Effect of Dry Processing of Coconut Oil on the Structure and Physicochemical Properties of Coconut Isolate Proteins
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Pre-Treatment of Raw Materials and Protein Extraction
2.3. Proximate Composition and Amino Acid Composition
2.4. Sodium Dodecylsulphate Polyacrylamine Gel Electrophoresis (SDS-PAGE)
2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.7. Scanning Electron Microscopy (SEM)
2.8. Sulfhydryl Group (SH) Contents Determination
2.9. Free Amino Content
2.10. Determination of Surface Hydrophobicity (H0)
2.11. Protein Solubility (PS)
2.12. Measurement of Water-Holding Capacity (WHC) and Fat Absorption Capacity (FAC)
2.13. Foaming Properties
2.14. Emulsification Properties
2.15. Statistical Analysis
3. Results and Discussions
3.1. Analysis of Proximate Composition and Amino Acid Composition
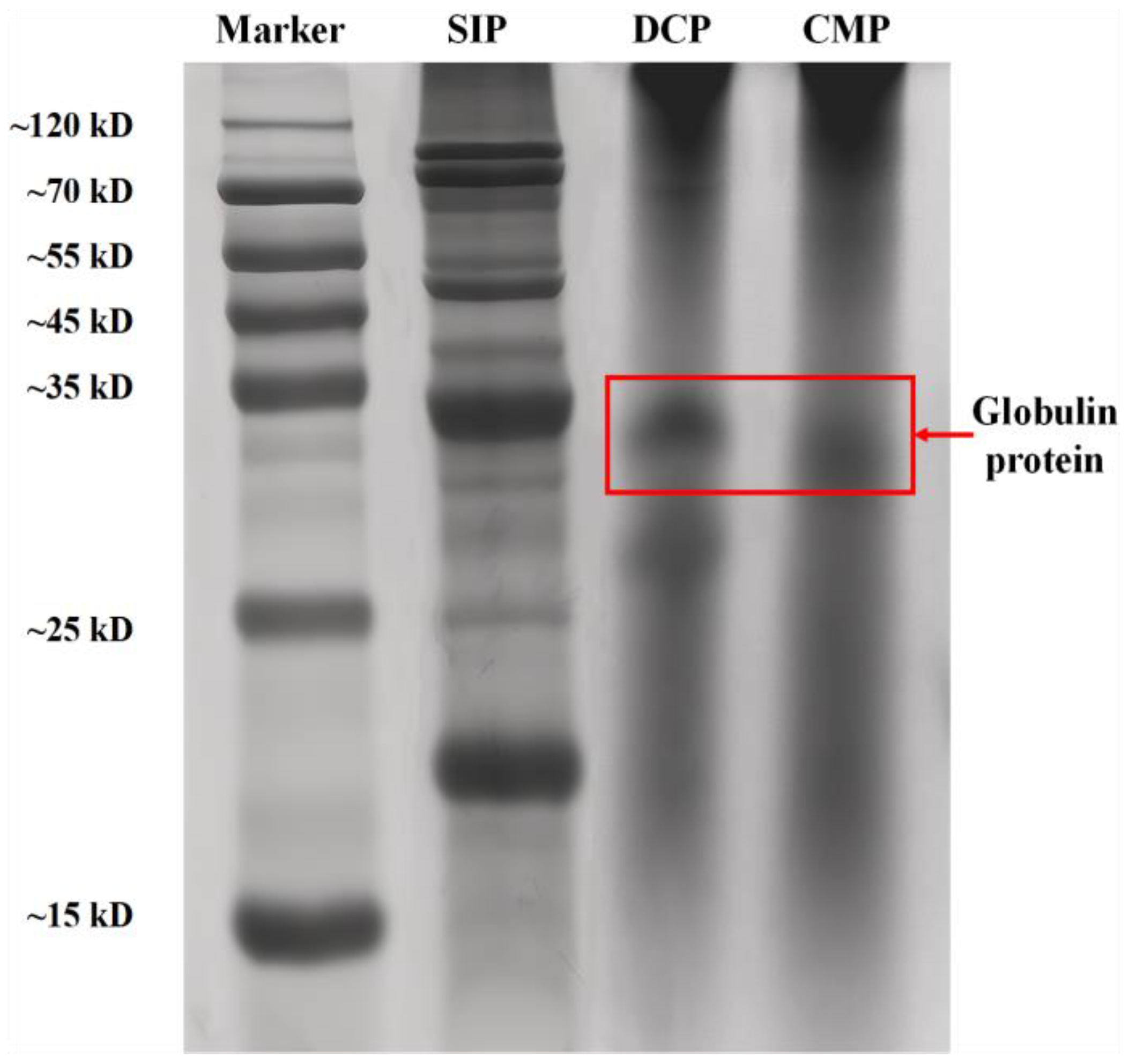
3.2. SDS-PAGE
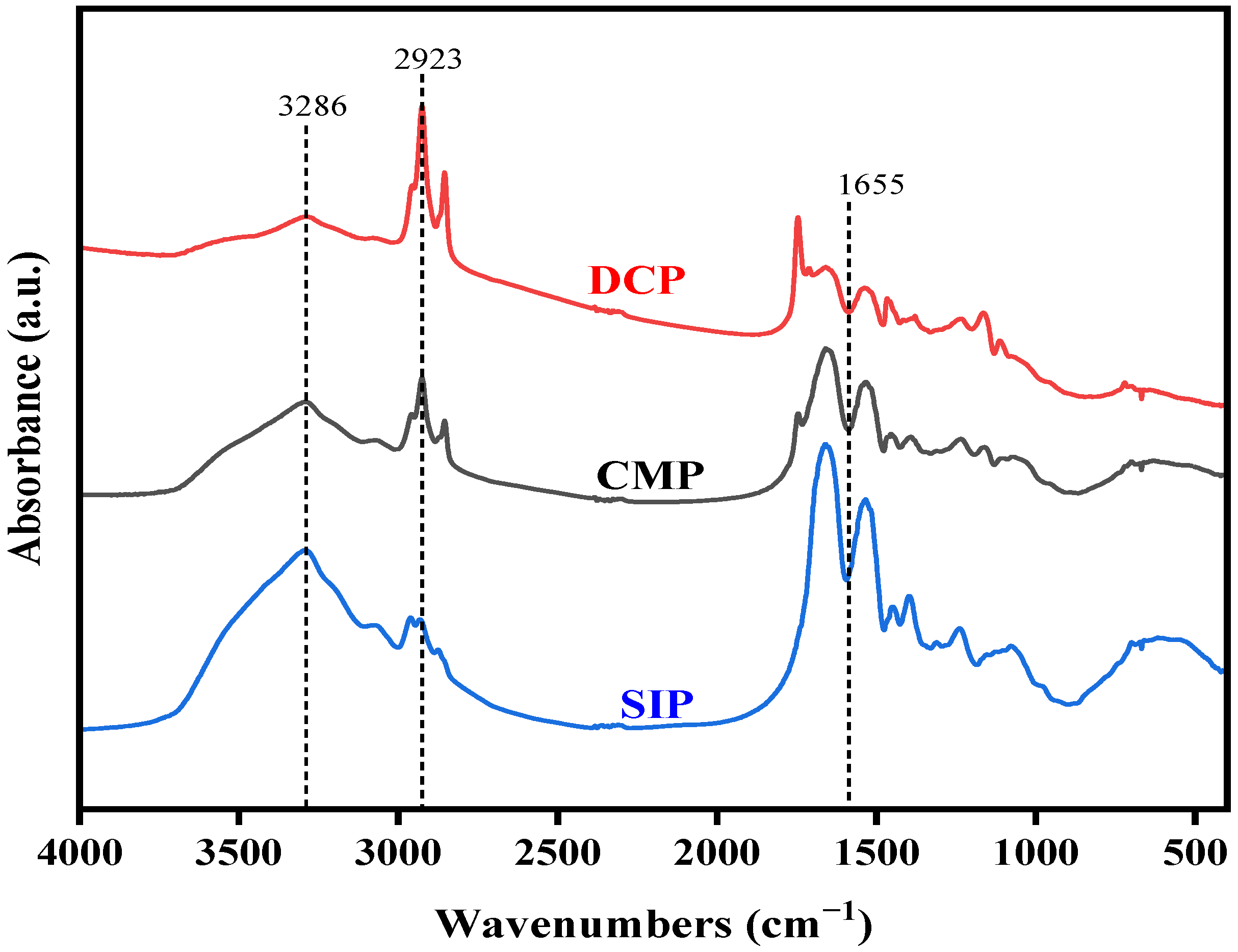
3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
3.4. Thermal Properties
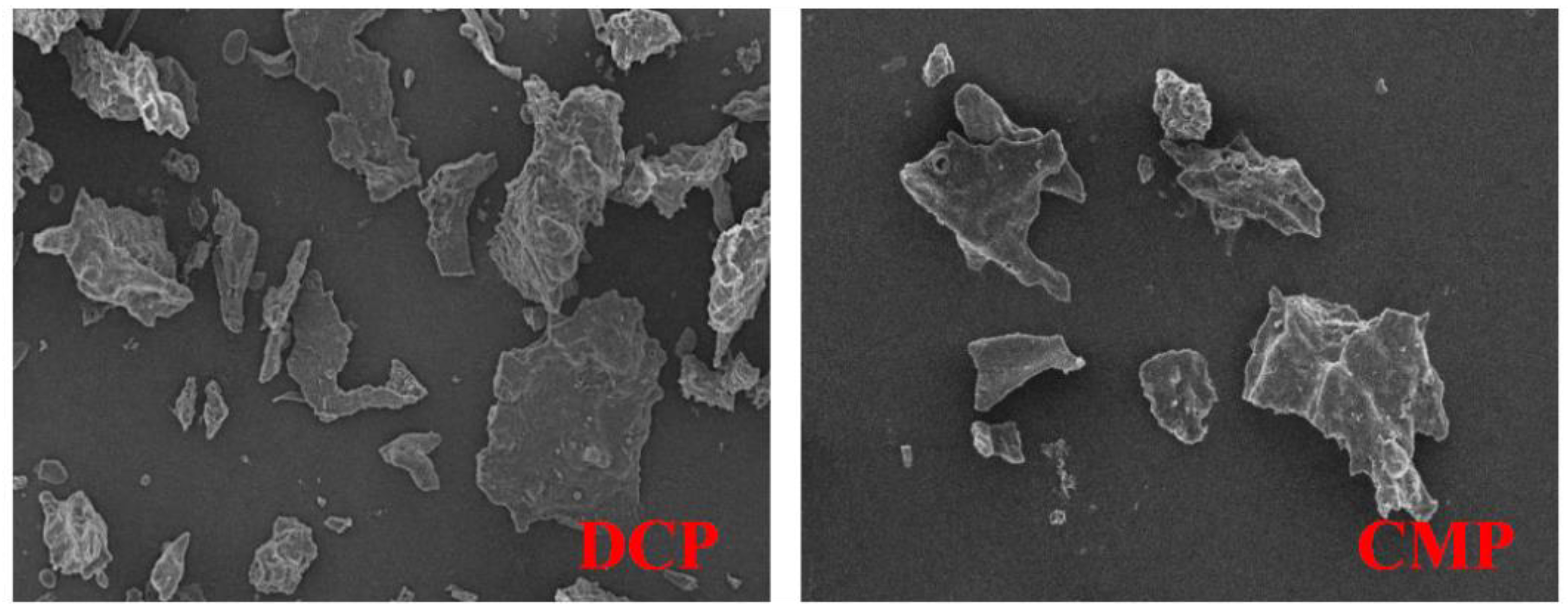
3.5. SEM
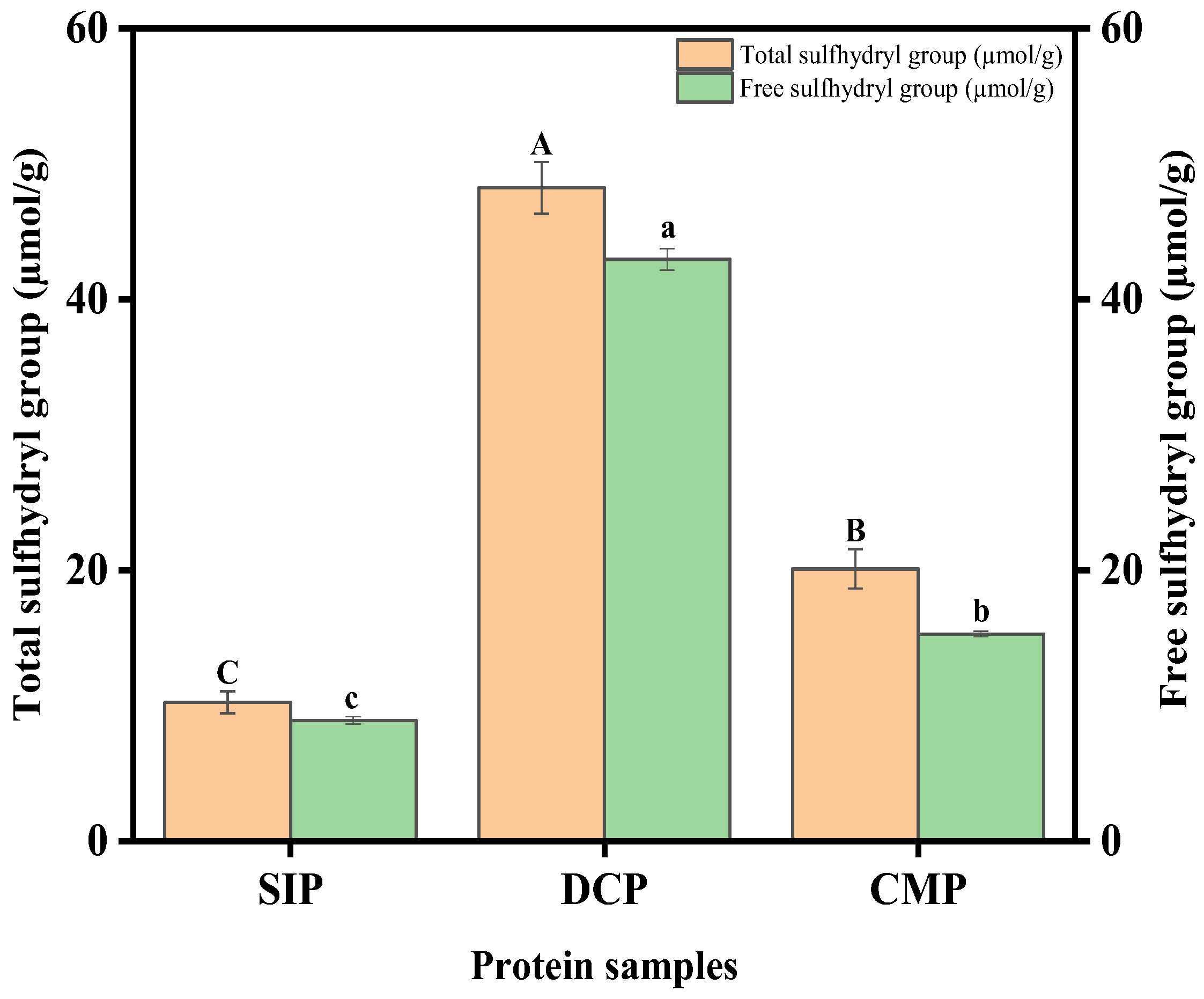
3.6. Analysis of Sulfhydryl (SH) Content
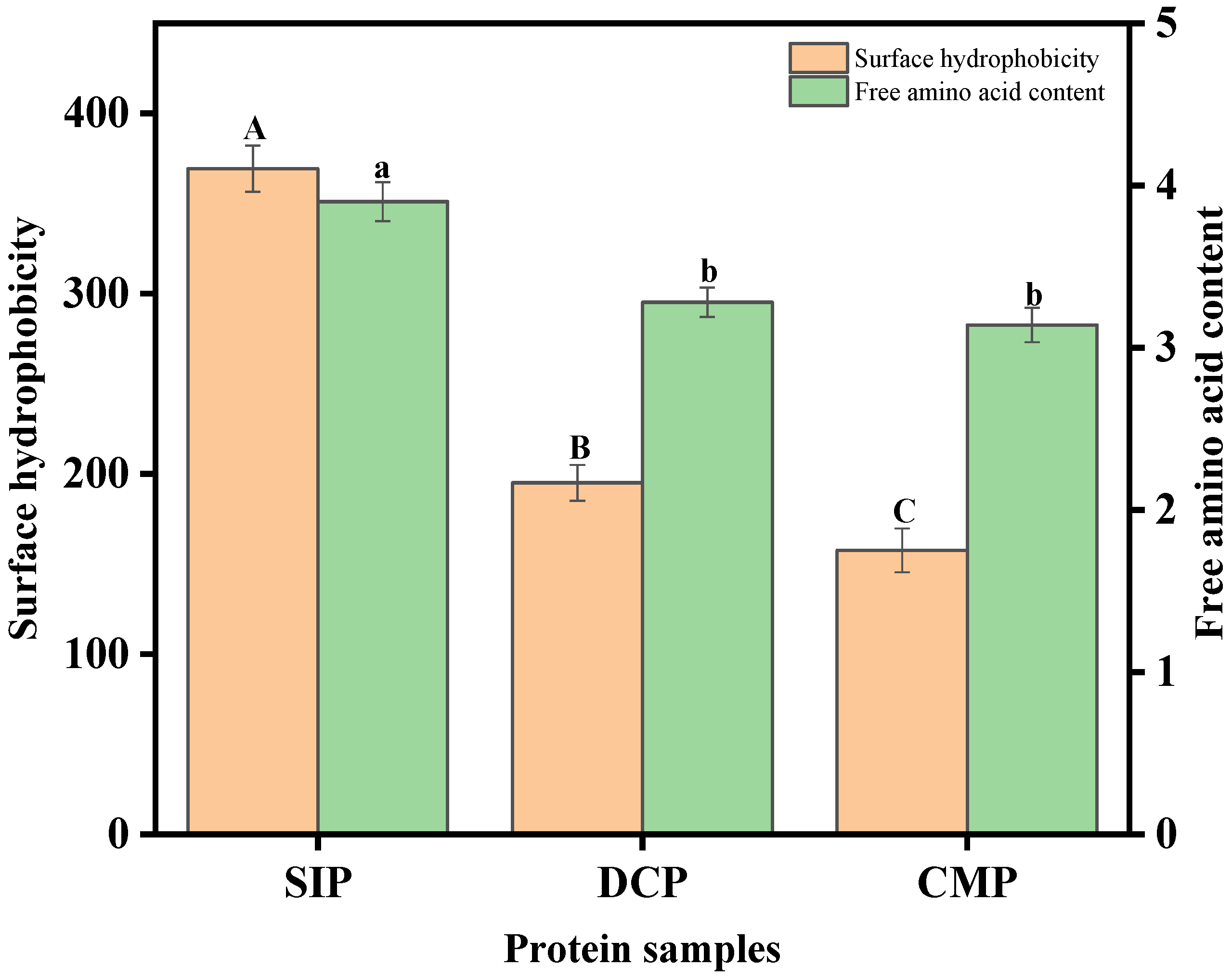
3.7. Analysis of Free Amino Content
3.8. Analysis of Surface Hydrophobicity
3.9. Functional Characteristics
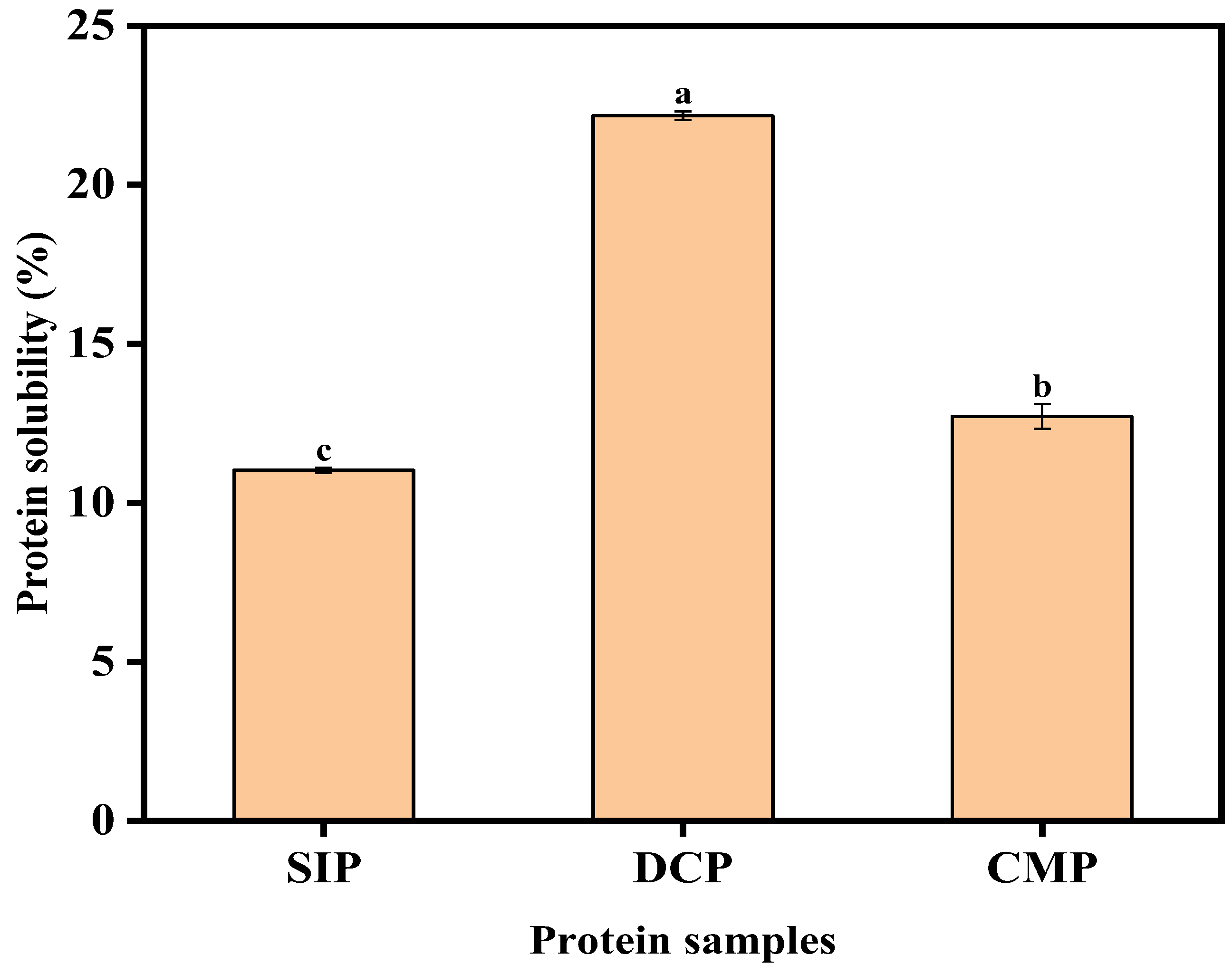
3.9.1. Analysis of Solubility
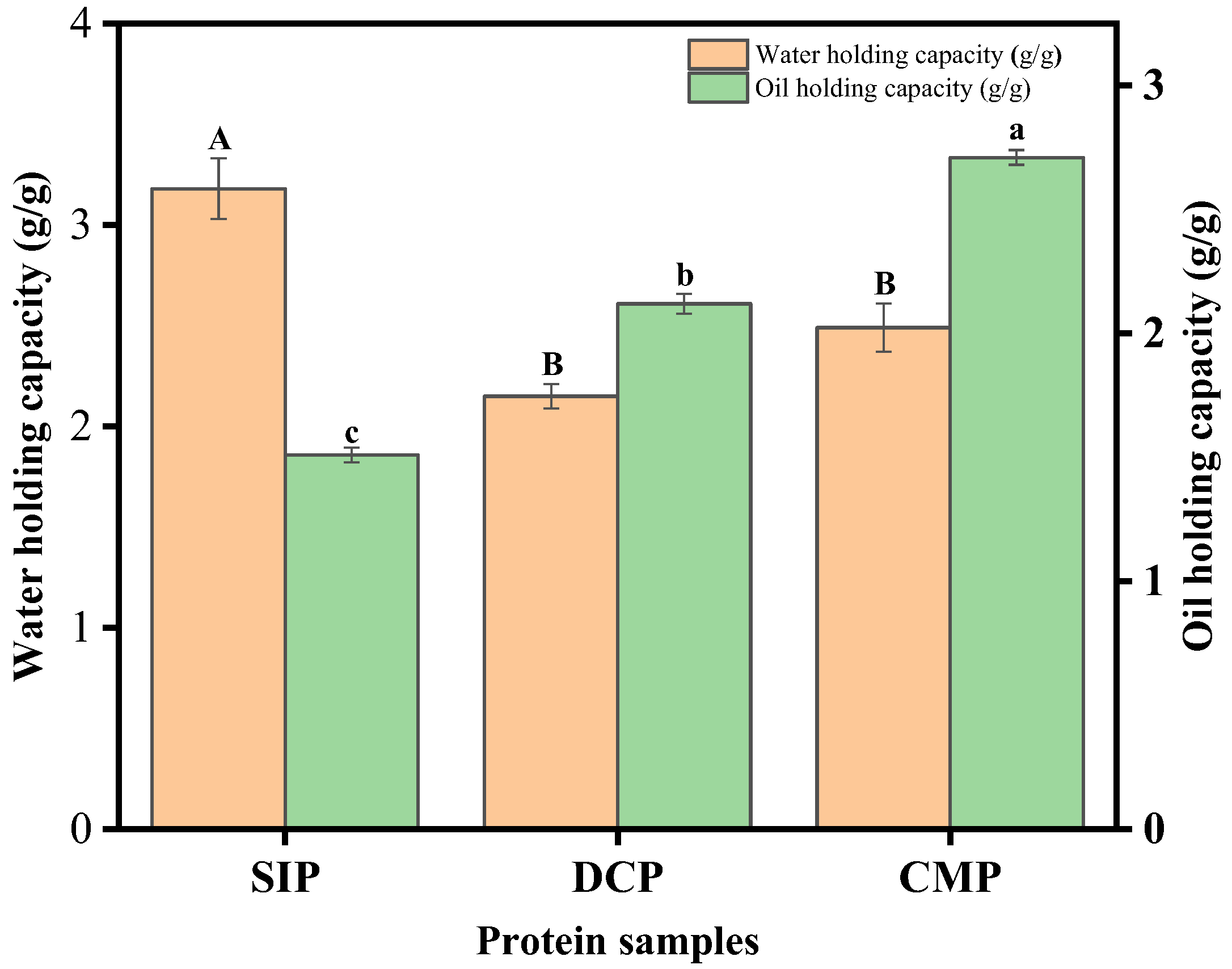
3.9.2. Water-Holding Capacity (WHC) and Fat-Holding Capacity (OHC)
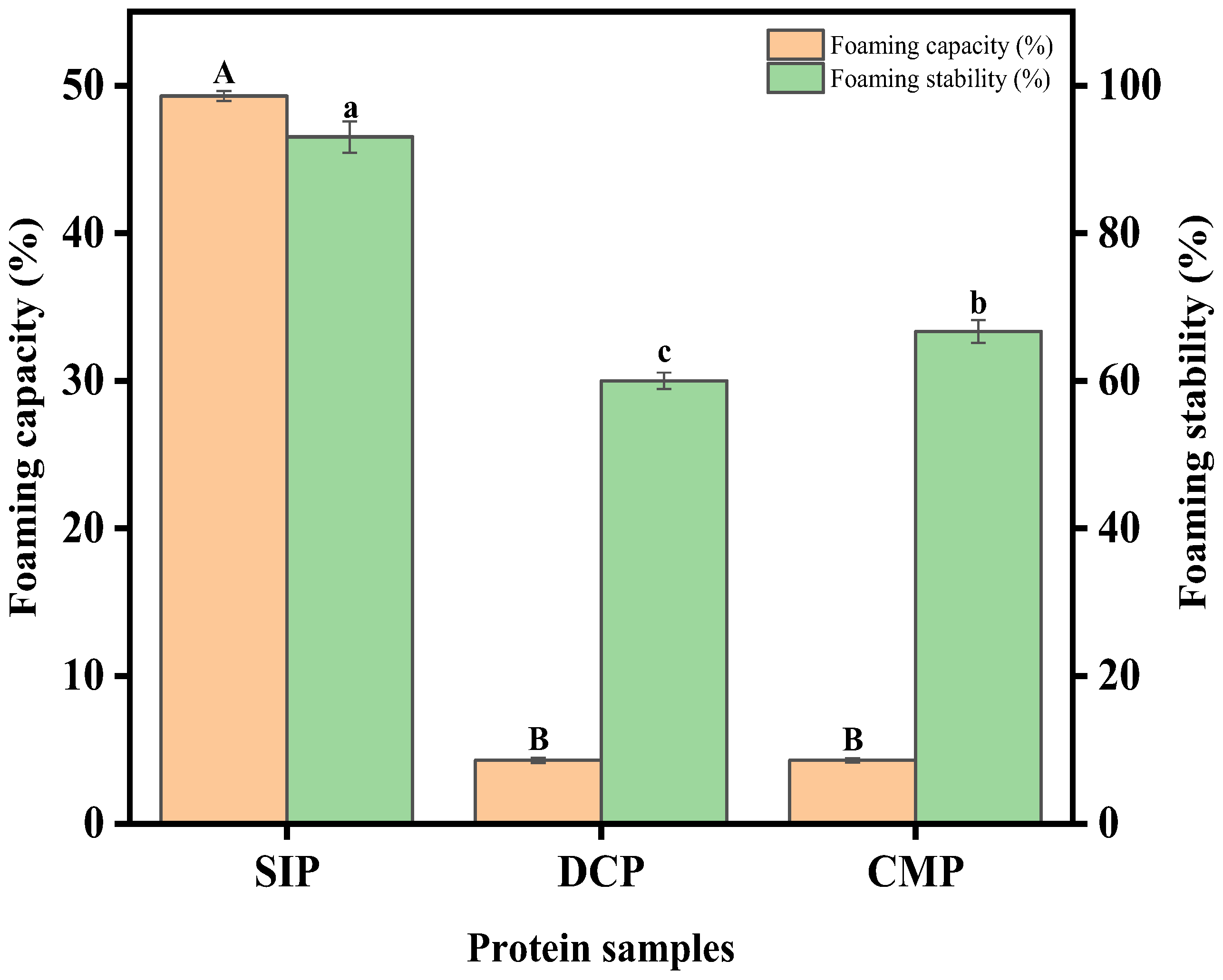
3.9.3. Foaming Properties
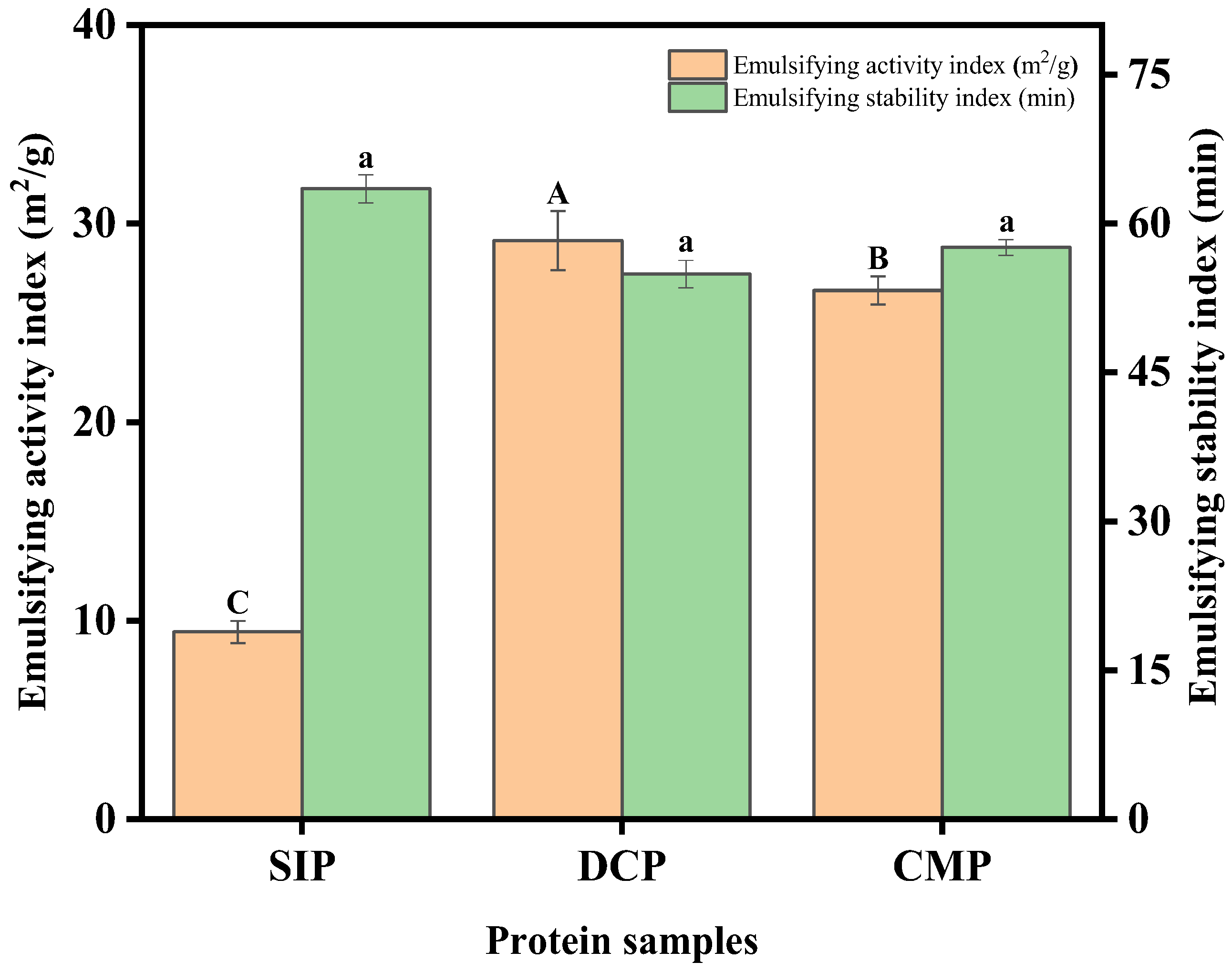
3.9.4. Emulsifying Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.F.; Kan, J.T.; Liu, X.Y.; Song, F.; Zhu, K.X.; Li, N. Chemical components, nutritional value, volatile organic compounds and biological activities in vitro of coconut (Cocos nucifera L.) water with different maturities. Foods 2024, 13, 863. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.; Carrascosa, C.; Ramos, F.; Raheem, D.; Lopes, M.; Raposo, A. Coconut sugar: Chemical analysis and nutritional profile; Health impacts; Safety and quality control; Food industry applications. Int. J. Environ. Res. Public Health 2023, 20, 3671. [Google Scholar] [CrossRef]
- Divya, P.M.; Roopa, B.S.; Manusha, C.; Balannara, P. A concise review on oil extraction methods, nutritional and therapeutic role of coconut products. J. Food Sci. Tech. Mys. 2022, 60, 441–452. [Google Scholar] [CrossRef]
- Wu, J.W.; Tang, Y.J.; Chen, W.X.; Chen, H.M.; Zhong, Q.P.; Pei, J.F. Mechanism for improving coconut milk emulsions viscosity by modifying coconut protein structure and coconut milk properties with monosodium glutamate. Int. J. Biol. Macromol. 2023, 252, 126139. [Google Scholar] [CrossRef]
- Chambal, B.; Bergenståhl, B.; Dejmek, P. Edible proteins from coconut milk press cake; one step alkaline extraction and characterization by electrophoresis and mass spectrometry. Food Res. Int. 2012, 47, 146–151. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, H.Y.; Ye, J. Variation of structures of ingredients of desiccated coconut during hydrolysis by hydrochloric acid at low temperature. Food Sci. Technol. 2017, 37, 593–598. [Google Scholar] [CrossRef][Green Version]
- Martínez-Padilla, L.P.; Hernández-Rojas, F.S.; Sosa-Herrera, M.G.; Juliano, P. Novel application of ultrasound and microwave-assisted methods for aqueous extraction of coconut oil and proteins. J. Food Sci. Technol. 2022, 59, 3857–3866. [Google Scholar] [CrossRef]
- Kumalasari, I.D.; Santosa, I.; Sulistiawati, E. Coconut Oil Production with Various Roasting Temperatures and Dried Grated Coconut as a By-Product. IOP Conf. Ser. Earth Environ. Sci. 2020, 515, 012026. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Li, Y.; Zhang, Y.L.; Zhao, S.L. Purification, characterization and synthesis of antioxidant peptides from enzymatic hydrolysates of coconut (Cocos nucifera L.) cake protein isolates. Rsc. Adv. 2016, 6, 54346–54356. [Google Scholar] [CrossRef]
- Wu, J.W.; Tang, Y.J.; Zhang, M.; Chen, W.X.; Chen, H.H.; Zhong, Q.P. Mechanism for improving the in vitro digestive properties of coconut milk by modifying the structure and properties of coconut proteins with monosodium glutamate. Food Res. Int. 2024, 185, 114288. [Google Scholar] [CrossRef]
- Yu, X.Y.; Zou, Y.; Zheng, Q.W.; Lu, F.X.; Li, D.H.; Guo, L.Q.; Lin, J.F. Physicochemical, functional and structural properties of the major protein fractions extracted from Cordyceps militaris fruit body. Food Res. Int. 2021, 142, 110211. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Physicochemical and functional properties of protein concentrate from by-product of coconut processing. Food Chem. 2018, 241, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Benjakul, S. Characteristics of albumin and globulin from coconut meat and their role in emulsion stability without and with proteolysis. Food Hydrocoll. 2017, 69, 220–228. [Google Scholar] [CrossRef]
- Deng, Y.J.; Huang, L.X.; Zhang, C.H.; Xie, P.J. Chinese quince seed proteins: Sequential extraction processing and fraction characterization. J. Food Sci. Technol. 2019, 57, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Liu, J.H.; Lv, J.R.; Li, Q.Y.; Oh, D.H.; Fu, X. Ultrasound-assisted glycation on ovalbumin fibrosis: A novel, efficient immobilization for lipase. Food Biosci. 2023, 56, 103358. [Google Scholar] [CrossRef]
- Pi, J.Z.; Wang, J.; Lv, J.R.; Jin, Y.G.; Oh, D.H.; Fu, X. Modification of ovalbumin by the enzymatic method: Consequences for foaming characteristics of fibrils. Food Hydrocoll. 2023, 139, 108492. [Google Scholar] [CrossRef]
- Naik, A.; Raghavendra, S.N.; Raghavarao, K.S.M.S. Production of coconut protein powder from coconut wet processing waste and its characterization. Appl. Biochem. Biotechnol. 2012, 167, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.G.; Thampi, B.S.; Menon, V.P.; Leelamma, S. Influence of dietary fiber from coconut kernel (Cocos nucifera) on the 1,2-dimethylhydrazine-induced lipid peroxidation in rats. J. Nutr. Biochem. 1999, 10, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Fathollahy, I.; Farmani, J.; Kasaai, M.R.; Hamishehkar, H. Characteristics and functional properties of Persian lime (Citrus latifolia) seed protein isolate and enzymatic hydrolysates. LWT-Food Sci. Technol. 2021, 140, 110765. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, M.; Shen, Y. Effects of ultrasound combined with preheating treatment to improve the thermal stability of coconut milk by modifying the physicochemical properties of coconut protein. Foods 2022, 11, 1042. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Ma, C.M.; Bian, X.; Liu, X.F.; Wang, Y.; Chen, F.l.; Wang, B.; Zhang, G.; Zhang, N. Characterization of the structure, antioxidant activity and hypoglycemic activity of soy (Glycine max L.) protein hydrolysates. Food Res. Int. 2023, 173, 113473. [Google Scholar] [CrossRef]
- Liu, F.F.; Li, Y.Q.; Wang, C.Y.; Liang, Y.; Zhao, X.Z.; He, J.X.; Mo, H.Z. Physicochemical, functional and antioxidant properties of mung bean protein enzymatic hydrolysates. Food Chem. 2022, 393, 133397. [Google Scholar] [CrossRef]
- Ai, M.M.; Tang, T.; Zhou, L.D.; Ling, Z.T.; Guo, S.G.; Jiang, A.M. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2019, 87, 933–942. [Google Scholar] [CrossRef]
- Mo, X.Q.; Wang, D.H.; Sun, X.S. Physicochemical properties of β and α′α subunits isolated from soybean β-conglycinin. J. Agric. Food Chem. 2011, 59, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Azargohar, R.; Nanda, S.; Rao, B.V.S.K.; Dalai, A.K. Slow pyrolysis of deoiled canola meal: Product yields and characterization. Energy Fuels 2013, 27, 5268–5279. [Google Scholar] [CrossRef]
- Li, Y.; Kong, B.; Xia, X. Structural changes of the myofibrillar proteins in common carp (Cyprinus carpio) muscle exposed to a hydroxyl radical-generating system. Process Biochem. 2013, 48, 863–870. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, X.; Gantumur, M.A.; Li, J.; Huang, Y.; Sukhbaatar, N. Extrusion of casein and whey protein isolate enhances anti-hardening and performance in high-protein nutrition bars. Food Chem. X 2023, 18, 100719. [Google Scholar] [CrossRef] [PubMed]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the physicochemical properties of pea protein by phshifting and ultrasound combined treatments. Ultrason. Sonochem. 2017, 38, 835–842. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, M.; Wang, Y.; Zhang, D.; Zhao, S.; Liu, J. Foaming properties of egg white proteins improved by enzymatic hydrolysis: The changes in structure and physicochemical properties. Food Hydrocoll. 2023, 141, 108681. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Du, H.; Lin, Y.; Stanton, C.; Daniloski, D.; Zannini, E.; Ross, R.P. Characterization and functional properties of pH- and heated time-induced aggregates from red lentil protein. Food Struc. 2023, 37, 100342. [Google Scholar] [CrossRef]
- Delahaije, R.J.B.M.; Gruppen, H.; Giuseppin, M.L.F.; Wierenga, P.A. Quantitative description of the parameters affecting the adsorption behaviour of globular proteins. Colloid. Surface B 2014, 123, 199–206. [Google Scholar] [CrossRef]
- Tian, Y.; Pi, J.; Lv, J.; Chen, Y.; Ma, M.; Fu, X. The impact of ultrasound treatment combined with flaxseed gum on the foaming properties of egg white. Food Hydrocoll. 2024, 148, 109507. [Google Scholar] [CrossRef]
- Kang, Z.L.; Bai, R.; Lu, F.; Zhang, T.; Gao, Z.S.; Zhao, S.M. Effects of high pressure homogenization on the solubility, foaming, and gel properties of soy 11S globulin. Food Hydrocoll. 2022, 124, 107261. [Google Scholar] [CrossRef]
- Ma, Y.G.; Zhang, J.; He, J.M.; Xu, Y.J.; Guo, X.B. Effects of high-pressure homogenization on the physicochemical, foaming, and emulsifying properties of chickpea protein. Food Res. Int. 2023, 170, 112986. [Google Scholar] [CrossRef]

| Defatted Desiccated Coconut | Defatted Copra Meal | DCP | CMP | |
|---|---|---|---|---|
| Moisture (%) | 5.32 ± 0.17 | 9.11 ± 0.34 | 7.26 ± 0.31 | 7.85 ± 0.26 |
| Carbohydrate (%) | 37.89 ± 1.23 | 30.91 ± 0.81 | 18.17 ± 0.11 | 13.94 ± 0.27 |
| Protein (%) | 43.50 ± 1.56 | 47.37 ± 1.07 | 56.28 ± 0.18 | 58.11 ± 2.14 |
| Fat (%) | 5.15 ± 0.15 | 3.24 ± 0.86 | 3.95 ± 0.34 | 1.99 ± 0.03 |
| Ash (%) | 3.13 ± 0.13 | 5.79 ± 0.37 | 3.04 ± 0.19 | 6.57 ± 1.38 |
| Amino Acid b | DCP | CMP | SIP a | WHO/FAO c | |
|---|---|---|---|---|---|
| Child | Adult | ||||
| Essential amino acid | |||||
| Thr | 37.53 | 36.83 | 30.72 | 34.00 | 9.00 |
| Val | 70.24 | 66.85 | 47.87 | 35.00 | 13.00 |
| Met | 29.08 | 26.73 | 15.41 | ||
| Ile | 48.77 | 44.61 | 60.78 | 28.00 | 13.00 |
| Leu | 85.57 | 82.98 | 63.39 | 66.00 | 19.00 |
| Phe | 55.72 | 56.97 | 45.15 | ||
| Lys | 28.89 | 33.18 | 68.92 | 58.00 | 5.00 |
| His | 22.87 | 23.95 | 22.14 | 19.00 | 16.00 |
| Total essential amino acid | 378.67 | 372.10 | 326.50 | ||
| Non-essential amino acid | |||||
| Asp | 93.84 | 95.59 | 121.68 | ||
| Ser | 46.25 | 45.55 | 55.79 | ||
| Glu | 181.61 | 197.80 | 180.94 | ||
| Gly | 49.69 | 49.56 | 42.44 | ||
| Ala | 51.63 | 50.95 | 43.74 | ||
| Cys | 4.31 | 4.18 | 18.89 | ||
| Arg | 119.97 | 113.32 | 83.03 | ||
| Tyr | 35.34 | 31.50 | 34.52 | ||
| Pro | 38.70 | 39.45 | 64.58 | ||
| EAA/TAA (%) | 37.87 | 37.21 | 35.44 | ||
| Amino acids with different characterization d | |||||
| Basic | 171.73 | 170.45 | 174.10 | ||
| Acidic | 275.44 | 293.39 | 302.62 | ||
| Charged polar | 447.17 | 463.84 | 476.72 | ||
| Hydrophobic | 379.71 | 368.54 | 340.93 | ||
| Sulfur-containing | 33.39 | 30.91 | 34.30 | ||
| Sample | Secondary Structures (%) | |||
|---|---|---|---|---|
| β-Sheet | α-Helix | β-Turn | Random | |
| SIP | 33.85 | 10.94 | 31.38 | 23.82 |
| DCP | 36.43 | 11.62 | 26.19 | 25.76 |
| CMP | 31.39 | 11.7 | 31.72 | 25.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, D.; Liu, W.; Kan, J.; Zhang, Y. Effect of Dry Processing of Coconut Oil on the Structure and Physicochemical Properties of Coconut Isolate Proteins. Foods 2024, 13, 2496. https://doi.org/10.3390/foods13162496
Liu X, Yang D, Liu W, Kan J, Zhang Y. Effect of Dry Processing of Coconut Oil on the Structure and Physicochemical Properties of Coconut Isolate Proteins. Foods. 2024; 13(16):2496. https://doi.org/10.3390/foods13162496
Chicago/Turabian StyleLiu, Xiaoyan, Duwei Yang, Wantong Liu, Jintao Kan, and Yufeng Zhang. 2024. "Effect of Dry Processing of Coconut Oil on the Structure and Physicochemical Properties of Coconut Isolate Proteins" Foods 13, no. 16: 2496. https://doi.org/10.3390/foods13162496
APA StyleLiu, X., Yang, D., Liu, W., Kan, J., & Zhang, Y. (2024). Effect of Dry Processing of Coconut Oil on the Structure and Physicochemical Properties of Coconut Isolate Proteins. Foods, 13(16), 2496. https://doi.org/10.3390/foods13162496






