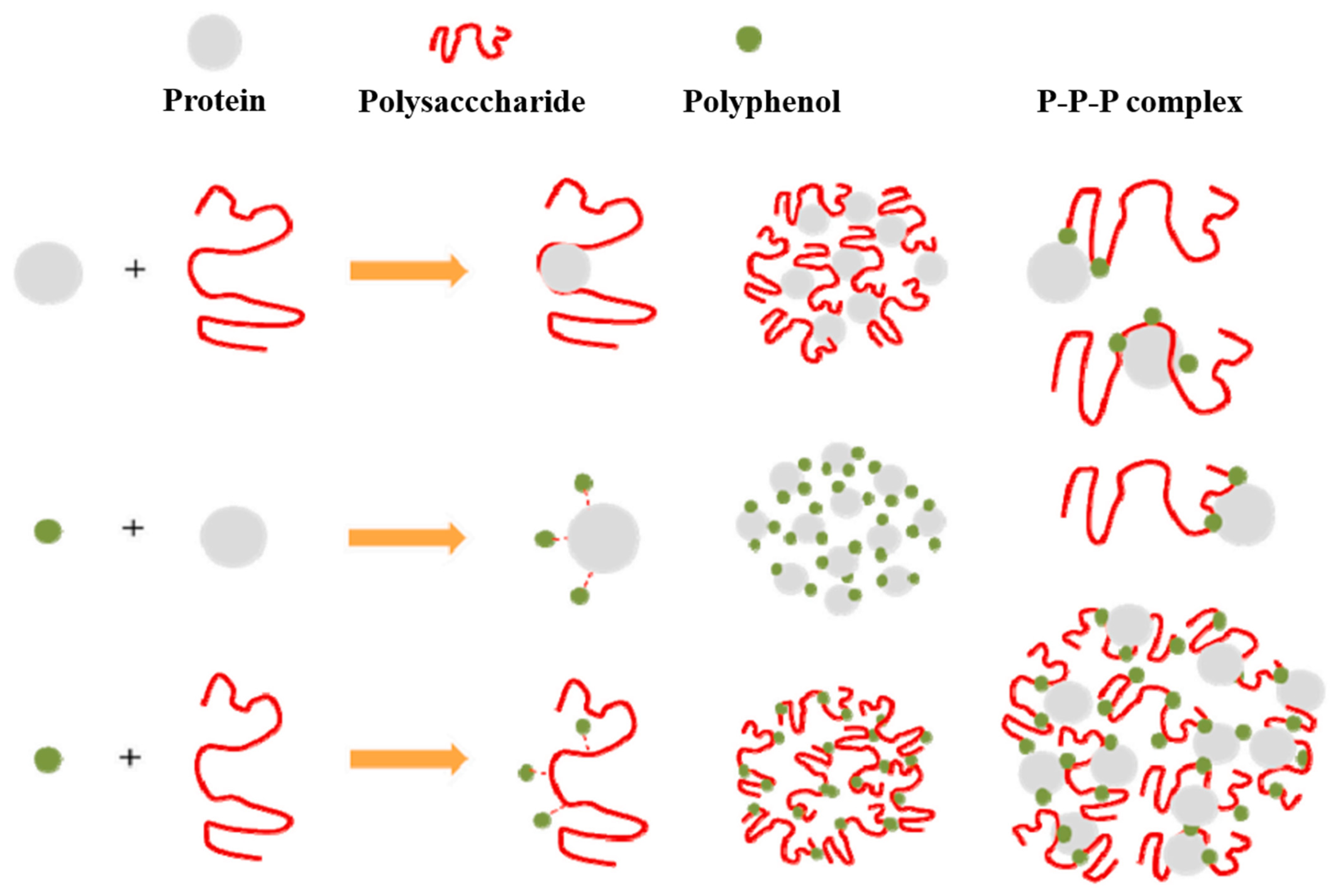
4.5. Protein–Carbohydrate–Polyphenol Interactions
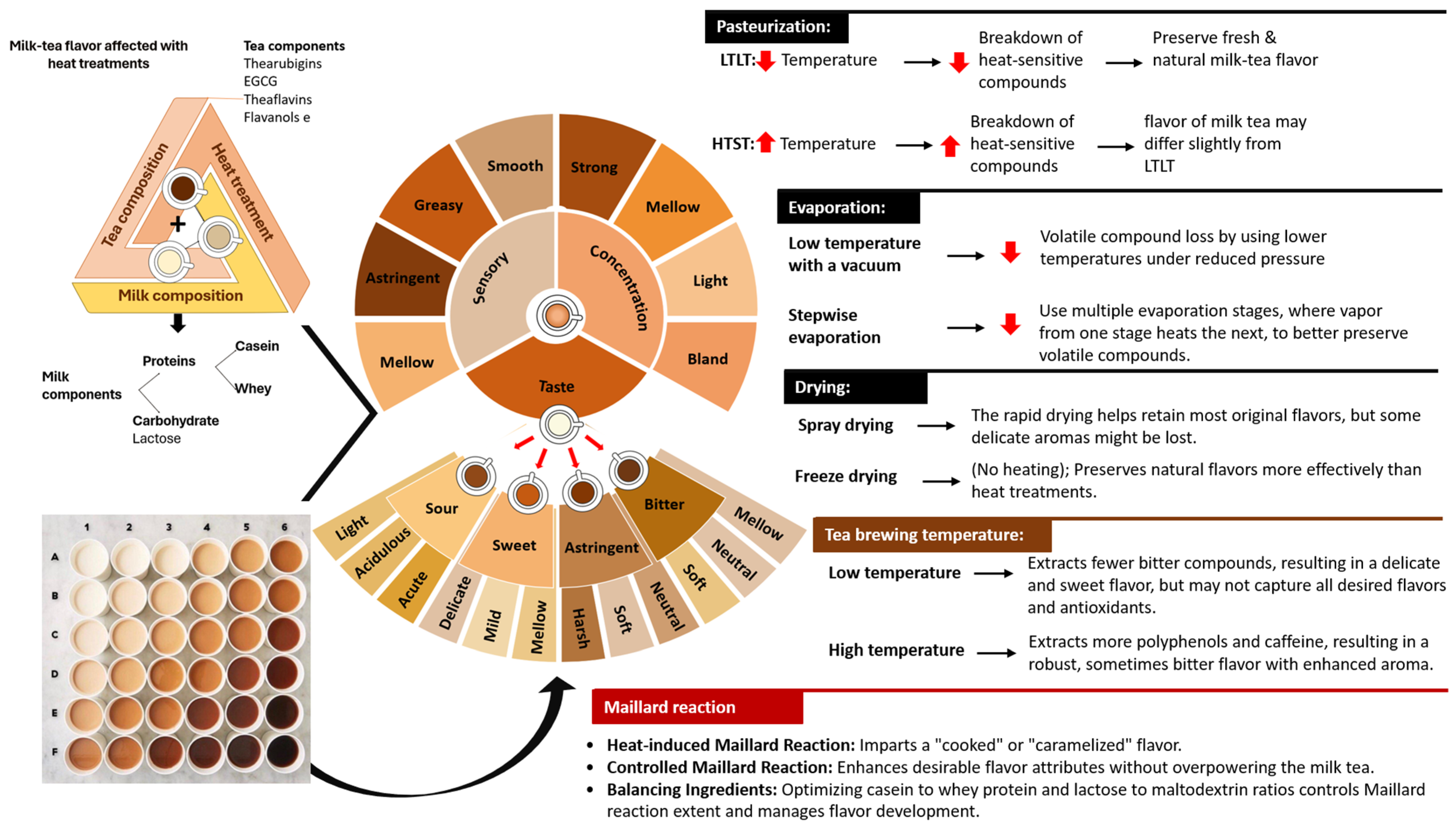
5. How Is the Overall Quality of Milk–Tea Affected by the Molecular Interactions of Its Composition?
6. Implications that Arise during the Current Milk–Tea Manufacturing Process
7. Future Trends
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, A.K.S.; Prasetyo, Y.T.; Libiran, M.A.D.C.; Lontoc, Y.M.A.; Lunaria, J.A.V.; Manalo, A.M.; Miraja, B.A.; Young, M.N.; Chuenyindee, T.; Persada, S.F.; et al. Consumer preference analysis on attributes of milk tea: A conjoint analysis approach. Foods 2021, 10, 1382. [Google Scholar] [CrossRef]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Wilms, L.C.; Beetstra, S.A.J.N.; Heijnen, C.G.M.; Voss, H.-P.; Bast, A. Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. J. Agric. Food Chem. 2002, 50, 1184–1187. [Google Scholar] [CrossRef]
- Weisburger, J.H. Tea and health: A historical perspective. Cancer Lett. 1997, 114, 315–317. [Google Scholar] [CrossRef]
- Du, S. The marketing strategy of the milk tea in china based on customer demand analysis. In Proceedings of the 2022 International Conference on Creative Industry and Knowledge Economy (CIKE 2022), Online, 25–27 March 2022; Volume 651, pp. 220–224. [Google Scholar] [CrossRef]
- Imran, A.; Sadiq Butt, M.; Saeed, F.; Sajid Arshad, M.; Sultan, T.; Sohaib, M. Effect of different time-solvent interactions on polyphenol content of milky tea. J. Food Process. Preserv. 2017, 41, e13039. [Google Scholar] [CrossRef]
- Hansika, T.; Wattage, P.; Lanka, S. Assessment on Factors Affecting towards Consumer Preference of Powdered Milk vs Fresh Milk in Sri Lanka: A Case of Rathnapura Divisional Secretariat Division. 2022; pp. 357–361. Available online: https://www.researchgate.net/publication/365669305_Assesment_on_factors_towards_consumer_preference_of_powdered_milk_vs_fresh_milk_in_Sri_Lanka_A_case_of_Rathnapura_divisional_secretariat_division (accessed on 4 March 2023).
- Kyle, J.A.M.; Morrice, P.C.; McNeill, G.; Duthie, G.G. Effects of infusion time and addition of milk on content and absorption of polyphenols from black tea. J. Agric. Food Chem. 2007, 55, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Addition of milk to tea infusions: Helpful or harmful? Evidence from in vitro and in vivo studies on antioxidant properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cui, C.; Zhu, Y.; Xia, X.; Jin, G.; Liu, C.; Li, Y.; Xue, X.; Hou, R. Effect of brewing conditions on the chemical and sensory profiles of milk tea. Food Chem. X 2022, 16, 100453. [Google Scholar] [CrossRef] [PubMed]
- Bista, A.; McCarthy, N.; O’Donnell, C.P.; O’Shea, N. Key parameters and strategies to control milk concentrate viscosity in milk powder manufacture. Int. Dairy J. 2021, 121, 105120. [Google Scholar] [CrossRef]
- Burke, N.; Zacharski, K.A.; Southern, M.; Hogan, P.; Ryan, M.P.; Adley, C.C. The dairy industry: Process, monitoring, standards, and quality. In Descriptive Food Science; IntechOpen: London, UK, 2018; p. 13. [Google Scholar] [CrossRef]
- Yildirim, N.; Genc, S. Energy and exergy analysis of a milk powder production system. Energy Convers. Manag. 2017, 149, 698–705. [Google Scholar] [CrossRef]
- Ackerman, D.L.; Craft, K.M.; Townsend, S.D. Infant food applications of complex carbohydrates: Structure, synthesis, and function. Carbohydr. Res. 2017, 437, 16–27. [Google Scholar] [CrossRef]
- Korir, M.W.; Wachira, F.N.; Wanyoko, J.K.; Ngure, R.M.; Khalid, R. The fortification of tea with sweeteners and milk and its effect on in vitro antioxidant potential of tea product and glutathione levels in an animal model. Food Chem. 2014, 145, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, K.P.; Chanin, R.; McDonald, C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes 2015, 6, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.Y.; Pranowo, D.; Sunarharum, W.B.; Prananto, Y.P.; Tansil, C.Z.L. Optimization on maltodextrin concentration and inlet spray drying temperature in producing edamame (glycine max l. Merr.) milk powder: Nutritional and microbiological profile. IOP Conf. Ser. Earth Environ. Sci. 2020, 515, 012064. [Google Scholar] [CrossRef]
- Jafari, S.; Jouki, M.; Soltani, M. Modification of physicochemical, structural, rheological, and organoleptic properties of sweetened condensed milk by maltodextrin, fructose, and lactose. J. Food Meas. Charact. 2021, 15, 3800–3810. [Google Scholar] [CrossRef]
- Al-Saadi, J.M.S.; Easa, A.M.; Deeth, H.C. Effect of lactose on cross-linking of milk proteins during heat treatments. Int. J. Dairy Technol. 2013, 66, 1–6. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A. Controlling milk protein interactions to enhance the reconstitution properties of whole milk powders—A minireview. Dairy Sci. Technol. 2010, 90, 123–136. [Google Scholar] [CrossRef]
- Stone, H. Example food: What are its sensory properties and why is that important? Npj Sci. Food 2018, 2, 11. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Halabi, A.; Deglaire, A.; Hamon, P.; Bouhallab, S.; Dupont, D.; Croguennec, T. Kinetics of heat-induced denaturation of proteins in model infant milk formulas as a function of whey protein composition. Food Chem. 2020, 302, 125296. [Google Scholar] [CrossRef]
- Li, H.; Zhao, T.; Li, H.; Yu, J. Effect of heat treatment on the property, structure, and aggregation of skim milk proteins. Front. Nutr. 2021, 8, 714869. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, M.; Zhang, X.; Li, X.; Chen, D.; Qin, Y.; Wang, J.; Wang, C. The effect of heat treatment on the microstructure and functional properties of whey protein from goat milk. J. Dairy Sci. 2020, 103, 1289–1302. [Google Scholar] [CrossRef]
- Gaonkar, A.G.; McPherson, A. Ingredient Interactions: Effects on Food Quality, 2nd ed.; Taylor & Francis Group: Abingdon, UK, 2006. [Google Scholar]
- Kong, F.; Kang, S.; Zhang, J.; Jiang, L.; Liu, Y.; Yang, M.; Cao, X.; Zheng, Y.; Shao, J.; Yue, X. The non-covalent interactions between whey protein and various food functional ingredients. Food Chem. 2022, 394, 133455. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Sun, Y.; Wang, C.; Guo, M. Fabrication and characterization of a cannabidiol-loaded emulsion stabilized by a whey protein-maltodextrin conjugate and rosmarinic acid complex. J. Dairy Sci. 2022, 105, 6431–6446. [Google Scholar] [CrossRef]
- Roy, A.; Shrivastava, S.L.; Mandal, S.M. Self-assembled carbohydrate nanostructures: Synthesis strategies to functional application in food. In Novel Approaches of Nanotechnology in Food; Elsevier: Amsterdam, The Netherlands, 2016; pp. 133–164. [Google Scholar] [CrossRef]
- Tzannis, S.T.; Prestrelski, S.J. Moisture effects on protein—Excipient interactions in spray-dried powders. Nature of destabilizing effects of sucrose. J. Pharm. Sci. 1999, 88, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Luo, H.; Wang, M.; Chen, J. Reducing the flocculation of milk tea using different stabilizers to regulate tea proteins. Foods 2023, 12, 1484. [Google Scholar] [CrossRef]
- Kent, R.M.; Fitzgerald, G.F.; Hill, C.; Stanton, C.; Paul Ross, R. Novel approaches to improve the intrinsic microbiological safety of powdered infant milk formula. Nutrients 2015, 7, 1217–1244. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, H.; Feng, Y.; Wu, Y.; Yang, L. Milk Tea Powder and Preparation Method Thereof. Patent No. CN103689088A. 2013. Available online: https://patents.google.com/patent/CN103689088A/en#patentCitations (accessed on 4 March 2023).
- Gao, Z.; Liu, H.; Zheng, J.; Chen, X. Milk Tea Powder. Patent No. CN1171889A. 1996. Available online: https://patents.google.com/patent/CN1171889A/en (accessed on 26 February 2023).
- Ferruzzi, M.G.; Green, R.J. Analysis of catechins from milk–tea beverages by enzyme assisted extraction followed by high performance liquid chromatography. Food Chem. 2006, 99, 484–491. [Google Scholar] [CrossRef]
- Haratifar, S.; Corredig, M. Interactions between tea catechins and casein micelles and their impact on renneting functionality. Food Chem. 2014, 143, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, P.; Wang, M. Effect of beta-glucosidase on the aroma of milky tea beverage. IOP Conf. Ser. Earth Environ. Sci. 2020, 512, 012075. [Google Scholar] [CrossRef]
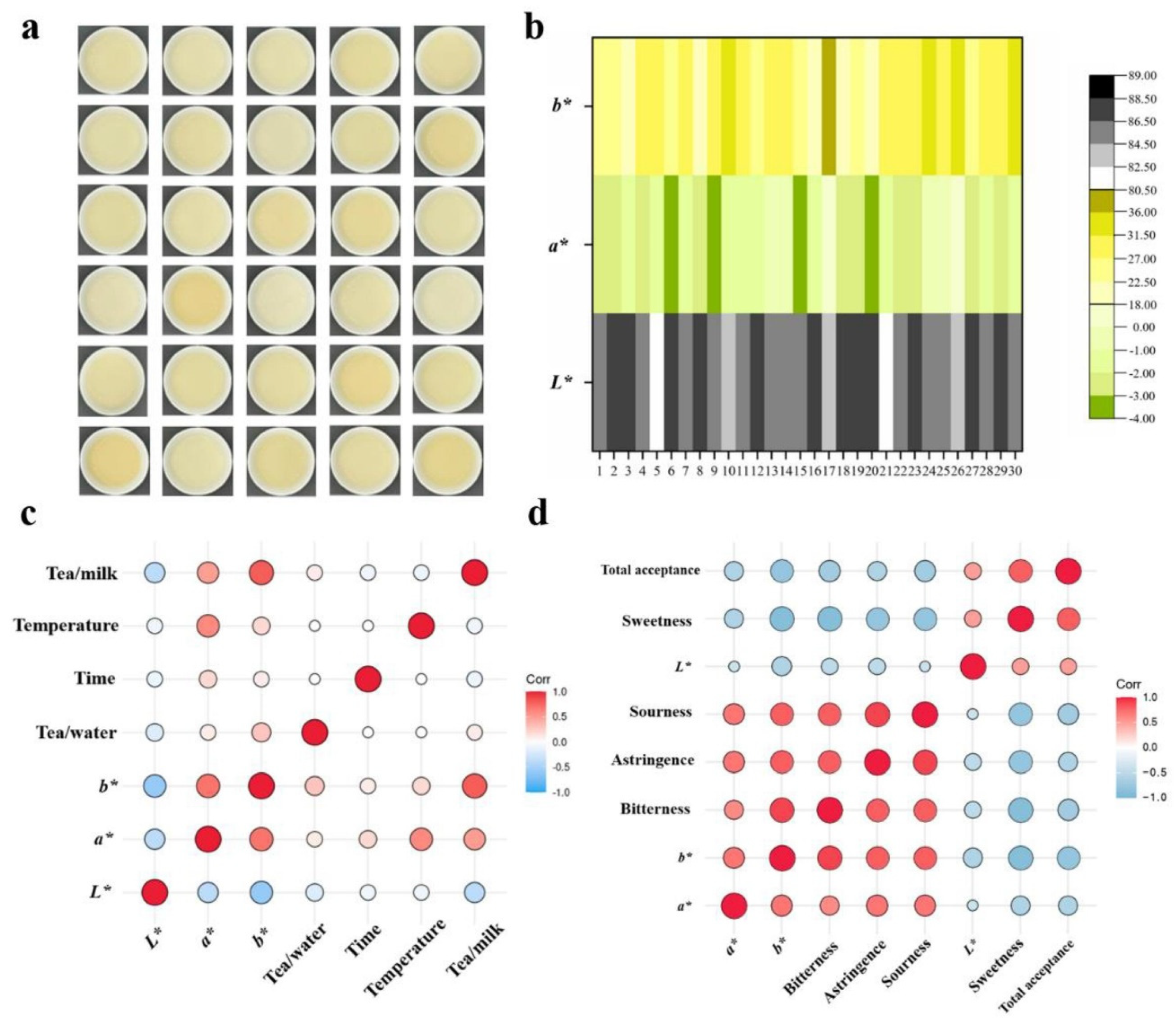
- Mao, Y.L.; Wang, J.Q.; Chen, G.S.; Granato, D.; Zhang, L.; Fu, Y.Q.; Gao, Y.; Yin, J.F.; Luo, L.X.; Xu, Y.Q. Effect of chemical composition of black tea infusion on the color of milky tea. Food Res. Int. 2021, 139, 109945. [Google Scholar] [CrossRef]
- Ye, J.; Fan, F.; Xu, X.; Liang, Y. Interactions of black and green tea polyphenols with whole milk. Food Res. Int. 2013, 53, 449–455. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Andren, A. Agents of Change; Kelly, A.L., Larsen, L.B., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Fox, P.F. Milk: An overview. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–54. [Google Scholar] [CrossRef]
- Park, Y.W.; Haenlein, G.F.W.; Wendorff, W.L. Overview of milk of non-bovine mammals. In Handbook of Milk of Non-Bovine Mammals, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Enzymology of milk and milk products. In Dairy Chemistry and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Jolles, P.; Fiat, A.M. The carbohydrate portions of milk glycoproteins. J. Dairy Res. 1979, 46, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Eliot, C. Aggregated casein gels: Interactions, rheology and microstructure. In Proceeding of the 3rd International Symposium on Food Rheology and Structure, Zurich, Switzerland, 9–13 February 2003; pp. 37–44. [Google Scholar]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Fox, P.F. Milk proteins as food ingredients. Int. J. Dairy Technol. 2001, 54, 41–55. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Review and update of casein chemistry. J. Dairy Sci. 1993, 76, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Thorn, D.C.; Ecroyd, H.; Carver, J.A.; Holt, C. Casein structures in the context of unfolded proteins. Int. Dairy J. 2015, 46, 2–11. [Google Scholar] [CrossRef]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- De Gobba, C.; Møller, M.S.; Rauh, V.; Svensson, B.; Lund, M.N. Casein–casein interactions in the presence of dairy associated carbohydrates analysed using surface plasmon resonance. Int. Dairy J. 2020, 105, 104686. [Google Scholar] [CrossRef]
- Gromiha, M.M.; Selvaraj, S. Inter-residue interactions in protein folding and stability. Prog. Biophys. Mol. Biol. 2004, 86, 235–277. [Google Scholar] [CrossRef]
- Lucey, J.A.; Horne, D.S. Perspectives on casein interactions. Int. Dairy J. 2018, 85, 56–65. [Google Scholar] [CrossRef]
- Creamer, L.K.; Bienvenue, A.; Nilsson, H.; Paulsson, M.; van Wanroij, M.; Lowe, E.K.; Anema, S.G.; Boland, M.J.; Jiménez-Flores, R. Heat-induced redistribution of disulfide bonds in milk proteins. 1. Bovine β-lactoglobulin. J. Agric. Food Chem. 2004, 52, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- deWit, J.N.; Klarenbeek, G. Effects of various heat treatments on structure and solubility of whey proteins. J. Dairy Sci. 1984, 67, 2701–2710. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Drapala, K.P.; Mulcahy, E.M.; Mulvihill, D.M. Whey protein−carbohydrate conjugates. In Whey Proteins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–280. [Google Scholar] [CrossRef]
- Qi, X.L.; Holt, C.; Mcnulty, D.; Clarke, D.T.; Brownlow, S.; Jones, G.R. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7, as determined by CD and IR spectroscopy: A test of the molten globule hypothesis. Biochem. J. 1997, 324, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Parkin, K.; Fennema, O. Fennema’s Food Chemistry; Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Fenelon, M.A.; Hickey, R.M.; Buggy, A.; McCarthy, N.; Murphy, E.G. Whey proteins in infant formula. In Whey Proteins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 439–494. [Google Scholar] [CrossRef]
- Krishna, T.C.; Najda, A.; Bains, A.; Tosif, M.M.; Papliński, R.; Kapłan, M.; Chawla, P. Influence of ultra-heat treatment on properties of milk proteins. Polymers 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.A.; Huppertz, T. Effects of high-pressure processing on structure and interactions of milk proteins. In Milk Proteins, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 243–267. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Trypsin and trypsin inhibitor bind milk beta-lactoglobulin: Protein–protein interactions and morphology. Int. J. Biol. Macromol. 2017, 96, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Maity, S.; Sardar, S.; Chakraborty, J.; Halder, U.C. Insight into the co-solvent induced conformational changes and aggregation of bovine β-lactoglobulin. Int. J. Biol. Macromol. 2016, 84, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Gambelli, L. Milk and its sugar-lactose: A picture of evaluation methodologies. Beverages 2017, 3, 35. [Google Scholar] [CrossRef]
- Idda, I.; Spano, N.; Ciulu, M.; Nurchi, V.M.; Panzanelli, A.; Pilo, M.I.; Sanna, G. Gas chromatography analysis of major free mono- and disaccharides in milk: Method assessment, validation, and application to real samples. J. Sep. Sci. 2016, 39, 4577–4584. [Google Scholar] [CrossRef]
- Amit, K.; Bandyopadhyay, P. Polysaccharide-protein interactions and their relevance in food colloids. In The Complex World of Polysaccharides; Issue tourism; InTech: Berlin, Germany, 2012; Volume 11, p. 13. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N. Protein-polysaccharide interactions for stabilization of food emulsions. J. Dispers. Sci. Technol. 2002, 23, 93–123. [Google Scholar] [CrossRef]
- McClements, D.J. Non-covalent interactions between proteins and polysaccharides. Biotechnol. Adv. 2006, 24, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Manab, A. Casein polysaccaharides interaction—A Review. Int. J. ChemTech Res. 2017, 10, 01–09. [Google Scholar]
- Prestes, A.A.; Helm, C.V.; Esmerino, E.A.; Silva, R.; Prudencio, E.S. Conventional and alternative concentration processes in milk manufacturing: A comparative study on dairy properties. Food Sci. Technol. 2022, 42, e08822. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; A’yun, Q.; Sedaghat Doost, A.; De Meulenaer, B.; Van der Meeren, P. Conjugation of milk proteins and reducing sugars and its potential application in the improvement of the heat stability of (recombined) evaporated milk. Trends Food Sci. Technol. 2021, 108, 287–296. [Google Scholar] [CrossRef]
- Giles, H.; Bull, S.P.; Lignou, S.; Gallagher, J.; Faka, M.; Methven, L. A narrative review investigating the potential effect of lubrication as a mitigation strategy for whey protein-associated mouthdrying. Food Chem. 2023, 137603. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Vorster, H.; Jerling, J.C.; Magee, E.; Mulligan, A.; Runswick, S.A.; Cummings, J.H. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br. J. Nutr. 1997, 78, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.J.; Brighenti, F.; Crozier, A. HPLC-MS analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.; Barreiro, C.; Deliza, R.; Gámbaro, A. Alternatives to reduce the bitterness, astringency and characteristic flavour of antioxidant extracts. Food Res. Int. 2009, 42, 871–878. [Google Scholar] [CrossRef]
- Dubrin, B. Tea Culture: History, Traditions, Celebrations, Recipes & More: History, Traditions, Celebrations, Recipes & More; Charlesbridge: Prague, Czech Republic, 2012. [Google Scholar]
- Spiro, M.; Chong, Y.Y. Kinetics and equilibria of tea infusion, Part 14. Surface films formed in hard water by black tea brews containing milk. Food Chem. 1997, 59, 247–252. [Google Scholar] [CrossRef]
- Das, T.; Mishra, S.; Nag, S.; Saha, K. Das. Green-synthesized gold nanoparticles from black tea extract enhance the chemosensitivity of doxorubicin in HCT116 cells via a ROS-dependent pathway. RSC Adv. 2022, 12, 8996–9007. [Google Scholar] [CrossRef]
- Sharangi, A.B. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Luczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S. Black tea and health. Nutr. Bull. 2008, 33, 91–101. [Google Scholar] [CrossRef]
- Mejares, C.T.; Huppertz, T.; Chandrapala, J. Heat-induced changes in blends of skimmed buffalo and bovine milk. Int. Dairy J. 2023, 141, 105627. [Google Scholar] [CrossRef]
- Malaka, R.; Maruddin, F.; Vargas, M.A.V.; Silva, A.J. Evaluation of the potential of matoa (Pometia pinnata) leaf extract as an antioxidant activity in pasteurized milk. Canrea J. Food Technol. Nutr. Culin. J. 2023, 6, 100–118. [Google Scholar] [CrossRef]
- Dumpler, J. On the Heat Stability of Concentrated Milk Systems-Kinetics of Heat-Induced Dissociation and Aggregation of Casein Micelles (Issue August 2017). 2017. Available online: https://www.researchgate.net/publication/340715991 (accessed on 24 March 2022).
- Velez-Ruiz, J.F.; Barbosa-Cánovas, G.V. Flow and structural characteristics of concentrated milk. J. Texture Stud. 2000, 31, 315–333. [Google Scholar] [CrossRef]
- Kalyankar, S.D.; Deshmukh, M.A.; Khedkar, C.D.; Deosarkar, S.S.; Sarode, A.R. Condensed Milk. In Encyclopedia of Food and Health, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 291–295. [Google Scholar] [CrossRef]
- Soltanizadeh, N.; Mirmoghtadaie, L.; Nejati, F.; Najafabadi, L.I.; Heshmati, M.K.; Jafari, M. Solid-state protein-carbohydrate interactions and their application in the food industry. Compr. Rev. Food Sci. Food Saf. 2014, 13, 860–870. [Google Scholar] [CrossRef]
- Fournaise, T.; Burgain, J.; Perroud, C.; Scher, J.; Gaiani, C.; Petit, J. Impact of formulation on reconstitution and flowability of spray-dried milk powders. Powder Technol. 2020, 372, 107–116. [Google Scholar] [CrossRef]
- Largo, E.; Cortes, M.; Ciro, H.J. Influence of maltodextrin and spray drying process conditions on sugarcane juice powder quality. Rev. Fac. Nac. Agron. Medellín 2015, 68, 7509–7520. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between phenolic acids, proteins, and carbohydrates—Influence on dough and bread properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Ha, C.-E. Three-dimensional structure of proteins and disorders of protein misfolding. In Essentials of Medical Biochemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–51. [Google Scholar] [CrossRef]
- Li, M.; Ritzoulis, C.; Du, Q.; Liu, Y.; Ding, Y.; Liu, W.; Liu, J. Recent progress on protein-polyphenol complexes: Effect on stability and nutrients delivery of oil-in-water emulsion system. Front. Nutr. 2021, 8, 765589. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Courtet-Compondu, M.-C.; Williamson, G.; Rezzi, S.; Kussmann, M.; Rytz, A. Non-covalent binding of proteins to polyphenols correlates with their amino acid sequence. Food Chem. 2012, 132, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, R.; Lei, D.; Huang, Y.; Cheng, S.; Zhu, Z.; Wu, Z.; Cravotto, G. Impact of ultrasound, microwaves and high-pressure processing on food components and their interactions. Trends Food Sci. Technol. 2021, 109, 1–15. [Google Scholar] [CrossRef]
- Nakano, T.; Ueki, M.; Mizoguchi, R.; Takeshita, M.; Arima, Y.; Aoki, T. Heat-induced aggregation of casein in reconstituted skimmed milk at pH 3.30–3.55. Food Sci. Technol. Res. 2017, 23, 249–254. [Google Scholar] [CrossRef]
- Kirchmeier, O.; El-Shobery, M.; Kamal, N.M. Heat-treatment of milk and development of SH-groups. Milchwiss.-Milk Sci. Int. 1984, 40, 722–723. [Google Scholar]
- De Wit, J.N.; Swinkels, G.A.M. A differential scanning calorimetric study of the denaturation of bovine b-lactoglobulin. Biochim. Biophys. Acta 1980, 624, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Interactions of milk proteins during the manufacture of milk powders. Le Lait 2007, 87, 413–423. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Senaratne, V.; Francois, S. Interactions between α-Lactalbumin and β-Lactoglobulin in the Early Stages of Heat Denaturation. J. Agric. Food Chem. 1997, 45, 3459–3464. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Mahomud, M.S.; Haque, M.E. Heat-induced interaction of milk proteins: Impact on yoghurt structure. Int. J. Food Sci. 2021, 2021, 5569917. [Google Scholar] [CrossRef]
- Choudhary, S.; Arora, S.; Kumari, A.; Narwal, V.; Sharma, V. Effect of type and quality of milk on heat induced protein–protein interactions in khoa. J. Food Sci. Technol. 2018, 55, 4321–4329. [Google Scholar] [CrossRef]
- Vasbinder, A.J.; Alting, A.C.; De Kruif, K.G. Quantification of heat-induced casein-whey protein interactions in milk and its relation to gelation kinetics. Colloids Surf. B Biointerfaces 2003, 31, 115–123. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. The mechanisms of the heat-induced interaction of whey proteins with casein micelles in milk. Int. Dairy J. 1999, 9, 233–236. [Google Scholar] [CrossRef]
- Hill, A.R. The β-lactoglobulin-χ-casein complex. Can. Inst. Food Sci. Technol. J. 1989, 22, 120–123. [Google Scholar] [CrossRef]
- Oka, D.; Ono, W.; Ohara, S.; Noguchi, T.; Takano, K. Effect of heat-induced κ-casein dissociation on acid coagulation of milk. J. Dairy Res. 2018, 85, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Zhong, Q. Physicochemical properties of skim milk powder dispersions after acidification to pH 2.4–3.0 and heating. Food Hydrocoll. 2020, 100, 105435. [Google Scholar] [CrossRef]
- Crowley, S.V.; Dowling, A.P.; Caldeo, V.; Kelly, A.L.; O’Mahony, J.A. Impact of α-lactalbumin:β-lactoglobulin ratio on the heat stability of model infant milk formula protein systems. Food Chem. 2016, 194, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; El-Bakry, M.; Neirynck, N.; Bogus, M.; Hoa, H.D.; Van der Meeren, P. Hydrophilic lecithins protect milk proteins against heat-induced aggregation. Colloids Surf. B Biointerfaces 2007, 60, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ye, A.; Dave, A.; Fraser, K.; Singh, H. Kinetics of heat-induced interactions among whey proteins and casein micelles in sheep skim milk and aggregation of the casein micelles. J. Dairy Sci. 2022, 105, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Roberts, R.; Felix, T.L.; Harte, F.M. Effect of high-pressure-jet processing on the viscosity and foaming properties of pasteurized whole milk. J. Dairy Sci. 2018, 101, 3887–3899. [Google Scholar] [CrossRef]
- Andersson, I.M.; Bergenståhl, B.; Millqvist-Fureby, A.; Alexander, M.; Paulsson, M.; Glantz, M. Particle morphology and rehydration properties of spray-dried microgels and fractal aggregates with varying fractions of native milk serum proteins. Int. Dairy J. 2021, 112, 104862. [Google Scholar] [CrossRef]
- Anema, S.G. Effect of milk concentration on the irreversible thermal denaturation and disulfide aggregation of β-lactoglobulin. J. Agric. Food Chem. 2000, 48, 4168–4175. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Regnault, S.; Gendreau, S.; Charbonneau, S.; Relkin, P. Enhancement of emulsifying properties of whey proteins by controlling spray-drying parameters. Food Hydrocoll. 2011, 25, 758–763. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. Influence of drying temperatures and storage parameters on the physicochemical properties of spray-dried infant milk formula powders. Int. Dairy J. 2020, 105, 104696. [Google Scholar] [CrossRef]
- Rogers, S.; Fang, Y.; Lin, S.X.Q.; Selomulya, C.; Chen, X.D. A monodisperse spray dryer for milk powder: Modelling the formation of insoluble material. Chem. Eng. Sci. 2012, 71, 75–84. [Google Scholar] [CrossRef]
- Fang, Y.; Rogers, S.; Selomulya, C.; Chen, X.D. Functionality of milk protein concentrate: Effect of spray drying temperature. Biochem. Eng. J. 2012, 62, 101–105. [Google Scholar] [CrossRef]
- Havea, P. Protein interactions in milk protein concentrate powders. Int. Dairy J. 2006, 16, 415–422. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Schuck, P.; Jeantet, R.; Bhandari, B.; Chen, X.D.; Perrone, Í.T.; de Carvalho, A.F.; Fenelon, M.; Kelly, P. Recent advances in spray drying relevant to the dairy industry: A comprehensive critical review. Dry. Technol. 2016, 34, 1773–1790. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Manning, M.C. Rational Design of Stable Protein Formulations; Carpenter, J.F., Manning, M.C., Eds.; Springer: New York, NY, USA, 2002; Volume 13. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Rielly, C.D.; Stapley, A.G.F. Effects of process variables on the denaturation of whey proteins during spray drying. Dry. Technol. 2007, 25, 799–807. [Google Scholar] [CrossRef]
- Semenova, M.G.; Moiseenko, D.V.; Grigorovich, N.V.; Anokhina, M.S.; Antipova, A.S.; Belyakova, L.E.; Polikarpov, Y.N.; Tsapkina, E.N. Protein–polysaccharide interactions and digestion of the complex particles. In Food Structures, Digestion and Health; Elsevier: Amsterdam, The Netherlands, 2014; pp. 169–192. [Google Scholar] [CrossRef]
- Broersen, K. Milk processing affects structure, bioavailability and immunogenicity of β-lactoglobulin. Foods 2020, 9, 874. [Google Scholar] [CrossRef]
- McMahon, D.J.; Brown, R.J. Composition, structure, and integrity of casein micelles: A Review. J. Dairy Sci. 1984, 67, 499–512. [Google Scholar] [CrossRef]
- Meltretter, J.; Wüst, J.; Pischetsrieder, M. Modified peptides as indicators for thermal and nonthermal reactions in processed milk. J. Agric. Food Chem. 2014, 62, 10903–10915. [Google Scholar] [CrossRef] [PubMed]
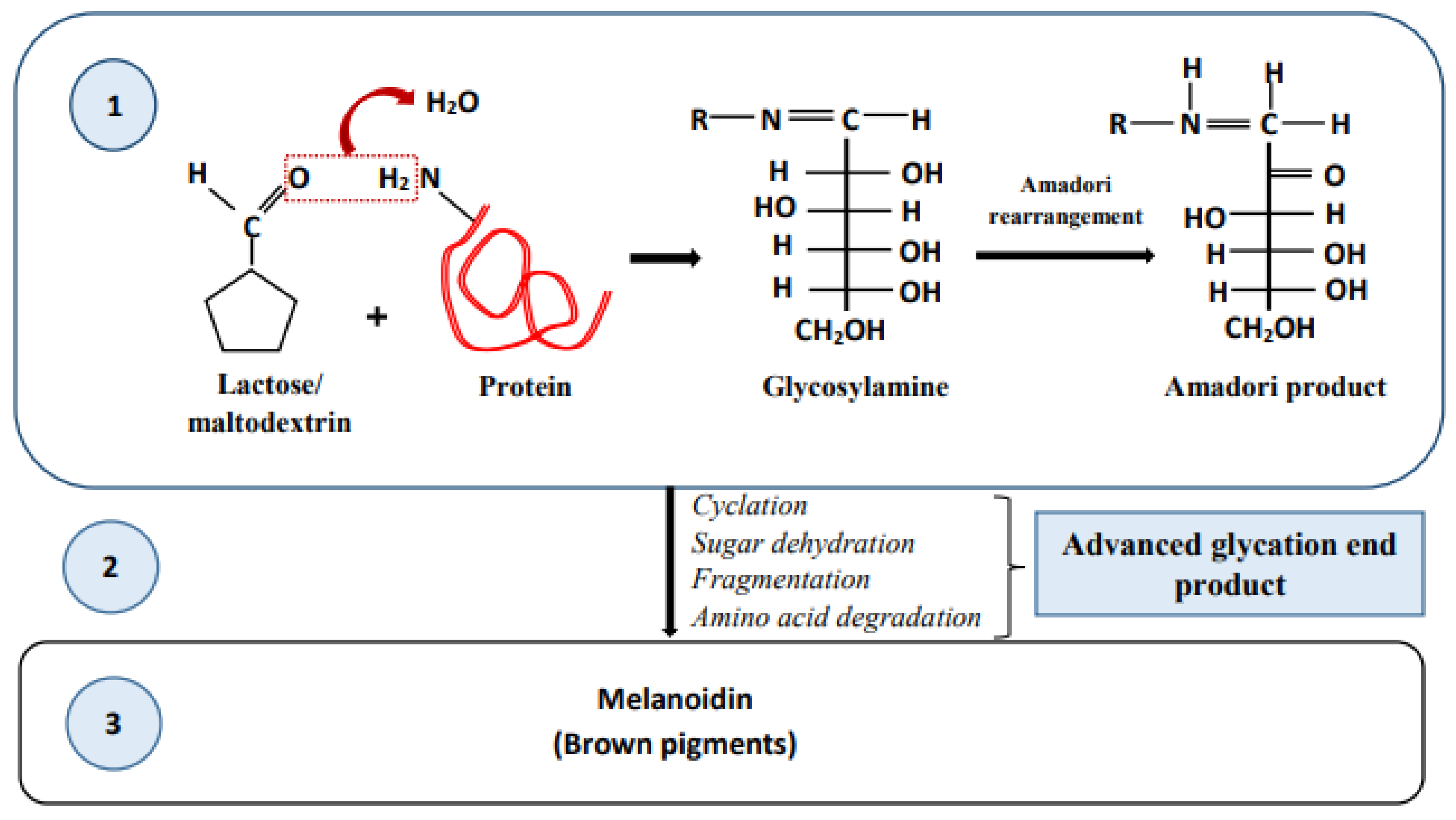
- Van Boekel, M.A.J.S. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- de Oliveira, F.C.; Coimbra, J.S.D.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins Via Maillard Reaction: Reaction Food Application. Foods 2021, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Uke, H. Maillard reaction in milk—Effect of heat treatment. In Milk Protein; Issue tourism; InTech: Berlin, Germany, 2012; Volume 11, p. 13. [Google Scholar] [CrossRef]
- Cortes Yanez, D.A.; Gagneten, M.; Leiva, G.E.; Malec, L.S. Antioxidant activity developed at the different stages of Maillard reaction with milk proteins. LWT 2018, 89, 344–349. [Google Scholar] [CrossRef]
- Leiva, G.E.; Naranjo, G.B.; Malec, L.S. A study of different indicators of Maillard reaction with whey proteins and different carbohydrates under adverse storage conditions. Food Chem. 2017, 215, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E. Properties and molecular interactions of whey protein concentrate upon storage. J. Dairy Sci. 1983, 66, 1843–1853. [Google Scholar] [CrossRef]
- ALjahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef]
- Hedegaard, R.V.; Skibsted, L.H. Shelf-life of food powders. In Handbook of Food Powders; Elsevier: Amsterdam, The Netherlands, 2013; pp. 409–434. [Google Scholar] [CrossRef]
- Hall, C. Oxidation of cereals and snack products. In Oxidation in Foods and Beverages and Antioxidant Applications; Woodhead Publishing Limited: Cambridgeshire, UK, 2010; pp. 369–390. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Naranjo, G.B.; Pereyra Gonzales, A.S.; Leiva, G.E.; Malec, L.S. The kinetics of Maillard reaction in lactose-hydrolysed milk powder and related systems containing carbohydrate mixtures. Food Chem. 2013, 141, 3790–3795. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, J.; Li, Z.; Chen, B.; Cui, L. Improving the mechanical and water-resistance properties of pea protein-based edible film via wet-heating Maillard reaction: Insights into the simultaneous effect of heating and Maillard reaction. Food Packag. Shelf Life 2023, 35, 101024. [Google Scholar] [CrossRef]
- Higa, F.A.; Nickerson, M.T. Plant protein-carbohydrate conjugates: A review of their production, functionality and nutritional attributes. Food Rev. Int. 2023, 39, 750–771. [Google Scholar] [CrossRef]
- Jing, H.; Kitts, D. Chemical and biochemical properties of casein–sugar Maillard reaction products. Food Chem. Toxicol. 2002, 40, 1007–1015. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0278691502000704 (accessed on 19 February 2023). [CrossRef] [PubMed]
- Zhang, S.; Wang, K.; Qin, Y.; Zhu, S.; Gao, Q.; Liu, D. The synthesis, biological activities and applications of protein–polysaccharide conjugates in food system: A review. Food Qual. Saf. 2023, 7, fyad006. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, F.; Wang, B.; Chen, L.; Liu, W.; Tan, S. A literature review on Maillard reaction based on milk proteins and carbohydrates in food and pharmaceutical products: Advantages, disadvantages, and avoidance strategies. Foods 2021, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Tang, G.; Wang, Q.; Zou, J.; Ma, M.; Huang, X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocoll. 2020, 100, 105384. [Google Scholar] [CrossRef]
- Baba, W.N.; McClements, D.J.; Maqsood, S. Whey protein–polyphenol conjugates and complexes: Production, characterization, and applications. Food Chem. 2021, 365, 130455. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Tang, J.; Xu, M.; Lyu, F.; Zhang, J.; Qiu, Y.; Liu, J.; Ding, Y. Whey protein and maltodextrin-stabilized oil-in-water emulsions: Effects of dextrose equivalent. Food Chem. 2021, 339, 128094. [Google Scholar] [CrossRef]
- Morell, P.; López-García, A.; Hernando, I.; Quiles, A. Improving pea protein emulsifying capacity by glycosylation to prepare high-internal-phase emulsions. Foods 2023, 12, 870. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Dickinson, E. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2019, 96, 209–223. [Google Scholar] [CrossRef]
- Kato, A. Industrial applications of maillard-type protein-polysaccharide conjugates. Food Sci. Technol. Res. 2002, 8, 193–199. [Google Scholar] [CrossRef]
- Perusko, M.; Ghnimi, S.; Simovic, A.; Stevanovic, N.; Radomirovic, M.; Gharsallaoui, A.; Smiljanic, K.; Van Haute, S.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Maillard reaction products formation and antioxidative power of spray dried camel milk powders increases with the inlet temperature of drying. LWT 2021, 143, 111091. [Google Scholar] [CrossRef]
- Dumpler, J.; Huppertz, T.; Kulozik, U. Invited review: Heat stability of milk and concentrated milk: Past, present, and future research objectives. J. Dairy Sci. 2020, 103, 10986–11007. [Google Scholar] [CrossRef] [PubMed]
- Dieplinger, J.; Pinto, J.T.; Dekner, M.; Brachtl, G.; Paudel, A. Impact of different saccharides on the in-process stability of a protein drug during evaporative drying: From sessile droplet drying to lab-scale spray drying. Pharm. Res. 2023, 40, 1283–1298. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.J. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef]
- Souillac, P.O.; Middaugh, C.R.; Rytting, J.H. Investigation of protein/carbohydrate interactions in the dried state. 2. Diffuse reflectance FTIR studies. Int. J. Pharm. 2002, 235, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, Z.; Nasirpour, A.; Scher, J.; Desobry, S. Interactions among lactose, β-lactoglobulin and starch in co-lyophilized mixtures as determined by Fourier Transform Infrared Spectroscopy. J. Food Sci. Technol. 2014, 51, 3376–3382. [Google Scholar] [CrossRef]
- Liu, W.R.; Langer, R.; Klibanov, A.M. Moisture-induced aggregation of lyophilized proteins in the solid state. Biotechnol. Bioeng. 1991, 37, 177–184. [Google Scholar] [CrossRef]
- Turner, L.G.; Swaisgood, H.E.; Hansen, A.P. Interaction of lactose and proteins of skim milk during ultra-high-temperature processing. J. Dairy Sci. 1978, 61, 384–392. [Google Scholar] [CrossRef]
- Acton, J.C.; Dawson, P.L. Impact of proteins on food colour. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2004; pp. 631–668. [Google Scholar] [CrossRef]
- Lea, C.H.; Hannan, R.S. Studies of the reaction between proteins and reducing sugars in the “dry” state. Biochim. Biophys. Acta 1949, 3, 313–325. [Google Scholar] [CrossRef]
- Zhou, Z.; Langrish, T. A review of Maillard reactions in spray dryers. J. Food Eng. 2021, 305, 110615. [Google Scholar] [CrossRef]
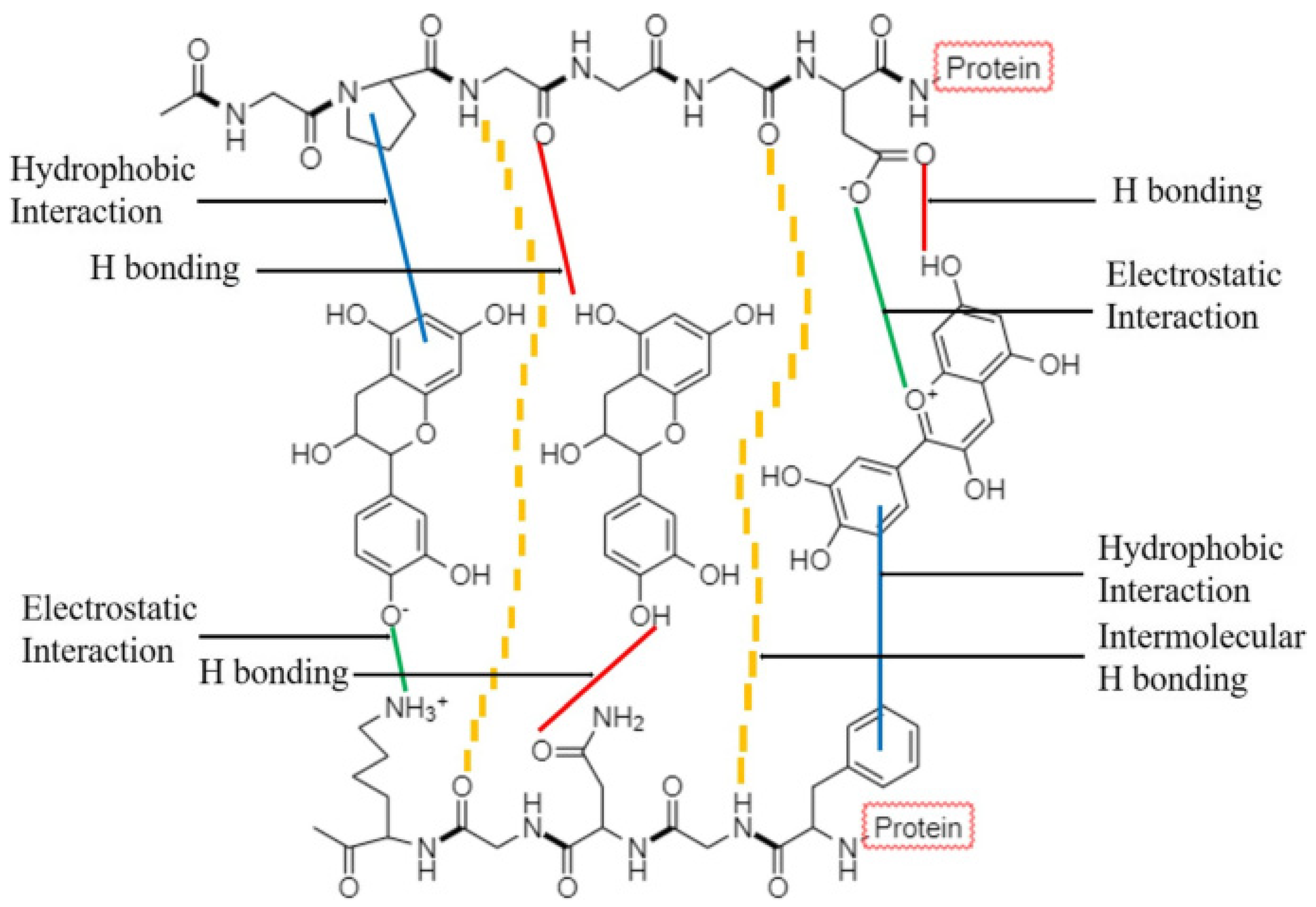
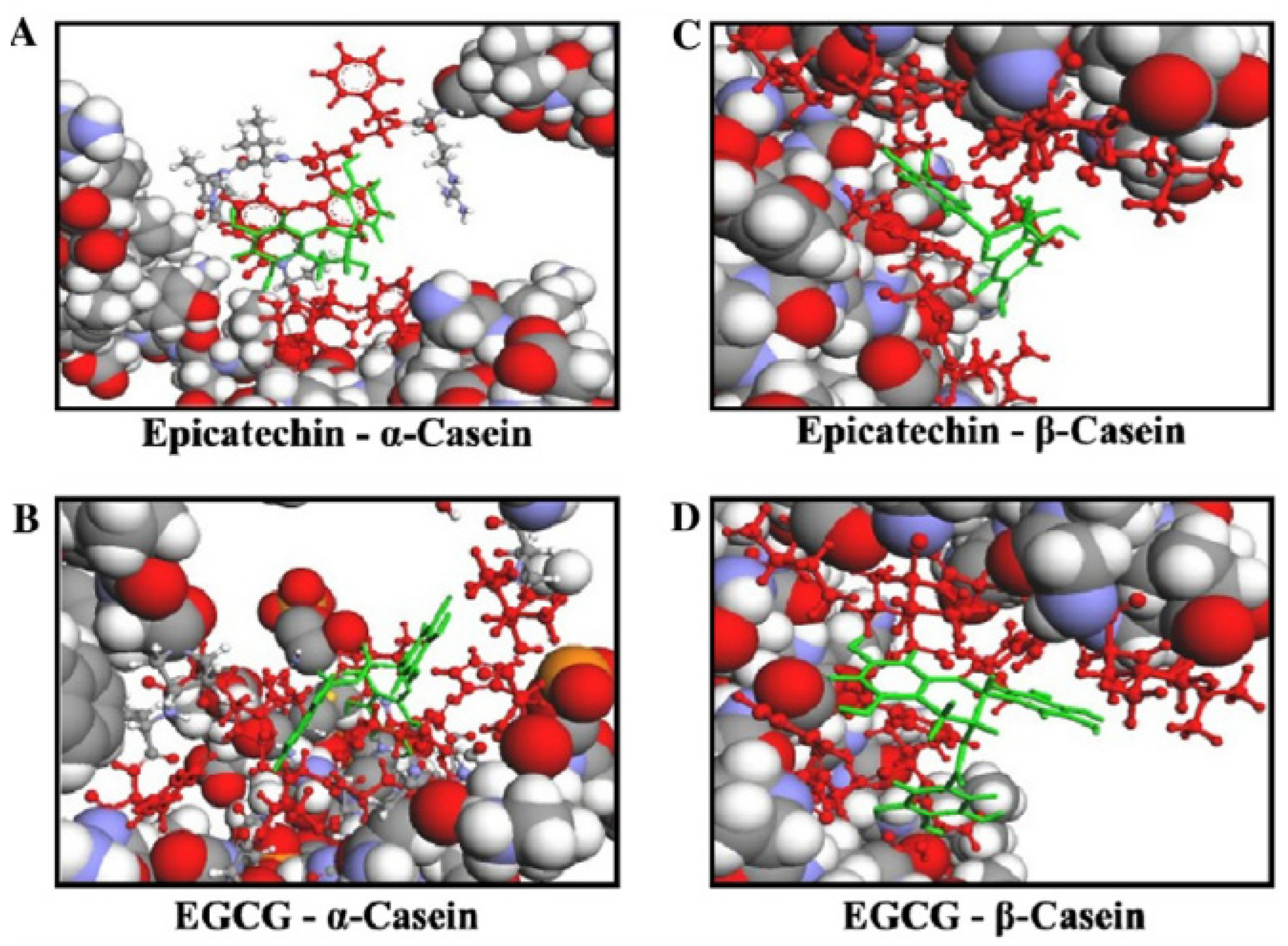
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.-A. Interaction of milk α- and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Y. Effect of extraction temperature on cream and extractability of black tea [Camellia sinensis (L.) O. Kuntze]. Int. J. Food Sci. Technol. 2003, 38, 37–45. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Emmambux, M.N. Tannin Binding of Kafirin and Its Effects on Kafirin Films. Ph.D. Thesis, University of Pretoria, Hatfield, Pretoria, 2004. [Google Scholar]
- Korpela, B. Food Protein Interactions with Plant Phenolics. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2022. [Google Scholar]
- Tazeddinova, D.; Rahman, M.R.; Hamdan, S.B.; Matin, M.M.; Bakri, M.B.; Rahman, M.M. Plant based polyphenol associations with protein: A prospective review. BioResources 2022, 17, 7110. [Google Scholar] [CrossRef]
- Haratifar, S. Nanoencapsulation of Tea Catechins in Casein Micelles: Effects on Processing and Biological Functionalities. 2012. Available online: http://hdl.handle.net/10214/4871 (accessed on 15 May 2022).
- Hofmann, T.; Glabasnia, A.; Schwarz, B.; Wisman, K.N.; Gangwer, K.A.; Hagerman, A.E. Protein binding and astringent taste of a polymeric procyanidin, 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose, castalagin, and grandinin. J. Agric. Food Chem. 2006, 54, 9503–9509. [Google Scholar] [CrossRef]
- Pascal, C.; Poncet-Legrand, C.; Cabane, B.; Vernhet, A. Aggregation of a proline-rich protein induced by epigallocatechin gallate and condensed tannins: Effect of protein glycosylation. J. Agric. Food Chem. 2008, 56, 6724–6732. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses—A review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Extraction kinetics of phytochemicals and antioxidant activity during black tea (Camellia sinensis L.) brewing. Nutr. J. 2015, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, N.; He, Q.; Sun, Q.; Gao, M.R.; Zeng, W.C. Effects of different phenolic compounds on the interfacial behaviour of casein and the action mechanism. Food Res. Int. 2022, 162, 112110. [Google Scholar] [CrossRef] [PubMed]
- Jobstl, E.; Howse, J.R.; Fairclough, J.P.A.; Williamson, M.P. Noncovalent cross-linking of casein by epigallocatechin gallate characterized by single molecule force microscopy. J. Agric. Food Chem. 2006, 54, 4077–4081. [Google Scholar] [CrossRef]
- Schwarz, B.; Hofmann, T. Is there a direct relationship between oral astringency and human salivary protein binding? Eur. Food Res. Technol. 2008, 227, 1693–1698. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; de Freitas, V. Interaction of different polyphenols with bovine serum albumin (bsa) and human salivary α-amylase (hsa) by fluorescence quenching. J. Agric. Food Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef]
- Brown, P.J.; Wright, W.B. An Investigation of the interactions between milk proteins and tea polyphenols. J. Chromatogr. A 1963, 11, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, D.; Sun, J.; Guo, H.; Ding, Q.; Liu, R.; Ren, F. Interaction of milk whey protein with common phenolic acids. J. Mol. Struct. 2014, 1058, 228–233. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Tabish Rehman, M.; AlAjmi, M.F.; Husain, F.M.; Hisamuddin, M.; Altwaijry, N. Molecular interaction of tea catechin with bovine β-lactoglobulin: A spectroscopic and in silico studies. Saudi Pharm. J. 2020, 28, 238–245. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Ishwarya, S.P. Selection of wall material for encapsulation by spray drying. In Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 77–100. [Google Scholar] [CrossRef]
- Li, M.; Cui, J.; Ngadi, M.O.; Ma, Y. Absorption mechanism of whey-protein-delivered curcumin using Caco-2 cell monolayers. Food Chem. 2015, 180, 48–54. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as carriers of polyphenols: Comparison of freeze-drying and spray-drying as encapsulation techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, D.; Sun, J.; Liu, X.; Jiang, L.; Guo, H.; Ren, F. Interaction of plant phenols with food macronutrients: Characterisation and nutritional–physiological consequences. Nutr. Res. Rev. 2014, 27, 1–15. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Amoako, D.; Awika, J.M. Polyphenol interaction with food carbohydrates and consequences on availability of dietary glucose. Curr. Opin. Food Sci. 2016, 8, 14–18. [Google Scholar] [CrossRef]
- Diaz-Montes, E. Wall materials for encapsulating bioactive compounds via spray-drying: A Review. Polymers 2023, 15, 2659. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, J.; Zhao, Q.; Niu, Y.; Zhao, D. Maltodextrin as wall material for microcapsules: A review. Carbohydr. Polym. 2022, 298, 120113. [Google Scholar] [CrossRef]
- Li, R.; Roos, Y.H.; Miao, S. Characterization of mechanical and encapsulation properties of lactose/maltodextrin/WPI matrix. Food Hydrocoll. 2017, 63, 149–159. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Ayora-Talavera, T.D.R.; Abud-Archila, M. Spray drying encapsulation of a native plant extract rich in phenolic compounds with combinations of maltodextrin and non-conventional wall materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Qu, P.; Luo, S.; Wang, C. Graduate Student Literature Review: Potential uses of milk proteins as encapsulation walls for bioactive compounds. J. Dairy Sci. 2022, 105, 7959–7971. [Google Scholar] [CrossRef]
- Ortega-Rivas, G.V.B.-C.E.; Juliano, P.; Yan, H. Food Powders. In Kluwer Academic/Plenum Publishers New York, Boston, Dordrecht, London, Moscow; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, M.; Wan, X.; Zhai, X.; Ho, C.T.; Zhang, L. Food polyphenols and Maillard reaction: Regulation effect and chemical mechanism. Crit. Rev. Food Sci. Nutr. 2024, 64, 4904–4920. [Google Scholar] [CrossRef]
- Borcherding, K.; Lorenzen, P.C.H.R.; Hoffmann, W. Effect of protein content, casein-whey protein ratio and pH value on the foaming properties of skimmed milk. Int. J. Dairy Technol. 2009, 62, 161–169. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Huppertz, T.; Chandrapala, J.; Adhikari, B.; Zisu, B. Physicochemical properties of spray-dried model infant milk formula powders: Influence of whey protein-to-casein ratio. Int. Dairy J. 2020, 100, 104565. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Chandrapala, J.; Adhikari, B.; Huppertz, T.; Zisu, B. Effect of lactose-to-maltodextrin ratio on emulsion stability and physicochemical properties of spray-dried infant milk formula powders. J. Food Eng. 2019, 254, 34–41. [Google Scholar] [CrossRef]
- Pessato, T.B.; de Morais, F.P.; de Carvalho, N.C.; Figueira, A.C.M.; Fernandes, L.G.R.; Zollner, R.D.L.; Netto, F.M. Protein structure modification and allergenic properties of whey proteins upon interaction with tea and coffee phenolic compounds. J. Funct. Foods 2018, 51, 121–129. [Google Scholar] [CrossRef]
- Walshe, E.J.; O’Regan, J.; O’Mahony, J.A. Influence of protein content and profile on the processing characteristics and physical properties of model infant formula powders. Int. J. Dairy Technol. 2021, 74, 592–599. [Google Scholar] [CrossRef]
- Ding, H.; Yan, H.; Yu, Z.; Liu, L. Spectroscopic analysis of the effect of glycation on casein structure and aggregation and its dependence on lactose concentration. Food Chem. 2023, 404, 134679. [Google Scholar] [CrossRef]
- Deng, S.; Zhou, X.; Dong, H.; Xu, Y.; Gao, Y.; Wang, B.; Liu, X. Mellow and thick taste of pu−erh ripe tea based on chemical properties by sensory−directed flavor analysis. Foods 2022, 11, 2285. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, E.M.; Park, C.W.; Drake, M.; Mulvihill, D.M.; O’Mahony, J.A. Improvement of the functional properties of whey protein hydrolysate by conjugation with maltodextrin. Int. Dairy J. 2016, 60, 47–54. [Google Scholar] [CrossRef]
- He, Z.; Yuan, B.; Zeng, M.; Tao, G.; Chen, J. Effect of simulated processing on the antioxidant capacity and in vitro protein digestion of fruit juice-milk beverage model systems. Food Chem. 2015, 175, 457–464. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein-phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Khalesi, M.; FitzGerald, R.J. Insolubility in milk protein concentrates: Potential causes and strategies to minimize its occurrence. Crit. Rev. Food Sci. Nutr. 2022, 62, 6973–6989. [Google Scholar] [CrossRef]
- Wang, Q.; He, L.; Labuza, T.P.; Ismail, B. Structural characterisation of partially glycosylated whey protein as influenced by pH and heat using surface-enhanced Raman spectroscopy. Food Chem. 2013, 139, 313–319. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, X.; Liu, M.; Xia, L.; Feng, K.; Wu, H.; Liu, Z. Reduced allergenicity of β-lactoglobulin in vitro by tea catechins binding. Food Agric. Immunol. 2013, 24, 305–313. [Google Scholar] [CrossRef]
- Park, C.W.; Drake, M. Condensed milk storage and evaporation affect the flavor of nonfat dry milk. J. Dairy Sci. 2016, 99, 9586–9597. [Google Scholar] [CrossRef]
- Ji, J.; Fitzpatrick, J.; Cronin, K.; Maguire, P.; Zhang, H.; Miao, S. Rehydration behaviours of high protein dairy powders: The influence of agglomeration on wettability, dispersibility and solubility. Food Hydrocoll. 2016, 58, 194–203. [Google Scholar] [CrossRef]
- Al-Abbasy, O.Y.; Younus, S.A.; Rashan, A.I.; Ahmad, O.A.S. Maillard reaction: Formation, advantage, disadvantage and control. A review. Food Sci. Appl. Biotechnol. 2024, 7, 145–161. [Google Scholar] [CrossRef]
- Kijewska, M.; Zawadzka, M.; Stefanowicz, P. High-Temperature, Solid-Phase Reaction of α-Amino Groups in Peptides with Lactose and Glucose: An Alternative Mechanism Leading to an α-Ketoacyl Derivative. J. Agric. Food Chem. 2023, 71, 5796–5803. [Google Scholar] [CrossRef]
- Turk-Gul, A.; Urgu-Ozturk, M.; Koca, N. The effects of different amounts of maltodextrin on the rheological behaviour and stability of white cheese emulsions, and the physical, microstructural, chemical and sensory properties of white cheese powders. Int. Dairy J. 2023, 138, 105552. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, H.V.; Rao, L.J.M. Influence of milk and sugar on antioxidant potential of black tea. Food Res. Int. 2008, 41, 124–129. [Google Scholar] [CrossRef]
- Deng, X.; Huang, G.; Tu, Q.; Zhou, H.; Li, Y.; Shi, H.; Wu, X.; Ren, H.; Huang, K.; He, X.; et al. Evolution analysis of flavor-active compounds during artificial fermentation of Pu-erh tea. Food Chem. 2021, 357, 129783. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.G.; Foegeding, E.A.; Drake, M.A. Invited review: Astringency in whey protein beverages. J. Dairy Sci. 2020, 103, 5793–5804. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Rauh, V.; Xiao, Y. The shelf life of heat-treated dairy products. Int. Dairy J. 2022, 125, 105235. [Google Scholar] [CrossRef]
- Early, R. Dairy products and milk-based food ingredients. In Natural Food Additives, Ingredients and Flavourings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 417–445. [Google Scholar] [CrossRef]
- Hammond, E. Filled and artificial dairy products and altered milk fats. In Modifying Lipids for Use in Food; Elsevier: Amsterdam, The Netherlands, 2006; pp. 462–487. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Jiang, Y.; Li, J.X.; Li, C.; Zhao, Y.; Li, C.; Jie, R.Q.D.; Zulewska, J.; Li, H.; et al. Application of tea polyphenols as additives in brown fermented milk: Potential analysis of mitigating Maillard reaction products. J. Dairy Sci. 2023, 106, 6731–6740. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.M.; Cheung, P.C.K. Overview of food chemistry. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–13. [Google Scholar] [CrossRef]
- Sharpe, E.; Hua, F.; Schuckers, S.; Andreescu, S.; Bradley, R. Effects of brewing conditions on the antioxidant capacity of twenty-four commercial green tea varieties. Food Chem. 2016, 192, 380–387. [Google Scholar] [CrossRef]
- Tong, L.; Yi, H.; Wang, J.; Pan, M.; Chi, X.; Hao, H.; Ai, N. Effect of preheating treatment before defatting on the flavor quality of skim milk. Molecules 2019, 24, 2824. [Google Scholar] [CrossRef]
- Pastoriza, S.; Pérez-Burillo, S.; Rufián-Henares, J.Á. How brewing parameters affect the healthy profile of tea. Curr. Opin. Food Sci. 2017, 14, 7–12. [Google Scholar] [CrossRef]
- Lee, A.P.; Barbano, D.M.; Drake, M.A. The influence of ultra-pasteurization by indirect heating versus direct steam injection on skim and 2% fat milks. J. Dairy Sci. 2017, 100, 1688–1701. [Google Scholar] [CrossRef]
- Beldie, A.A.; Moraru, C.I. Forward osmosis concentration of milk: Product quality and processing considerations. J. Dairy Sci. 2021, 104, 7522–7533. [Google Scholar] [CrossRef]
- Harizi, N.; Madureira, J.; Zouari, A.; Ayadi, M.A.; Cabo Verde, S.; Boudhrioua, N. Effects of spray drying, freeze drying and gamma irradiation on the antioxidant activities of camel and cow milk fractions. Processes 2023, 11, 897. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D.; Niemira, B.A. Pasteurization of foods with ultrasound: The present and the future. Appl. Sci. 2022, 12, 416. [Google Scholar] [CrossRef]
- Chavez-Martinez, A.; Reyes-Villagrana, R.A.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Tirado-Gallegos, J.M.; Bolivar-Jacobo, N. Low and high-intensity ultrasound in dairy. Foods 2020, 9, 1688. [Google Scholar] [CrossRef] [PubMed]
- Alalam, S.; Chamberland, J.; Gravel, A.; Perreault, V.; Britten, M.; Pouliot, Y.; Labrie, S.; Doyen, A. Valorization of concentrated dairy white wastewater by reverse osmosis in model cheese production. Dairy 2022, 3, 248–261. [Google Scholar] [CrossRef]
- Keshani, S.; Daud, W.R.W.; Nourouzi, M.M.; Namvar, F.; Ghasemi, M. Spray drying: An overview on wall deposition, process and modeling. J. Food Eng. 2015, 146, 152–162. [Google Scholar] [CrossRef]
| Factor | Method and Conditions | Effect on the Maillard Reaction | Reference |
|---|---|---|---|
| Heating method | Wet heating: Uses high temperatures ~90 °C for 2–96 h | High heat alters the protein structure inevitably and affects protein functionality, including foaming and emulsifying properties. | [138] |
| Dry heating: Uses a temperature in the range of 0–80 °C for a longer time | Protein denaturation and aggregation rarely occur due to the use of mild heat treatment, resulting in improved stability during long-term storage. | [139] | |
| Temperature | Occurs above 35 °C | At a temperature of 35 °C, the Maillard reaction occurs slowly and accelerates at or above 55 °C. The structure of proteins changes at high temperatures due to denaturation, aggregation, and precipitation, which reduces the number of amino groups that can participate in the reaction. | [140,141] |
| pH | When pH > 7 | The earlier stage of the Maillard reaction progresses more quickly. | [141] |
| When pH is up to 10 | The polysaccharides in open-chain form, which are usually favored at higher pH levels up to 9–10, exhibit the maximum reactivity. | ||
| Water activity | In the range of 0.60–0.85 | The Maillard reaction demonstrates the highest possible reaction rate. | [142] |
| Water content | In the range of 30–75% | Increasing the water content accelerates the Maillard reaction. | |
| Carbohydrate type | Monosaccharaides/disaccharides/ oligosaccharides /polysaccharides | The degree of the Maillard reaction can be limited by substituting polysaccharides for the reducing sugars because they have fewer reducing groups and a higher molecular weight. | [143] |
| Sample | Treatment Conditions | Type and Impact of Interactions | Functionality Modifications | References |
|---|---|---|---|---|
| Liquid milk formula that maintained a C:W ratio of proteins (5.5% total protein) at 40:60 with increasing α-La:β-Lg ratios at 0:1, 0:5, 1:3, 2:1, and 4:6 | Stability at high temperature (140 °C and pH 6.6–6.9) and viscosity changes during HTST treatment were observed | Protein–protein interactions | Protein–protein interactions (whey–casein association) were decreased with increasing α-La:β-Lg ratios. This increased heat stability and showed a less extensive increase in particle size, viscosity, and covalent interactions between proteins after thermal applications | [107] |
| Liquid milk formula that maintained a C:W ratio of proteins (1.45% total protein) at 94:6, 90:20, 60:40, 40:60, 20:80, and 7:93 | Forming properties of skim milk against heating at pH 6.6 were investigated | Columbic interactions (electrostatic interactions between electric charges) | Increased bubble diameter (d10) and higher foam density in the range of 0.15–0.16 g/cm3 were observed in the 60:40 and 20:80 samples. A C:W ratio of 20:80 and a pH of ≤6.7 exhibited attractive foaming properties | [193] |
| Liquid infant milk formula produced by maintaining the C:W ratio of proteins (15% w/w protein from 20% w/w TS) at 40:60, 50:50, and 60:40 | Particle size, zeta potential, and viscosity of UHT pasteurized (100 °C for 30 min) and homogenized (at 55 °C with first- and second-stage pressures of 13.8 MPa and 3.5 MPa, respectively) wet mix of pH 6.8 was evaluated | Electrostatic repulsion | The particle size and viscosity did not significantly differ with different C:W ratios. The particle size of all samples was below 1 µm and reflected better emulsification properties for both casein and whey protein. The sample at 40:60 reported the highest net negative charge, and it decreased significantly with the increase in casein fractions. Sufficient electrostatic repulsion between droplets maintains wet mixes stable by preventing attractive interactions between droplets | [194] |
| Liquid infant milk formula produced by maintaining the L:M ratio of carbohydrates (59% w/w) at 100:0, 85:15, and 70:30. The formula contained 15% w/w of protein at C:W ratio of 40:60 | Droplet size, zeta potential, and viscosity of the UHT pasteurized (100 °C for 30 min) and homogenized (at 55 °C with first- and second-stage pressure of 13.8 MPa and 3.5 MPa, respectively) wet mix of pH 6.8 was evaluated | Electrostatic repulsion | Droplet sizes were not significantly different with L:M ratios. The particle size of all samples was below 1 µm. The net negative charge was the greatest for the sample without maltodextrin, followed by L:M ratios of 85:15 and 70:30. All samples had sufficient electrostatic repulsion to prevent droplet attraction associations. There was an insignificant increase in the viscosity of samples with the increase in the maltodextrin concentration | [195] |
| Liquid whey–phenol solution prepared using WPI and EGCG by maintaining the WPI concentration at 5 mg/mL while varying the ECCG concentration to obtain WPI:EGCG ratios of 1:1, 1:0.5, 1:0.2, and 1:0.1 | The solution was tested for its allergenic properties at two different pH values at 3.5 (acidic) and 7.0 (neutral) and temperature at 25 °C | Non-covalent interactions occurred as a consequence of whey protein’ secondary and tertiary structure modifications | The WPI-EGCG complexes at a molar ratio of 1:1 at both pH levels showed a lower IgE binding to β-Lg and BSA, which are two allergens in milk (but not to α-La). The complexation of EGCG causes the formation of hypoallergenic products and, therefore, effectively reduces the allergenicity of β-Lg and BSA | [196] |
| Concentrated infant milk formula produced by maintaining the C:W ratio of proteins (15% w/w protein from 20% w/w TS) at 40:60, 50:50, and 60:40 | Particle size, zeta potential, and viscosity of evaporated (TS—50 ± 2%) wet mix at pH 6.8 was evaluated | Electrostatic repulsion | The volume mean diameter of all C:W ratios increased with evaporation due to the coalescence of emulsion droplets. The concentrated sample, which had a C:W ratio of 60:40, showed the largest particle size, followed by 50:50 and 40:60. The net negative charge was the highest for 40:60 and decreased with the increase in casein fractions. Sufficient electrostatic repulsion between droplets helped to keep the wet mixes stable. The viscosity of the samples decreased with the increase in casein content and was attributed to a higher whey protein denaturation | [194] |
| Concentrated infant milk formula produced by maintaining an L:M ratio of carbohydrates (59% w/w) at 100:0, 85:15, and 70:30. The formula contained 15% w/w of proteins at a C:W ratio of 40:60 | Droplet size, zeta potential, and viscosity of the concentrated (TS 50 ± 2%, pH 6.8) infant formula using falling film evaporator was analyzed | Electrostatic repulsion | Although the droplet size of the samples was not different for L:M ratios, the concentration showed an increment. The net negative charge was the greatest for the sample without maltodextrin, followed by the samples bearing L:M ratios of 85:15 and 70:30. However, the negative charge decreased after evaporation. Sufficient electrostatic repulsion between droplets helped to keep the wet mixes stable. The apparent viscosity of the concentrate increased with the increase in the maltodextrin concentration | [195] |
| Concentrated milk powder formulas that maintained a C:W ratio of proteins (modulating protein contents 10, 14, and 18/100g) at 60:40, 40:60, and 80:20 | Viscosity, bulk density, and particle size were observed in spray-dried (inlet temperature—185 °C, outlet temperature—90 °C, water evaporation rate—20 L/h) powders | Polymer–polymer interactions | Increasing the protein content and decreasing the whey protein to casein ratio were observed to increase viscosity during processing. At the C:W ratio of 80:20, particle size, viscosity, and bulk densities were higher than in the samples that had a C:W ratio of 40:60 | [197] |
| Infant milk formula powder produced by maintaining a C:W ratio of proteins (15% w/w) at 40:60, 50:50, and 60:40 | Particle size, particle morphology, water activity, color, bulk and surface composition, crystallinity, and solubility of spray-dried (inlet temperature—180 °C, outlet temperature—90 °C, rotational speed of rotary atomizer—21,500 rpm, feed temperature—55 °C) infant formulas were observed | Covalent disulphide bonds | The power bulk compositions (total protein, fat, carbohydrate, and ash) were not significantly different after spray drying at different C:W ratios. The volume mean diameter of the powders increased with the increase in the C:W ratios. A lower water activity was reported by the C:W ratio at 60:40. Glass transition temperatures (Tg), crystallinity, surface composition, color, and solubility of the powders were not significantly affected by the C:W ratios. The surface morphology of all freshly prepared powders was mostly smooth, spherical, and with little or no agglomerations. Whey–casein covalent disulfide bonds showed up to a little extent, and hence, showed some degree of aggregation | [194] |
| Infant milk formula powder produced by maintaining an L:M ratio of carbohydrates (59% w/w) at 100:0, 85:15, and 70:30. The formula contained 15% w/w of proteins at a C:W ratio of 40:60 | Powder composition, particle size, water activity, glass transition temperature, crystanillity, surface morphology, surface composition, free fat, color, and solubility of spray-dried powder (inlet temperature—180 °C, outlet temperature—90 °C, atomizer pressure—0.3 MPa) were analyzed | Covalent disulphide bonds | The powder composition remained unchanged in contrast to the initial composition. The moisture content, crystallinity, and yellowness of the powders gradually decreased with the increase in the maltodextrin content. The particle size, water activity, solubility, and surface composition (proteins, carbohydrates, fats) did not significantly differ among the L:M ratios. The smallest particles were observed at the L:M ratios of 100:0 (50 µm), 85:15 (51 µm), and 70:30 (51.3 µm). Tg significantly increased with the increase in the maltodextrin concentration. The surface morphology of all freshly prepared powders was mostly smooth, spherical, and with little or no agglomerations. The presence of aggregations indicated the formation of covalent disulphide bonds | [195] |
| Casein–lactose model powder matrix prepared with a C:W ratio at 1:0, 1:1.5, 1.2, and 1:2.5 | Color, protein aggregation, and protein structure changes were investigated in terms of the effect on spray-dried powder (inlet temperature—175 °C, outlet temperature—80 °C, peristaltic pump speed—11 mL/min) | Non covalent bonding (hydrogen and hydrophobic interactions) | Casein glycation was not dependent on the relative lactose amounts, and there was no difference in the browning index after spray drying. However, glycation resulted in larger molecules in the 1:1.5 C:L fortified powder, associated with α-casein and β-casein glycation sites. Both the 1:2 and 1:15 C:L ratios showed fluorescence of casein lower than 2.0 × 1010 M-1S-1, indicating the presence of non-covalent bonds between casein and lactose molecules. Upon glycation, new hydroxyl groups were introduced to the structure. The ratio of 1:1.5 reported the highest glycation. Glycation reduced the casein hydrophobicity, and particle distribution followed with casein aggregation | [198] |
| Powder Property | Associated Interaction | Correlation of Desired Powder Property with Associated Interaction | References |
|---|---|---|---|
| Flavor quality | Protein–protein interactions (whey–casein interactions) | Decrease the flavor quality with the increase in protein–protein interactions due to losing proteins with precipitation | [25] |
| Polyphenol–protein–polysaccharides | Reduce the astringency taste of polyphenols with polysaccharides–flavonol–BSA interactions as BSA α-helical structures become curled irregular and, hence, precipitate. Therefore, polyphenol–protein–polysaccharides interactions thicken and mellow the taste of the medium | [199] | |
| Color | Protein–polyphenol complexes (theaflavins, thearubigins, and EGCG–milk protein) | Modify interactions with tea polyphenols/pigments and proteins provide redness to the milk–tea where there was no visible red in tea before mixing in milk | [37] |
| Whey–maltodextrin conjugates | Provide yellowish to dark brown color depend on the heating time due to Maillard conjugation | [200] | |
| Antioxidant capacity | Protein–polyphenol interactions (β-Lg-EGCG) | Change the antioxidant capacity mainly due to structural changes in the β-Lg molecule because binding polyphenols to proteins changes its secondary structure with the increase in β-sheets and α-helix followed by the structure stabilization of proteins | [178] |
| Protein–polyphenol interactions β-casein-EGCG complexes | Binding β-casein with EGCG reduces the antioxidant properties of EGCG due to effects on the electron donation ability of polyphenol by reducing its available free hydroxyl groups to oxidize | [2,201] | |
| Protein–polyphenol conjugates | Binding proteins with phenols increases the antioxidant ability of proteins in contrast to proteins alone | [21] | |
| Nutritional availability | Protein–polyphenol complexes | Nutritional properties of proteins are reduced with protein–polyphenol complexes due to lowering the availability of amino acids | [202] |
| Protein–carbohydrate interactions (through the Maillard reaction) | Modify the available lysine’s residence in proteins during the Maillard reaction, resulting in the lower availability of amino acids, and hence, a lower nutritional value | [126,129] | |
| Foaming properties | Protein–polysaccharide complexes | Foaming properties obtained with protein–polysaccharide complexes are considerably higher than protein alone due to the increase in the stability of interfacial liquid film. These viscoelastic properties of protein–polysaccharide complexes entrap air to form stable foams in the system. Increase in the viscosity in the liquid film with these complexes in the mixture also increases the foam stability due to lowering the air diffusion, entrapped inside the foam | [66] |
| Solubility | Protein–protein and protein–carbohydrate interactions (casein–casein, casein–whey, and protein–lactose) | Casein–casein and casein–whey interactions are the main cause of the insolubility of powders. A greater insolubility is then promoted by protein–lactose during the Maillard reaction | [203] |
| Whey–carbohydrate interactions | Partially glycosylated whey enhanced solubility and heat stability due to suppressing inter-molecular interactions, thereby resulting in resistance to denaturation and reduced surface hydrophobicity. The intermolecular interaction reduction is caused with unique glycosylation sites and lowering sulfhydryl sites | [204] | |
| Whey–maltodextrin conjugates | Significantly improved protein solubility of whey–maltodextrin conjugates are attributed to enhanced protein hydration. Furthermore, whey–maltodextrin conjugates provide a superior thermal stability to proteins in an added salt environment | [200] | |
| Protein–polyphenol complexes | The solubility of the protein is decreased due to the increase in the molecular weight of proteins by protein–polyphenol conjugation | [205] | |
| Allergenicity | Protein–polyphenol complexes (β-Lg-catechin) | Binding β-Lg with tea catechin yielded a lower allergenicity due to shielding epitopes of β-Lg, which lower the binding capacity of IgE and IgG | [206] |
| Milk–Tea Processing Stage | Implication | Scientific Explanation | Reference |
|---|---|---|---|
| Ingredient consistency | Difficulty of maintaining the composition of tea and milk | Variations in the levels of polyphenols and the degree of fermentation of catechins in tea leaves can lead to significant differences in the astringency, bitterness, and overall flavour profile of milk–tea. The composition of polyphenols in tea is highly dependent on the type of tea, tea growing conditions, and processing methods. Variations in milk composition, such as the presence of fat, being skimmed, or using fermented milk, affect the overall quality, richness, and texture of the final product. Milk fat composition is significantly influenced by short-chain fatty acids, notably butyric acid, which contributes to the characteristic flavour and creaminess of milk. | [213,217,218,219,220,221,222] |
| Processing conditions | Brewing temperatures of tea Mixing ratio of milk: tea Method of blending | Different brewing temperatures affect the extraction of polyphenols, catechins, and other compounds from tea leaves, impacting the flavour, aroma, and health benefits of the final milk–tea product. Higher temperatures typically extract more polyphenols, leading to a stronger, more astringent flavour, while lower temperatures extract fewer polyphenols, resulting in a milder taste. The ratio of milk to tea can influence the balance of flavors and the overall sensory experience of the milk–tea. A higher proportion of milk can mellow the astringency and bitterness of tea, while a lower proportion of milk preserves the robustness of the tea flavors. The optimal ratio depends on the desired sensory attributes of the final product. The method of combining milk and tea can significantly modulate the astringency of the final product. Adding milk to strong black tea is recommended due to its higher ability to mellow out tea tannins, resulting in less bitterness. This approach optimizes the interaction between milk proteins and tea polyphenols, effectively reducing astringency and enhancing flavour balance. | [35,76,223] |
| Heat treatment | Method and the temperature of pasteurization | The method and temperature of pasteurization can affect the quality and safety of milk–tea. Pasteurization is essential to eliminate pathogenic microorganisms and extend shelf life. However, excessive heat treatment can lead to undesirable changes in the sensory attributes and nutritional profile of the final product. | [224] |
| Method of concentration | Evaporation reduces the final product quality due to prolonged heat exposure, negatively affecting color, taste, and nutritional value. Reverse osmosis (RO) has a lower energy consumption and less heat exposure, improving product properties, but is affected by membrane fouling and fat globule damage, which increases free fatty acids, challenging for whole milk. | [225] | |
| Method of drying | Drying method affects the total phenolic content. High temperatures in spray drying degrade phenolic compounds, reducing the total phenolic content. Freeze-drying at low temperatures preserves phenolic compounds, maintaining a higher total phenolic content. | [226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijegunawardhana, D.; Wijesekara, I.; Liyanage, R.; Truong, T.; Silva, M.; Chandrapala, J. Process-Induced Molecular-Level Protein–Carbohydrate–Polyphenol Interactions in Milk–Tea Blends: A Review. Foods 2024, 13, 2489. https://doi.org/10.3390/foods13162489
Wijegunawardhana D, Wijesekara I, Liyanage R, Truong T, Silva M, Chandrapala J. Process-Induced Molecular-Level Protein–Carbohydrate–Polyphenol Interactions in Milk–Tea Blends: A Review. Foods. 2024; 13(16):2489. https://doi.org/10.3390/foods13162489
Chicago/Turabian StyleWijegunawardhana, Dilema, Isuru Wijesekara, Rumesh Liyanage, Tuyen Truong, Mayumi Silva, and Jayani Chandrapala. 2024. "Process-Induced Molecular-Level Protein–Carbohydrate–Polyphenol Interactions in Milk–Tea Blends: A Review" Foods 13, no. 16: 2489. https://doi.org/10.3390/foods13162489







