Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animal Grouping, Drug Administration, and Sample Collection
2.3. Gastric Emptying and Small Intestinal Propulsion Assessments
2.4. Quantitative Assessment of the Gastrointestinal Hormone Concentrations in the Murine Serum
2.5. Spontaneous Activity of the Isolated Gastrointestinal Tissues
2.6. Western Blot Analysis
2.7. 16S rDNA Sequencing
2.8. Data Analysis
3. Results
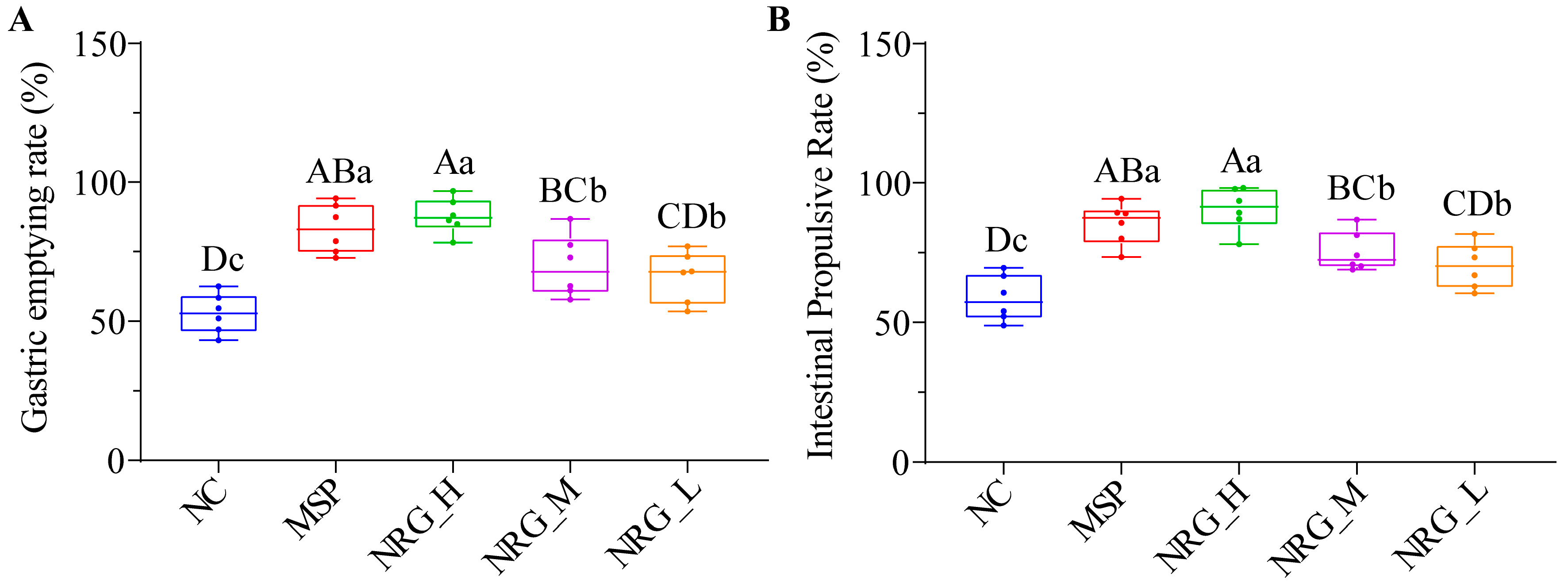
3.1. NRG Enhances Gastric Emptying and Small Intestinal Propulsion in Normal Mice
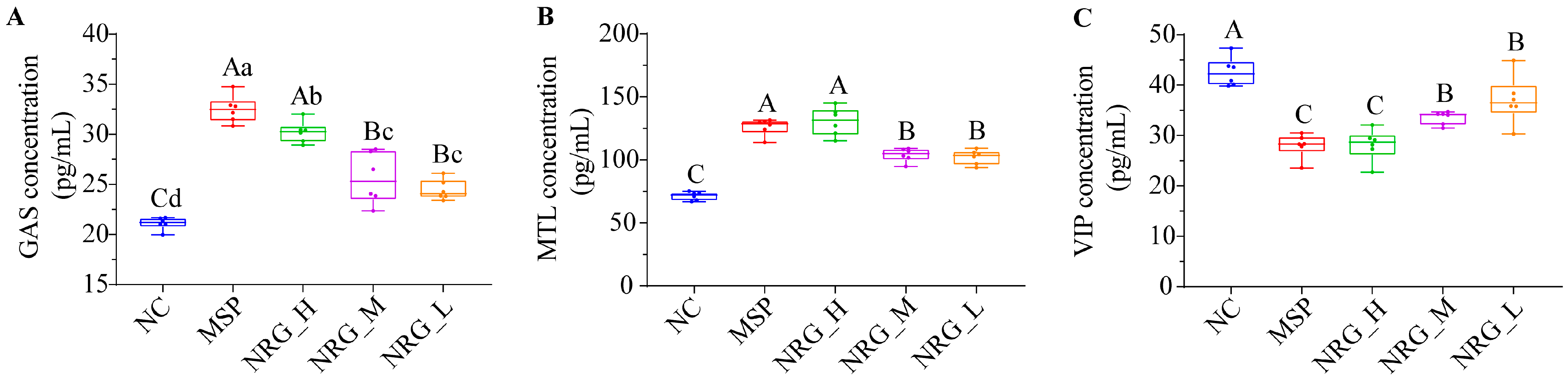
3.2. NRG Regulates Three Gastrointestinal Hormone Expression Levels in Serum
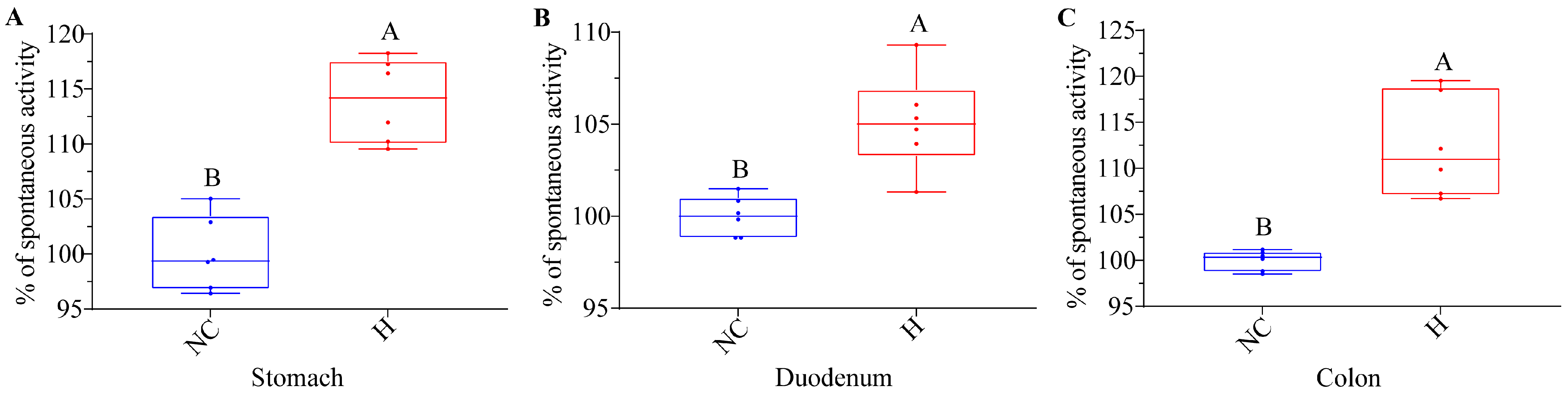
3.3. NRG Boosts the Spontaneous Activity of Isolated Gastrointestinal Tissues
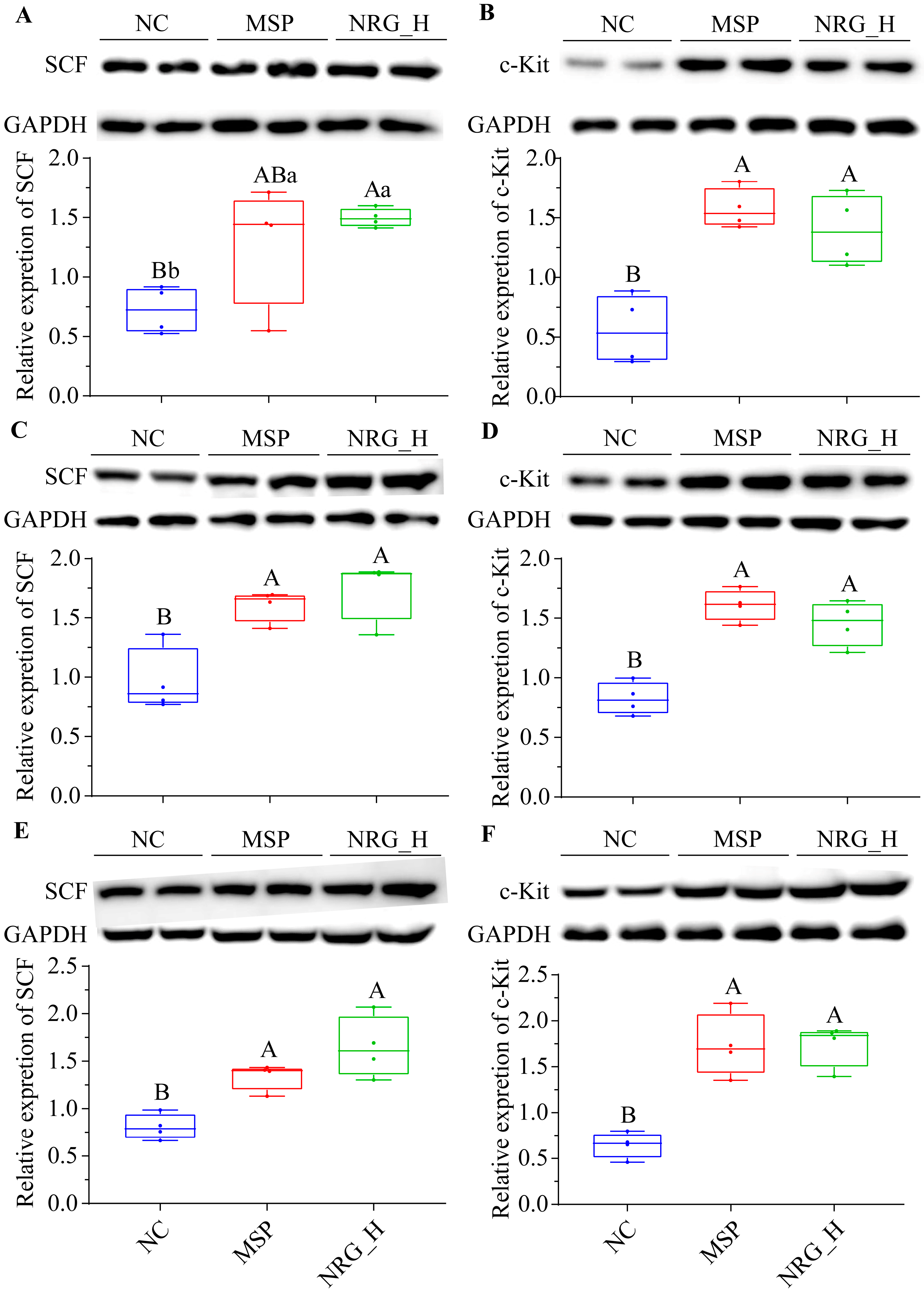
3.4. NRG Enhances the Expression of SCF and c-Kit Proteins in Gastrointestinal Tissues
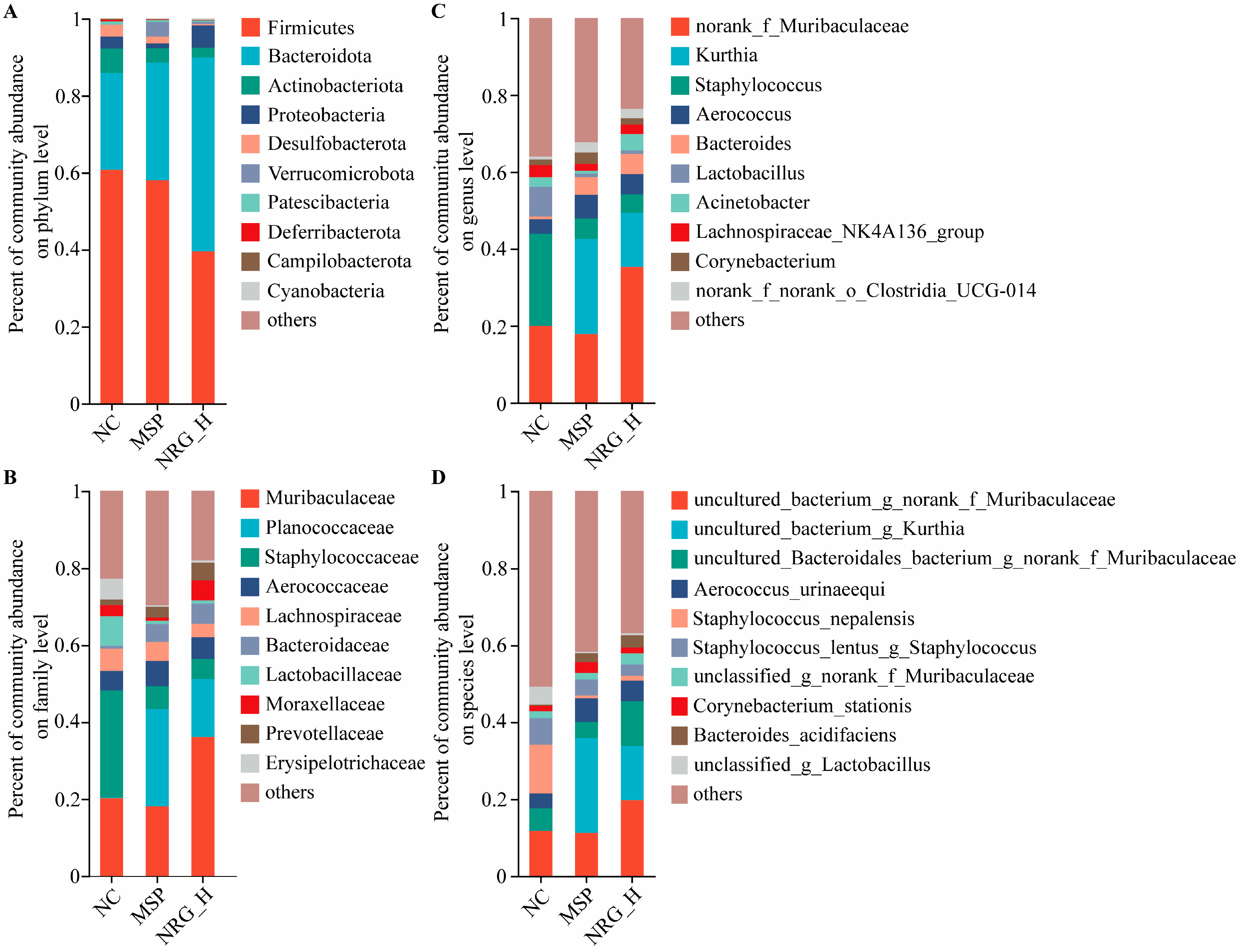
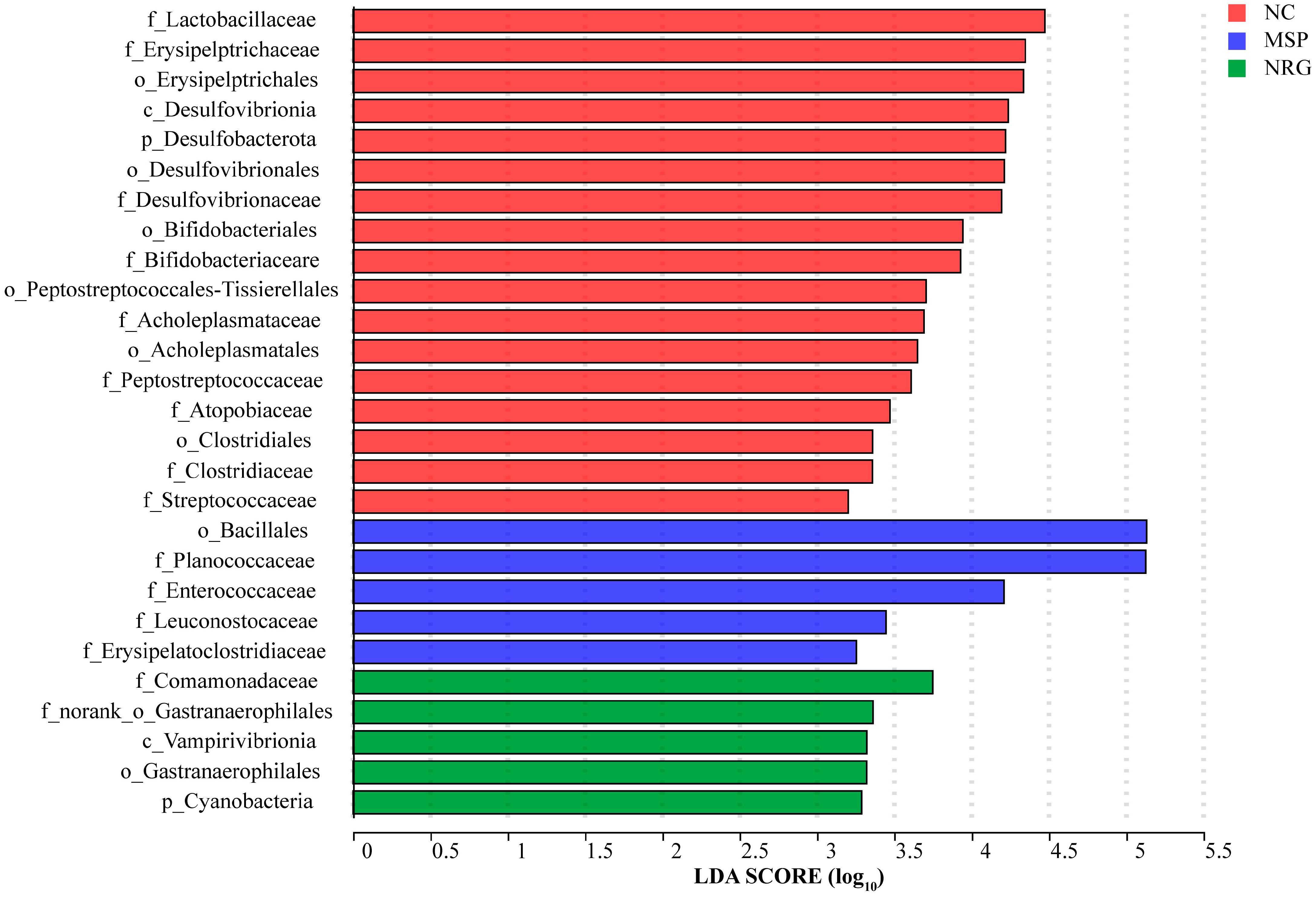
3.5. NRG Modulates Gut Microbiota Composition
4. Discussion
4.1. Regulating the Secretion of Related Hormones
4.2. Improving the Expression Levels of Proteins Associated with the SCF/c-kit Signaling Pathway
4.3. Influencing the Composition of Gut Microbiota
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-therapeutic potential of naringenin (4′,5,7-Trihydroxyflavonone): Experimental evidence and mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymanska, N.; Kozlowska, J. Naringenin and its derivatives-health-promoting phytobiotic against resistant bacteria and fungi in humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Markle, J.M.; Hegele, R.A.; Huff, M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, J.; Vanisree, A.J. Naringenin sensitizes resistant C6 glioma cells with a repressive impact on the migrating ability. Ann. Neurosci. 2020, 27, 114–123. [Google Scholar]
- Orrego-Lagaron, N.; Martinez-Huelamo, M.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M.; Escribano-Ferrer, E. High gastrointestinal permeability and local metabolism of naringenin: Influence of antibiotic treatment on absorption and metabolism. Br. J. Nutr. 2015, 114, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef]
- Chin, L.H.; Hon, C.M.; Chellappan, D.K.; Chellian, J.; Madheswaran, T.; Zeeshan, F.; Awasthi, R.; Aljabali, A.A.; Tambuwala, M.M.; Dureja, H.; et al. Molecular mechanisms of action of naringenin in chronic airway diseases. Eur. J. Pharmacol. 2020, 879, 173139. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Krishnamurthy, B.; Bhatia, J.; Sharma, C.; Kamal, M.A.; Ojha, S.; Arya, D.S. Pharmacological properties and therapeutic potential of naringenin: A citrus flavonoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 4341–4359. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical rial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Chao, P.D.L.; Hou, Y.C.; Hsiu, S.L.; Wen, K.C.; Tsai, S.Y. Pharmacokinetics and conjugation metabolism of naringin and naringenin in rats after single dose and multiple dose administrations. J. Food Drug Anal. 2020, 14, 247. [Google Scholar] [CrossRef]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef]
- Stickney, J.C.; Northup, D.W. Effect of gastric emptying upon propulsive motility of small intestine of rats. Proc. Soc. Exp. Biol. Med. 1959, 101, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jin, X.; Zheng, C.; Ma, F.; Zhang, X.; Gao, P.; Gao, J.; Zhang, L. Bidirectional effects of Mao Jian Green Tea and its flavonoid glycosides on gastrointestinal motility. Foods 2023, 12, 854. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yue, M.; Cheng, Y.; Sullivan, M.A.; Chen, W.; Yu, H.; Li, F.; Wu, S.; Lv, Y.; Zhai, X.; et al. Naringenin prevents non-alcoholic steatohepatitis by modulating the host metabolome and intestinal microbiome in MCD diet-fed mice. Food Sci. Nutr. 2023, 11, 7826–7840. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Jia, X.; Hu, M. Disposition of formononetin via enteric recycling: Metabolism and excretion in mouse intestinal perfusion and Caco-2 cell models. Mol. Pharm. 2005, 2, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.; Thevenin, C. Physiology, Gastrointestinal Hormonal Control. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Jang, Y.; Kim, T.K.; Shim, W.S. Naringin exhibits in vivo prokinetic activity via activation of ghrelin receptor in gastrointestinal motility dysfunction rats. Pharmacology 2013, 92, 191–197. [Google Scholar] [CrossRef]
- Park, M.; Kim, K.; Lee, Y.M.; Rhyu, M.R.; Kim, H.Y. Naringenin stimulates cholecystokinin secretion in STC-1 cells. Nutr. Res. Pract. 2014, 8, 146–150. [Google Scholar] [CrossRef]
- Blair, P.J.; Rhee, P.L.; Sanders, K.M.; Ward, S.M. The significance of interstitial cells in neurogastroenterology. J. Neurogastroenterol. Motil. 2014, 20, 294–317. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Yang, Z.; Panf, A.; Zuo, W.; Guo, J.; Zhou, W. Relaxant effect of flavonoid naringenin on contractile activity of rat colonic smooth muscle. J. Ethnopharmacol. 2014, 155, 1177–1183. [Google Scholar] [CrossRef]
- Lopez-Pingarron, L.; Almeida, H.; Pereboom-Maicas, D.; Garcia, J.J. Pathophysiological implications of interstitial Cajal-like cells (ICC-like) in uterus: A comparative study with gastrointestinal ICCs. Curr. Issues Mol. Biol. 2023, 45, 7557–7571. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.Y.; Choi, S.H.; Son, C.G. Interstitial cells of Cajal: Potential targets for functional dyspepsia treatment using medicinal natural products. Evid.-Based Complement. Altern. Med. 2021, 2021, 9952691. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M. Spontaneous electrical activity and rhythmicity in gastrointestinal smooth muscles. Adv. Exp. Med. Biol. 2019, 1124, 3–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shui, L.; Liu, Y.; Zheng, M. C-Kit, a double-edged sword in liver regeneration and diseases. Front. Genet. 2021, 12, 598855. [Google Scholar] [CrossRef]
- Sheikh, E.; Tran, T.; Vranic, S.; Levy, A.; Bonfil, R.D. Role and significance of c-KIT receptor tyrosine kinase in cancer: A review. Bosn J. Basic Med. Sci. 2022, 22, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Huang, Y.; Tang, H.; Tu, X.; He, J.; Wang, T.; Zhang, Q.; Xiong, F.; Li, D.; Qiu, Z. Role of stem cell growth factor/c-Kit in the pathogenesis of irritable bowel syndrome. Exp. Ther. Med. 2017, 13, 1187–1193. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chen, Y.T.; Gao, W.Y.; Wu, M.J.; Yen, J.H. Nobiletin down-regulates c-KIT gene expression and exerts antileukemic effects on human acute myeloid leukemia Cells. J. Agric. Food Chem. 2018, 66, 13423–13434. [Google Scholar] [CrossRef]
- Sakai-Kashiwabara, M.; Abe, S.; Asano, K. Suppressive activity of quercetin on the production of eosinophil chemoattractants from eosinophils in vitro. In Vivo 2014, 28, 515–522. [Google Scholar] [PubMed]
- Wu, Y.X.; Yang, X.Y.; Han, B.S.; Hu, Y.Y.; An, T.; Lv, B.H.; Lian, J.; Wang, T.Y.; Bao, X.L.; Gao, L.; et al. Naringenin regulates gut microbiota and SIRT1/PGC-1a signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Biomed. Pharmacother. 2022, 153, 113286. [Google Scholar] [CrossRef]
- Chang, S.C.; Shen, M.H.; Liu, C.Y.; Pu, C.M.; Hu, J.M.; Huang, C.J. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer. Oncol. Lett. 2020, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Agraib, L.M.; Yamani, M.I.; Tayyem, R.; Abu-Sneineh, A.T.; Rayyan, Y.M. Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: A pilot, randomized, double-blind, placebo-controlled study. Clin. Nutr. ESPEN 2022, 51, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Tanabe, S.; Suzuki, T. Naringenin enhances intestinal barrier function through the expression and cytoskeletal association of tight junction proteins in Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 2019–2028. [Google Scholar] [CrossRef]
- Yu, R.; Gu, Y.; Zheng, L.; Liu, Z.; Bian, Y. Naringenin prevents NAFLD in the diet-induced C57BL/6J obesity model by regulating the intestinal barrier function and microbiota. J. Funct. Foods 2023, 105, 105578. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Argoty, G.A.; Zhang, L.; Tomasula, P.; Wang, M.; Pontious, S.; Kobori, M.; Xiao, W. Analysis of temporal changes in growth and gene expression for commensal gut microbes in response to the polyphenol naringenin. Microbiol. Insights 2018, 11, 1178636118775100. [Google Scholar] [CrossRef]
- Liang, S.; He, Z.P.; Liang, Z.P.; Wang, K.; Du, B.; Guo, R.X.; Li, P. Prunus persica (L.) Batsch blossom soluble dietary fiber synergia polyphenol improving loperamide-induced constipation in mice via regulating stem cell factor/C-kit, NF-κB signaling pathway and gut microbiota. Food Res. Int. 2024, 192, 114761. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, B.; Du, A.Q.; Zhou, Z.Y.; Lu, D.Z.; Zhu, Z.H.; Ke, S.Z.; Wang, S.J.; Yu, Y.L.; Chen, J.W.; et al. Saccharina japonica fucan suppresses high fat diet-induced obesity and enriches fucoidan-degrading gut bacteria. Carbohydr. Polym. 2022, 290, 119411. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Sasaki, T.; Itoh, K.; Kitahara, T.; Takema, Y.; Hiramatsu, K.; Ishikawa, D.; Shibuya, T.; Kobayashi, O.; Osada, T.; et al. A soluble fiber diet increases bacteroides fragilis group abundance and immunoglobulin a production in the gut. Appl. Environ. Microbiol. 2020, 86, e00405–e00420. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Guo, H.; Li, J.; Li, Z.; Yan, Z.; Wei, L.; Hua, Y.; Lin, L.; Tian, Y. Exploring the mechanism of action of Sanzi formula in intervening colorectal adenoma by targeting intestinal flora and intestinal metabolism. Front. Microbiol. 2022, 13, 1001372. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Du, Y.; Ren, D.; Yang, X.; Zhao, Y. Gut microbiota-dependent catabolites of tryptophan play a predominant role in the protective effects of turmeric polysaccharides against DSS-induced ulcerative colitis. Food Funct. 2021, 12, 9793–9807. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Niu, Y.; Ren, B.; Wang, S.; Song, Y.; Wang, X.; Zhao, K.; Yue, Z.; Li, Y.; Gao, J. Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota. Foods 2024, 13, 2520. https://doi.org/10.3390/foods13162520
Wu L, Niu Y, Ren B, Wang S, Song Y, Wang X, Zhao K, Yue Z, Li Y, Gao J. Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota. Foods. 2024; 13(16):2520. https://doi.org/10.3390/foods13162520
Chicago/Turabian StyleWu, Lei, Yao Niu, Boyang Ren, Shengyu Wang, Yuhong Song, Xingyu Wang, Kai Zhao, Zhao Yue, Yaru Li, and Jianhua Gao. 2024. "Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota" Foods 13, no. 16: 2520. https://doi.org/10.3390/foods13162520
APA StyleWu, L., Niu, Y., Ren, B., Wang, S., Song, Y., Wang, X., Zhao, K., Yue, Z., Li, Y., & Gao, J. (2024). Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota. Foods, 13(16), 2520. https://doi.org/10.3390/foods13162520




