Influence of Penicillium lanosum and Staphylococcus equorum on Microbial Diversity and Flavor of Mianning Hams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. High-Throughput Sequencing
2.3. Determination of Volatile Flavor Substances
2.4. Determination of Free Amino Acids
2.5. Data Analysis
3. Results and Analysis
3.1. High-Throughput Sequencing
3.1.1. Microbial Diversity
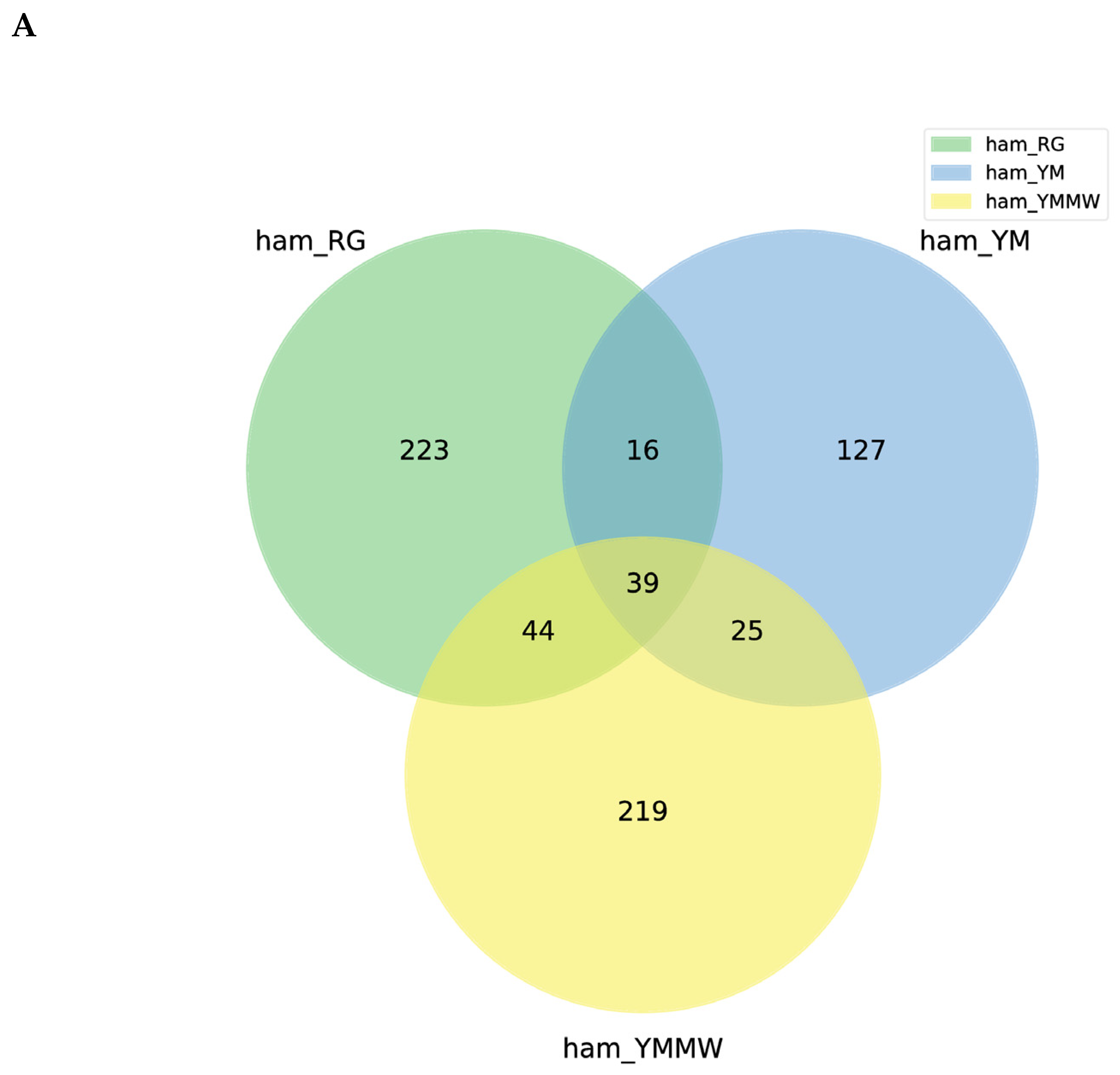
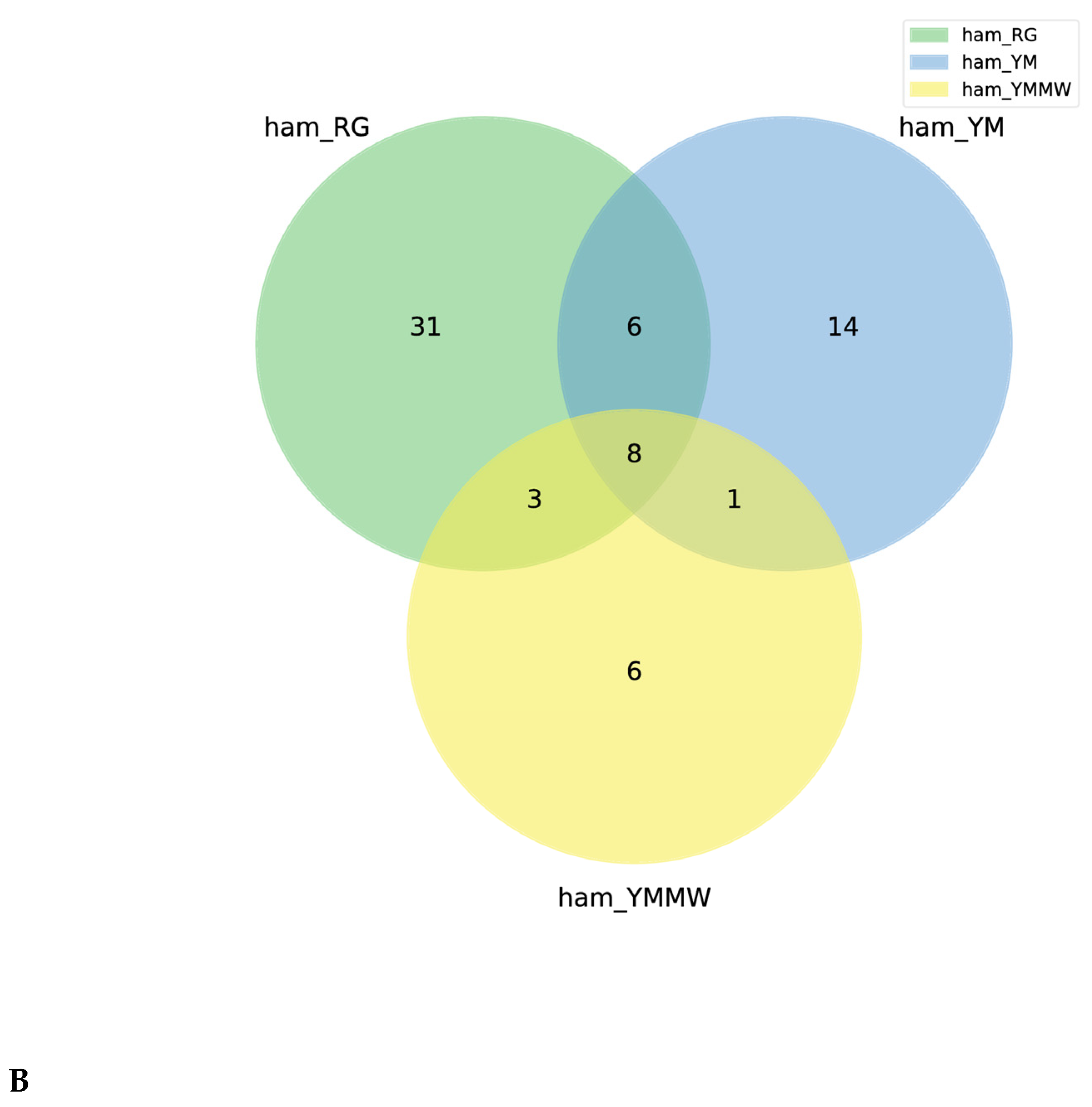
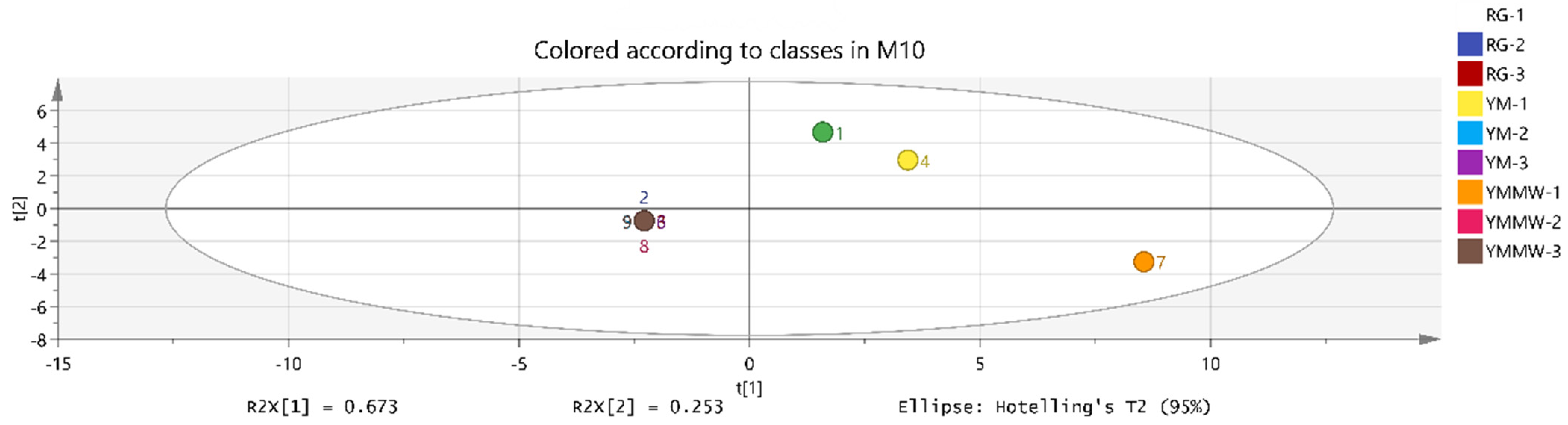
3.1.2. Differences in Microbial Communities
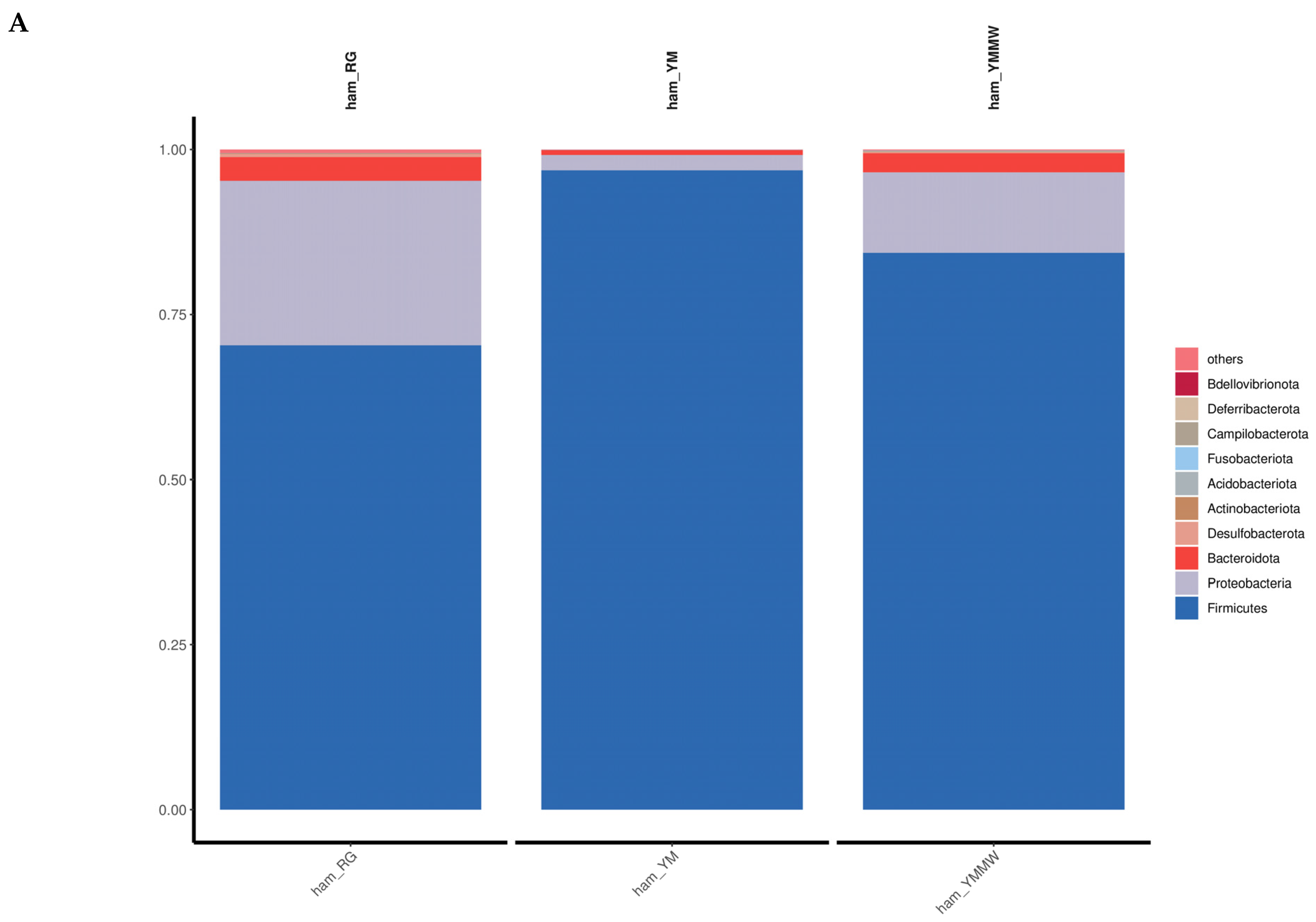
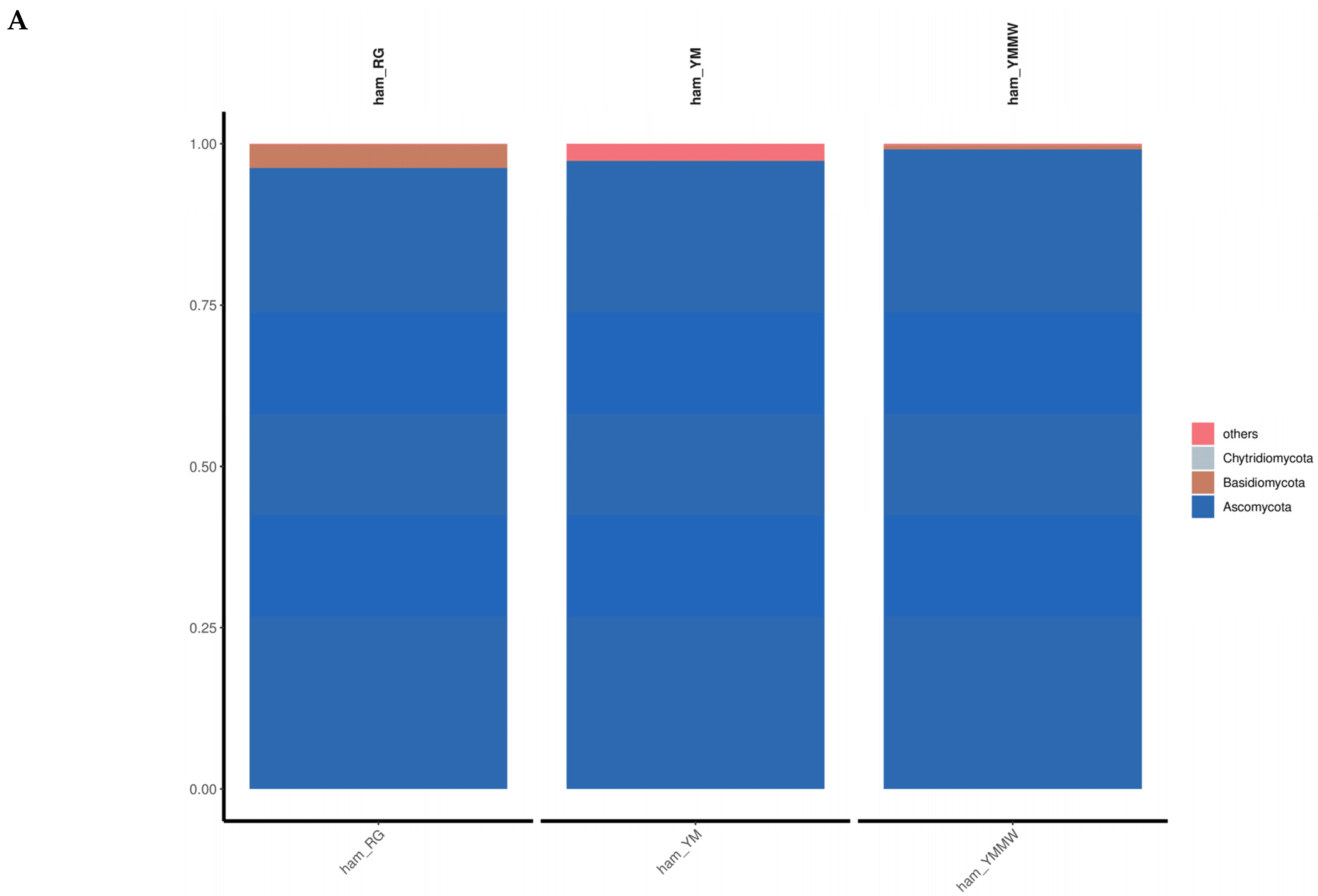
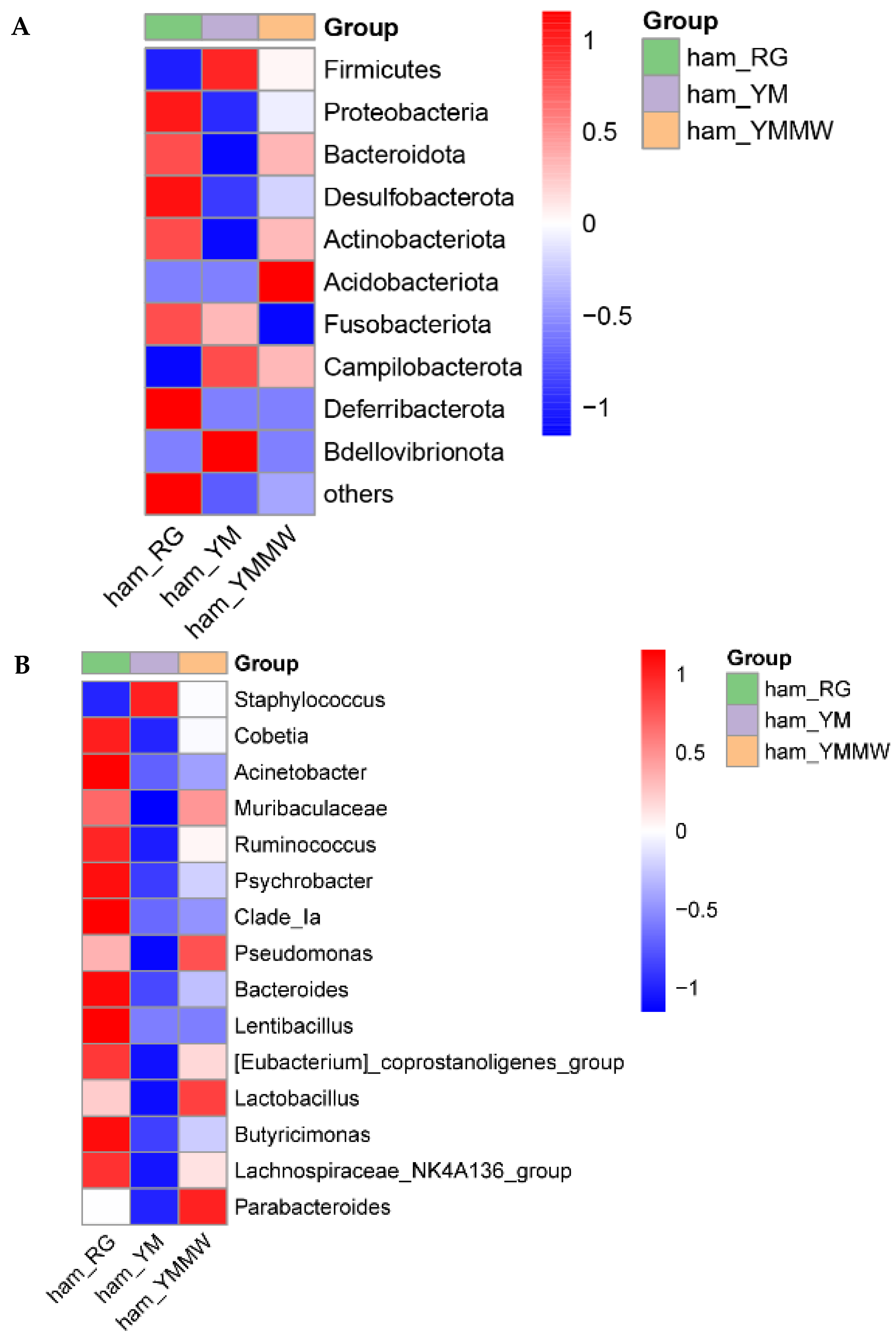
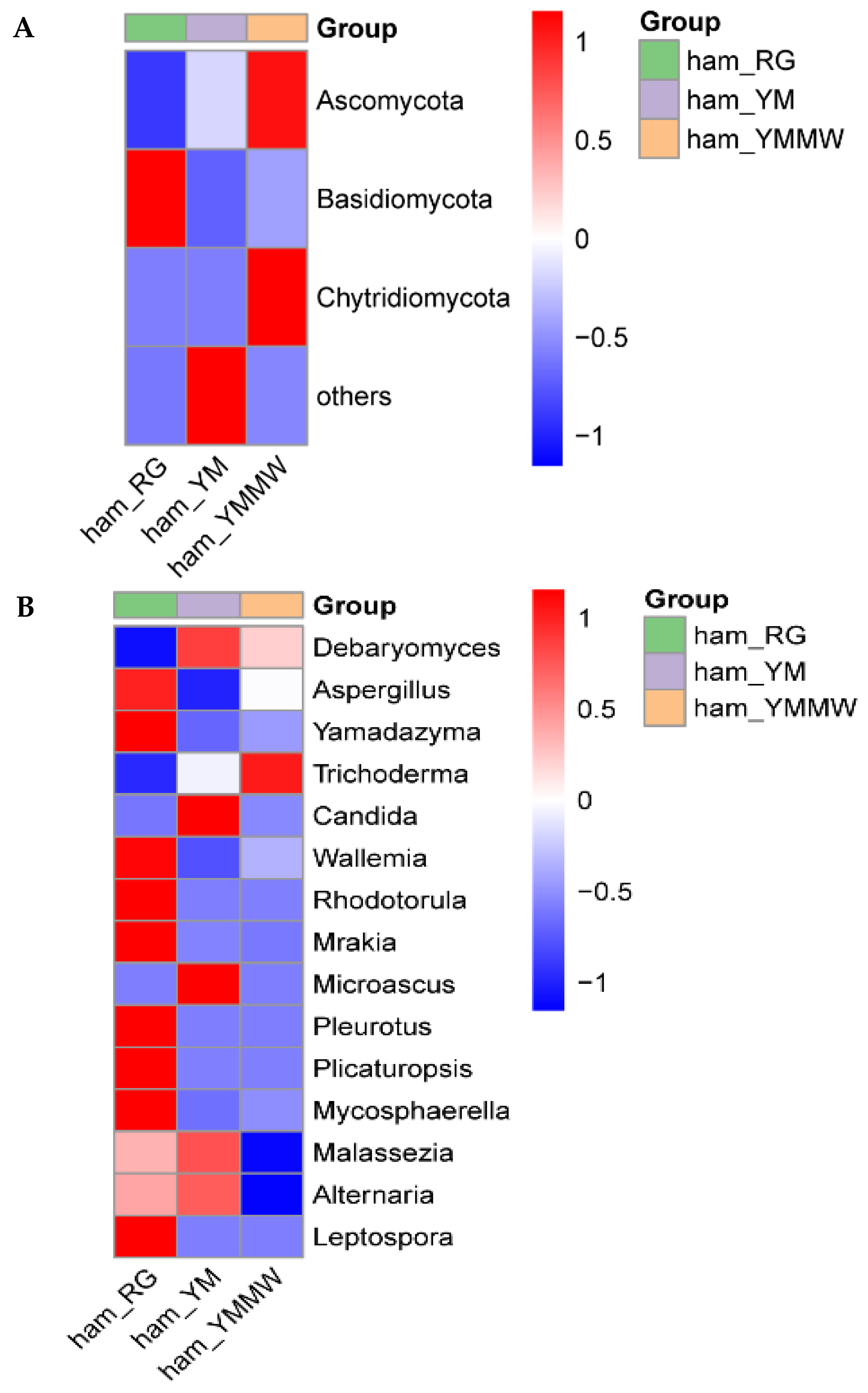
3.1.3. Species Distribution
3.2. Volatile Flavor Substances
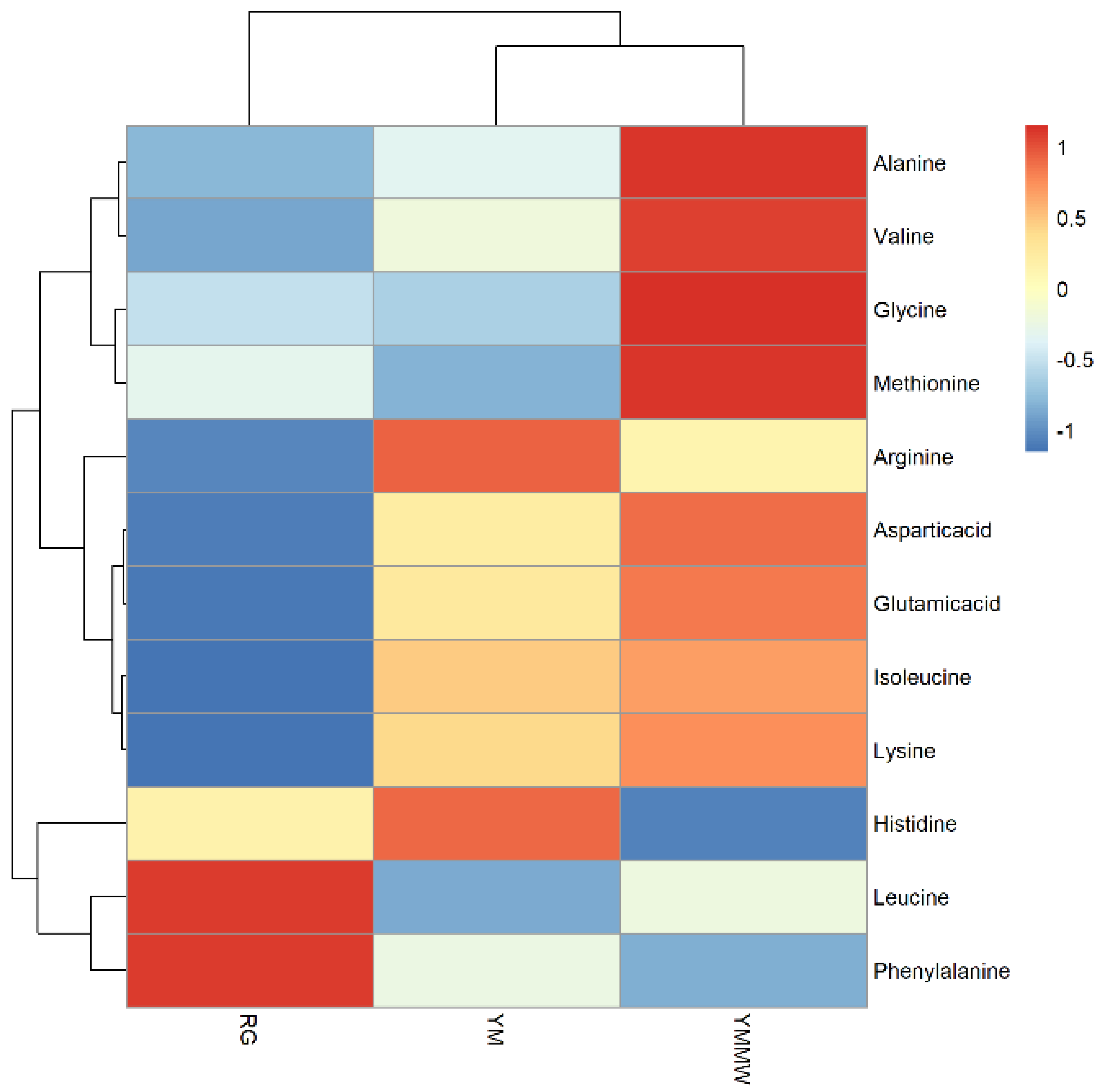
3.3. Free Amino Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talon, R.; Leroy, S.; Lebert, I. Microbial ecosystems of traditional fermented meat products: The importance of indigenous starters. Meat Sci. 2007, 77, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pisacane, V.; Callegari, M.L.; Puglisi, E.; Dallolio, G.; Rebecchi, A. Microbial analyses of traditional Italian salami reveal microorganisms transfer from the natural casing to the meat matrix. Int. J. Food Microbiol. 2015, 207, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of Starter Cultures on the Safety of Fermented Meat Products. Frontiers 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yu, J.; Yu, J.; Pan, Y.; Ou, Y. Isolation and Screening of Staphylococcus xylosus P2 from Chinese Bacon: A Novel Starter Culture in Fermented Meat Products. Int. J. Food Eng. 2019, 12, 5. [Google Scholar] [CrossRef]
- Zhu, Y. Study on the Influence of Starter Culture on the Quality of Mianning Ham. Bachelor’s Thesis, Chengdu University, Chengdu, China, 2023. [Google Scholar]
- Wang, M.; Wang, C.; Yang, C.; Peng, L.; Xie, Q.; Zheng, R.; Dai, Y.; Liu, S.; Peng, X. Effects of Lactobacillus plantarum C7 and Staphylococcus warneri S6 on flavor quality and bacterial diversity of fermented meat rice, a traditional Chinese food. Food Res. Int. 2021, 150, 110745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xia, Q.; Du, L.; He, J.; Sun, Y.; Dang, Y.; Geng, F.; Pan, D.; Cao, J.; Zhou, G. Recent developments in off-odor formation mechanism and the potential regulation by starter cultures in dry-cured ham. Crit. Rev. Food Sci. 2023, 63, 8781–8795. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhen, Z.; Li, H.; Zhou, G.; Zhang, J. Study on the microbiota of Jinhua ham during the fermentation process. Food Sci. 2008, 29, 190–195. [Google Scholar]
- Wang, L. Progress in the study of the role of microorganisms in the production of dry-cured hams. Meat Res. 2008, 10, 16–18. [Google Scholar]
- Long, Q.; Nie, Q.; Qian, Z.; Liu, C. Current status of research on functional fermentation agents for fermented meat products. Food Sci. 2016, 37, 263–269. [Google Scholar]
- Bruna, J.M.; Hierro, E.M.; de la Hoz, L.; Mottram, D.S.; Fernández, M.; Ordóñez, J.A. Changes in selected biochemical and sensory parameters as affected by the superficial inoculation of Penicillium camemberti on dry fermented sausages. Int. J. Food Microbiol. 2003, 85, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Toldrá, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Technol. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; He, L.; Zhang, Y.; Huang, G.; Bao, X.; Qiu, Z. Surface microbial community succession pattern during the fermentation of Xuanen ham. Food Ferment. Ind. 2021, 47, 34–42. [Google Scholar]
- Marty, E.; Bodenmann, C.; Buchs, J.; Hadorn, R.; Eugster-Meier, E.; Lacroix, C.; Meile, L. Prevalence of antibiotic resistance in coagulase-negative staphylococci from spontaneously fermented meat products and safety assessment for new starters. Int. J. Food Microbiol. 2012, 159, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, C.; Cui, W.; Zhao, G.; Jiao, Y.; Yin, F.; Li, J.; Han, M.; Zhang, W. Progress in the isolation, screening and application of microbial fermentation agents in fermented meat products. Meat Res. 2019, 33, 61–66. [Google Scholar]
- Chen, L.; Wang, Z.; Ji, L.; Zhang, J.; Zhao, Z.; Zhang, R.; Bai, T.; Hou, B.; Wang, W. Flavor Composition and Microbial Community Structure of Mianning Ham. Front. Microbiol. 2020, 11, 623775. [Google Scholar] [CrossRef] [PubMed]
- GBT. Microbiological Examination of Food Hygiene-Examination of Meat and Meat Produets; GBT: Beijing, China, 2003. [Google Scholar]
- Nossa, C.W.; Oberdorf, W.E.; Yang, L.; Aas, J.A.; Paster, B.J.; Desantis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroentero. 2010, 16, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Wu, L.; Qin, Y.; Van Nostrand, J.D.; Ning, D.; Sun, B.; Xue, K.; Liu, F.; Deng, Y.; Liang, Y.; et al. Evaluation of the reproducibility of amplicon sequencing with Illumina MiSeq platform. PLoS ONE 2017, 12, e0176716. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Che, L.; Zhang, W.; Huang, Q.; Li, C.; Xu, B. Insight into the autochthonous bacterial strains as starter cultures for improving the flavor profiles of dry-cured duck: Changes in microbial diversity and metabolic profiles. Food Chem. 2024, 443, 138446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Chen, J.; Sun, Z.; Wang, F.; Wang, C.; Fu, L. High-throughput sequencing-based characterization of the predominant microbial community associated with characteristic flavor formation in Jinhua Ham. Food Microbiol. 2021, 94, 103643. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, S.; Wang, P.; Chen, W.; Liu, L.; Yao, S. Diversity analysis of epidermal fungi flora in different grape varieties. China Brew. 2022, 41, 122–127. [Google Scholar]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Parikh, H.I.; Koparde, V.N.; Bradley, S.P.; Buck, G.A.; Sheth, N.U. MeFiT: Merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinform. 2016, 17, 491. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Rai, S.N.; Qian, C.; Pan, J.; Rai, J.P.; Song, M.; Bagaitkar, J.; Merchant, M.; Cave, M.; Egilmez, N.K.; McClain, C.J. Microbiome data analysis with applications to pre-clinical studies using QIIME2: Statistical considerations. Genes Dis. 2021, 8, 215–223. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y.; Rideout, J.R.; Bolyen, E.; Li, H.; Albert, P.S.; Caporaso, J.G. q2-longitudinal: Longitudinal and Paired-Sample Analyses of Microbiome Data. MSystems 2018, 3, 10–11. [Google Scholar] [CrossRef]
- Bi, J.; Yang, Z.; Li, Y.; Li, B.; Gao, Y.; Ping, C.; Chen, Z.; Li, C. Effects of different cooking methods on volatile flavor compounds in garlic. Int. J. Gastron. Food Sci. 2023, 31, 100642. [Google Scholar] [CrossRef]
- Zhang, J. Clinical Characterisation of Patients with Aortic Coarctation and Serum Multi-Omics Combined with Gut Flora Characterisation. Bachelor’s Thesis, China Medical University, Taichung, China, 2022. [Google Scholar]
- Shao, X.; Wang, H.; Song, X.; Xu, N.; Sun, J.; Xu, X. Effects of different mixed starter cultures on microbial communities, taste and aroma compounds of traditional Chinese fermented sausages. Food Chem. X 2024, 21, 101225. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Lu, H.; Yan, C.; Yan, Q.; Yao, X.; Wang, W.; Kang, D.; Liu, Y. Characteristics of microbial community in Linyi fermented pork sausage and their correlation with quality: Effects of the single Lactobacillus starter. LWT 2023, 185, 115169. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Q.; Liu, B.; Lei, Y.; Tang, H.; Zhang, S.; Zhang, J.; Li, H. Dynamic changes of bacterial communities during processing of Norden hams. Food Ind. Sci. Technol. 2021, 42, 83–89. [Google Scholar]
- Zhang, Y.; Shu, X.; Huang, X.; Lv, T.; Wang, Y.L.; Zhang, Y.; Zhang, Z.; Luo, G.; Li, X.; Li, X.; et al. Application of 16S rDNA to analyse surface and internal microbial diversity of Saba hams. Meat Res. 2020, 34, 26–32. [Google Scholar]
- Wang, Z. Study on Microbial Community Structure and Flavor Succession of Mianning Ham at Different Processing Stages. Bachelor’s Thesis, Chengdu University, Chengdu, China, 2022. [Google Scholar]
- Mu, Y.; Shu, W.; Mu, Y. Microbial Diversity and Volatile Flavour Analysis of Panxian Hams. Food Res. Dev. 2019, 40, 77–85. [Google Scholar]
- Zou, Y.; Liu, S.Y.; Wang, G.Y.; Pu, Y.H.; Ge, C.R.; Liao, G.Z. Analysis of fungal community structure in Xuanwei ham based on PCR-DGGE technique. Food Ferment. Ind. 2020, 46, 269–274. [Google Scholar]
- Iacumin, L.; Chiesa, L.; Boscolo, D.; Manzano, M.; Cantoni, C.; Orlic, S.; Comi, G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009, 26, 65–70. [Google Scholar] [CrossRef]
- Wan, J. Screening of Traditional Xuanwei Ham Yeast and Its Effect on the Quality of Fermented Sausages. Bachelor’s Thesis, Dalian University of Technology, Dalian, China, 2019. [Google Scholar]
- Li, C.; Zou, Y.; Liao, G.; Yang, Z.; Gu, D.; Pu, Y.; Ge, C.; Wang, G. Variation of microbiological and small molecule metabolite profiles of Nuodeng ham during ripening by high-throughput sequencing and GC-TOF-MS. Food Sci. Hum. Well. 2024, 13, 2187–2196. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Han, Q.; Xie, Y.; Zhou, H.; Zhou, K.; Li, X.; Xu, B. Comprehensive insights into the evolution of microbiological and metabolic characteristics of the fat portion during the processing of traditional Chinese bacon. Food Res. Int. 2022, 155, 110987. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, G.; Wan, D.; Kong, W.; Li, C.; Gu, D.; Pu, Y.; Ge, C.; Wang, G. Combined application of high-throughput sequencing and LC-MS/MS-based metabolomics to evaluate the formation of Zn-protoporphyrin in Nuodeng ham. Food Res. Int. 2022, 162, 112209. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Z.; Wang, G.; Chen, G.; Zhou, N.; Zhong, Y.; Yang, Y.; Wu, H.; Yang, C.; Liao, G. Revealing the intrinsic relationship between microbial communities and physicochemical properties during ripening of Xuanwei ham. Food Res. Int. 2024, 186, 114377. [Google Scholar] [CrossRef]
- Deng, J.; Xu, H.; Li, X.; Wu, Y.; Xu, B. Correlation of characteristic flavor and microbial community in Jinhua ham during the post-ripening stage. LWT 2022, 171, 114067. [Google Scholar] [CrossRef]
- Petričević, S.; Radovčić, N.M.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Brewer, M.S.; Vega, J.D. Detectable Odor Thresholds of Selected Lipid Oxidation Compounds in a Meat Model System. J. Food Sci. 2010, 3, 592–595. [Google Scholar] [CrossRef]
- García-González, D.L.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T. Relationship between sensory attributes and volatile compounds qualifying dry-cured hams. Meat Sci. 2008, 2, 315–325. [Google Scholar] [CrossRef]
- Mu, Y.; Su, W.; Mu, Y. Analysis of Microbial Diversity and Key Volatile Flavor Compounds of Panxian Dry-cured Ham. Food Res. Dev. 2019, 15, 77–85. [Google Scholar]
- Liu, D.; Zhou, G.; Xu, X. The main flavor components of Jinhua ham and their determination methods. J. Nanjing Agric. Univ. 2009, 32, 173–176. [Google Scholar]
- Liu, D.Y.; Zhou, G.H.; Xu, X.L. A new method for identifying key flavor compounds in foods: The “ROAV” method. Food Sci. 2008, 7, 370–374. [Google Scholar]
- Wang, L.; Zhang, H.; Chen, D.; Deng, Y.; Yang, Z.; Wu, Y. Analysis of volatile flavour substances in different parts of muscle of northern Guizhou sheep. Meat Res. 2021, 35, 47–52. [Google Scholar]
- Zhao, F.; Xu, P.; Zeng, S.; Yang, X. Analysis and evaluation of volatile flavoring substances in the traditional fermentation process of sturgeon. Food Sci. 2019, 40, 236–242. [Google Scholar]
- Bu, L.; Xie, H.; Zhang, X.; Li, X.; Ou, X.; Jing, S.; Zhong, Z. A study on the effect of free amino acids on the flavour of pork from 10-month-old Rongchang pigs. Agro-Process. 2016, 11, 29–31. [Google Scholar]
- Zhong, S.; Liu, D.; Wang, X.; Zhang, Q. Advances in the Study of Flavour Presenting Substances in Foods. Food Ind. Sci. Technol. 2020, 41, 333–339. [Google Scholar]
- Li, H.; Li, C.B.; Xu, X.L.; Zhou, G.H. Effects of illumination and packaging on non-heme iron and color attributes of sliced ham. Meat Sci. 2012, 91, 521–526. [Google Scholar] [CrossRef]
- Dan, H.; Liu, Y.; Yu, H.; Mao, X.; Liu, D.; Cheng, H.; Wang, L. Isolation and Fermentation Characteristics of Lactic Acid Bacteria from Traditional Fermented Meat in Sichuan Province, China. Food Ind. Sci. Technol. 2016, 37, 149–152. [Google Scholar]
- Niu, X. Screening of Aroma-Producing Staphylococci in Fermented Ham and Their Application in Fermented Sausages. Bachelor’s Thesis, Harbin University of Commerce, Harbin, China, 2020. [Google Scholar]


| Samples | Observed OTUs | Shannon | Chao1 | Simpson | ACE | Sequence Coverage | |
|---|---|---|---|---|---|---|---|
| RG | Bacterial 16S rDNA | 135.63 | 3.245 | 135.63 | 0.66 | 135.36 | 0.99 |
| Fungal ITS | 20.67 | 2.26 | 20.67 | 0.72 | 26.5 | 1 | |
| YM | Bacterial 16S rDNA | 92.63 | 1.54 | 92.74 | 0.36 | 92.67 | 0.99 |
| Fungal ITS | 14.3 | 1.277 | 14.3 | 0.4 | 14 | 0.99 | |
| YMMW | Bacterial 16S rDNA | 143.3 | 2.93 | 143.87 | 0.61 | 143.5 | 0.99 |
| Fungal ITS | 11.33 | 1.68 | 11.33 | 0.6 | 13.5 | 0.99 |
| Chemical Compound | Absolute Content/(μg/kg) | |||
|---|---|---|---|---|
| RG | YM | YMMW | Total | |
| Aldehyde | 5291.94 | 6661.41 | 5013.34 | 16,966.69 |
| Ketone | 149.62 | 208.88 | 543.36 | 901.86 |
| Acids | 486.02 | 431.8 | 225.61 | 1143.43 |
| Salts | 1023.83 | 430.23 | 627.97 | 2082.03 |
| Alcohol | 2169.96 | 1700.73 | 1122.37 | 4993.06 |
| Hydrocarbons | 796.82 | 636.09 | 868.29 | 2301.2 |
| Others | 939.23 | 1506.44 | 1156.33 | 3602 |
| Total | 10,857.42 | 11,575.58 | 9557.37 | 31,990.37 |
| Chemical Compound | Threshold (μg/kg) | OAV Value (OVA ≥ 1) | ||
|---|---|---|---|---|
| RG | YM | YMMW | ||
| hexanal | 7.50 | 357.83 | 199.55 | 115.49 |
| heptanal | 10.00 | 23.769 | 18.80 | 24.78 |
| 3-Methylthiopropionaldehyde | 0.04 | - | - | 532.75 |
| benzaldehyde C6H5CHO, the simplest aromatic aldehyde | 50.00 | - | 1.57 | 4.33 |
| octanal | 0.10 | 3318.90 | 3855.30 | 5535.60 |
| phenylacetaldehyde (PAG) | 9.00 | - | 3.97 | 14.95 |
| nonanal | 3.50 | 327.17 | 342.08 | 468.43 |
| trans-2-Nonanal | 0.07 | 2507.00 | 1616.86 | 1430.43 |
| decanal | 0.90 | 59.68 | 94.28 | 318.33 |
| trans,trans-2,4-Nonadienal | 0.06 | 663.50 | 423.00 | 212.67 |
| undecanal | 14.00 | - | 0.82 | 1.82 |
| trans,trans-2,4-Decadienal | 0.03 | 1359.00 | 1275.00 | 1005.67 |
| dodecanal | 1.07 | - | 13.34 | 30.71 |
| 2-Heptanone | 70.00 | - | 1.55 | 2.84 |
| 2-Nonanone | 25.00 | - | - | 7.09 |
| 2-Undecanone | 10.00 | - | - | 2.62 |
| hexanoic acid | 200.00 | 1.36 | 0.37 | 0.05 |
| hexanol | 200.00 | 0.16 | 1.07 | 0.69 |
| 1-Octen-3-ol | 2.00 | 213.32 | 297.59 | 137.27 |
| octanol | 54.00 | - | 4.28 | - |
| linalool | 1.50 | - | 11.03 | 65.96 |
| nonanal | 2.00 | - | - | 15.27 |
| Amino Acid Name | Flavor Properties | Amino Acid Content/(g/100 g) | ||
|---|---|---|---|---|
| RG | YM | YMMW | ||
| Aspartic (Asp) | Umami | 2.42 | 2.69 | 2.83 |
| Glutamic (Glu) | Umami | 3.57 | 4.13 | 4.36 |
| Threonine (Thr) | Sweetness | 1.39 | 1.31 | 1.26 |
| Serine (Ser) | Sweetness | 1.14 | 1.17 | 1.04 |
| Glycine (Gly) | Sweetness | 1.22 | 1.21 | 1.35 |
| Histidine (His) | Bitterness | 1.38 | 1.54 | 1.13 |
| Arginine (Arg) | Bitterness | 1.55 | 1.87 | 1.74 |
| Alanine (Ala) | Sweetness | 1.42 | 1.78 | 2.97 |
| Proline (Pro) | Sweetness | 1.16 | 1.21 | 1.01 |
| Tyrosine (Tyr) | Bitterness | 0.98 | 0.96 | 0.87 |
| Valine (Val) | Sweetness | 1.28 | 1.33 | 1.42 |
| Methionine (Met) | Sweetness | 0.73 | 0.64 | 0.98 |
| Isoleucine (Ile) | Bitterness | 1.20 | 1.35 | 1.37 |
| Leucine (Leu) | Bitterness | 2.44 | 2.32 | 2.36 |
| Phenylalanine (Phe) | Bitterness | 1.25 | 1.16 | 1.12 |
| Lysine (Lys) | Sweetness | 2.26 | 2.48 | 2.53 |
| Total amino acids (TAAs) | 25.39 | 27.15 | 28.34 | |
| Amino Acid Name | Threshold (mg/g) | TAV Value (TAV ≥ 1) | ||
|---|---|---|---|---|
| RG | YM | YMMW | ||
| Aspartic | 1 | 2.42 | 2.69 | 2.83 |
| Glutamic | 0.3 | 11.9 | 13.77 | 14.53 |
| Glycine | 1.3 | 0.94 | 0.93 | 1.04 |
| Histidine | 0.2 | 6.9 | 7.7 | 5.65 |
| Arginine | 0.5 | 3.1 | 3.74 | 3.48 |
| Alanine | 0.6 | 2.37 | 2.97 | 4.95 |
| Valine | 0.4 | 3.2 | 3.33 | 3.55 |
| Methionine | 0.3 | 2.43 | 2.13 | 3.27 |
| Isoleucine | 0.9 | 1.33 | 1.5 | 1.52 |
| Leucine | 1.9 | 1.28 | 1.22 | 1.24 |
| Phenylalanine | 0.9 | 1.39 | 1.29 | 1.24 |
| Lysine | 0.5 | 4.52 | 4.96 | 5.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhu, Y.; Wang, W.; Zhang, J.; He, D.; Ji, L.; Chen, L. Influence of Penicillium lanosum and Staphylococcus equorum on Microbial Diversity and Flavor of Mianning Hams. Foods 2024, 13, 2494. https://doi.org/10.3390/foods13162494
Wang W, Zhu Y, Wang W, Zhang J, He D, Ji L, Chen L. Influence of Penicillium lanosum and Staphylococcus equorum on Microbial Diversity and Flavor of Mianning Hams. Foods. 2024; 13(16):2494. https://doi.org/10.3390/foods13162494
Chicago/Turabian StyleWang, Wenli, Yanli Zhu, Wei Wang, Jiamin Zhang, Daolin He, Lili Ji, and Lin Chen. 2024. "Influence of Penicillium lanosum and Staphylococcus equorum on Microbial Diversity and Flavor of Mianning Hams" Foods 13, no. 16: 2494. https://doi.org/10.3390/foods13162494






