Canavanine Content Quantification in Processed Bitter Vetch (Vicia ervilia) and Its Application as Flour in Breads: An Analysis of Nutritional and Sensory Attributes
Abstract
1. Introduction
2. Methods
2.1. Chemicals
2.2. Seed Samples
2.3. Processing
2.4. Basic Analysis
2.5. Bread Preparation
2.6. Determination of Nutritional Composition
2.7. Calculations of Protein-Digestibility-Corrected Amino Acid Scores (PDCAASs)
2.8. Determination of Mineral Composition
2.9. Sensory Evaluation of the Bitter Vetch Breads
2.10. Extraction of Canavanine
2.11. Precolumn Derivatization
2.12. HPLC Analysis
2.13. Statistical Analyses
3. Results and Discussion
3.1. Bitter Vetch’s Nutritional Value
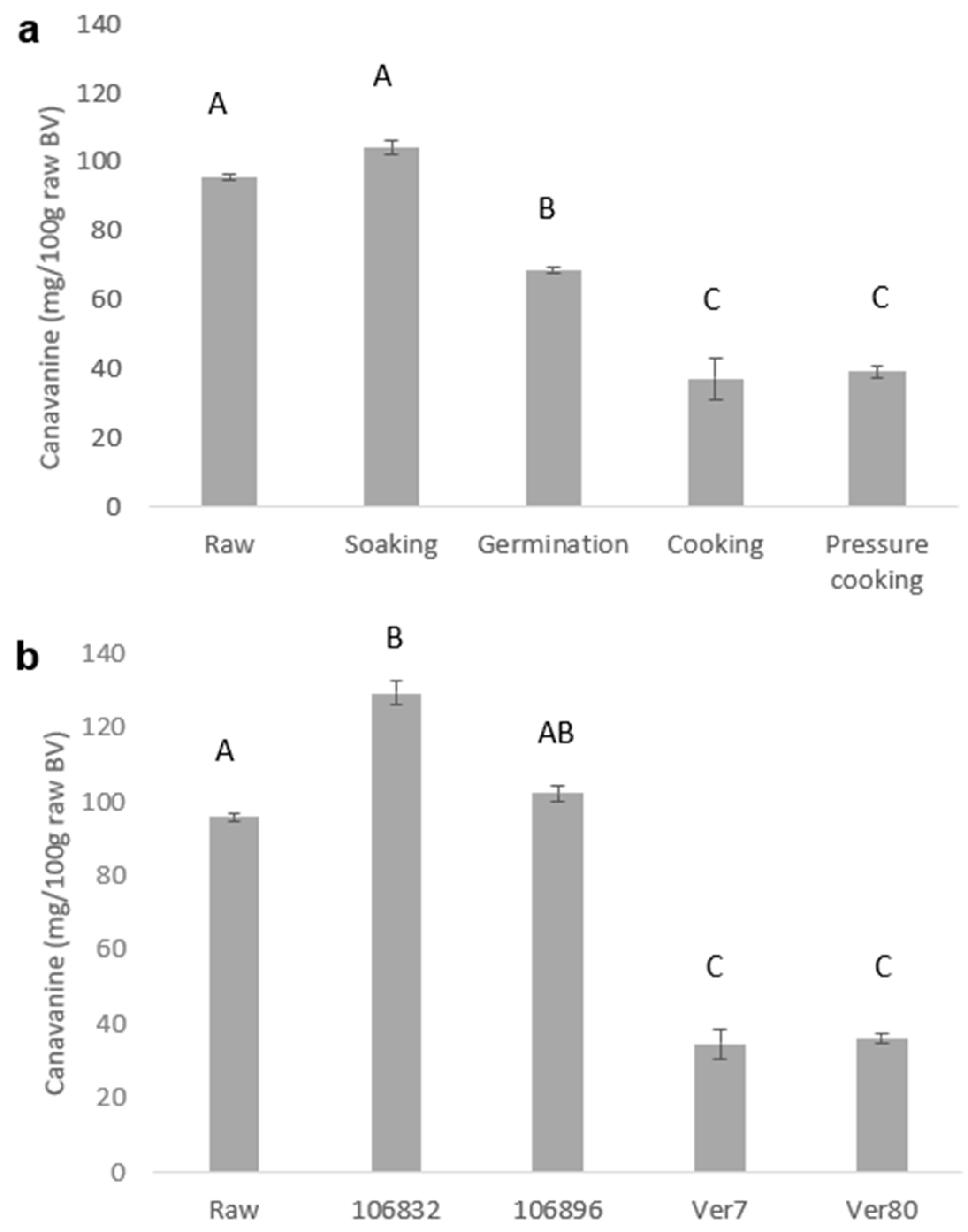
3.2. Effects of Processing on Canavanine Content
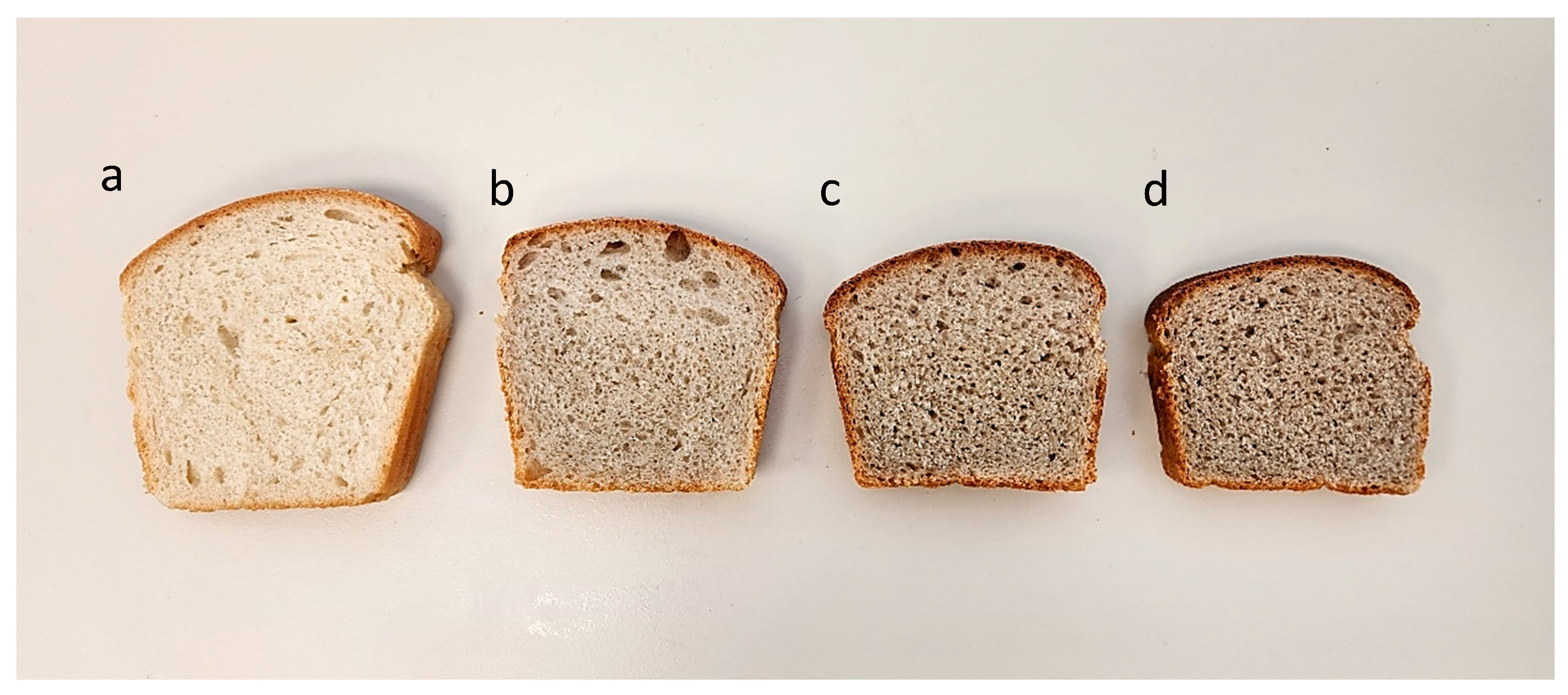
3.3. Bitter Vetch Breads
3.4. Nutritional Values, Canavanine Contents, and Mineral Contents of Bitter Vetch Breads
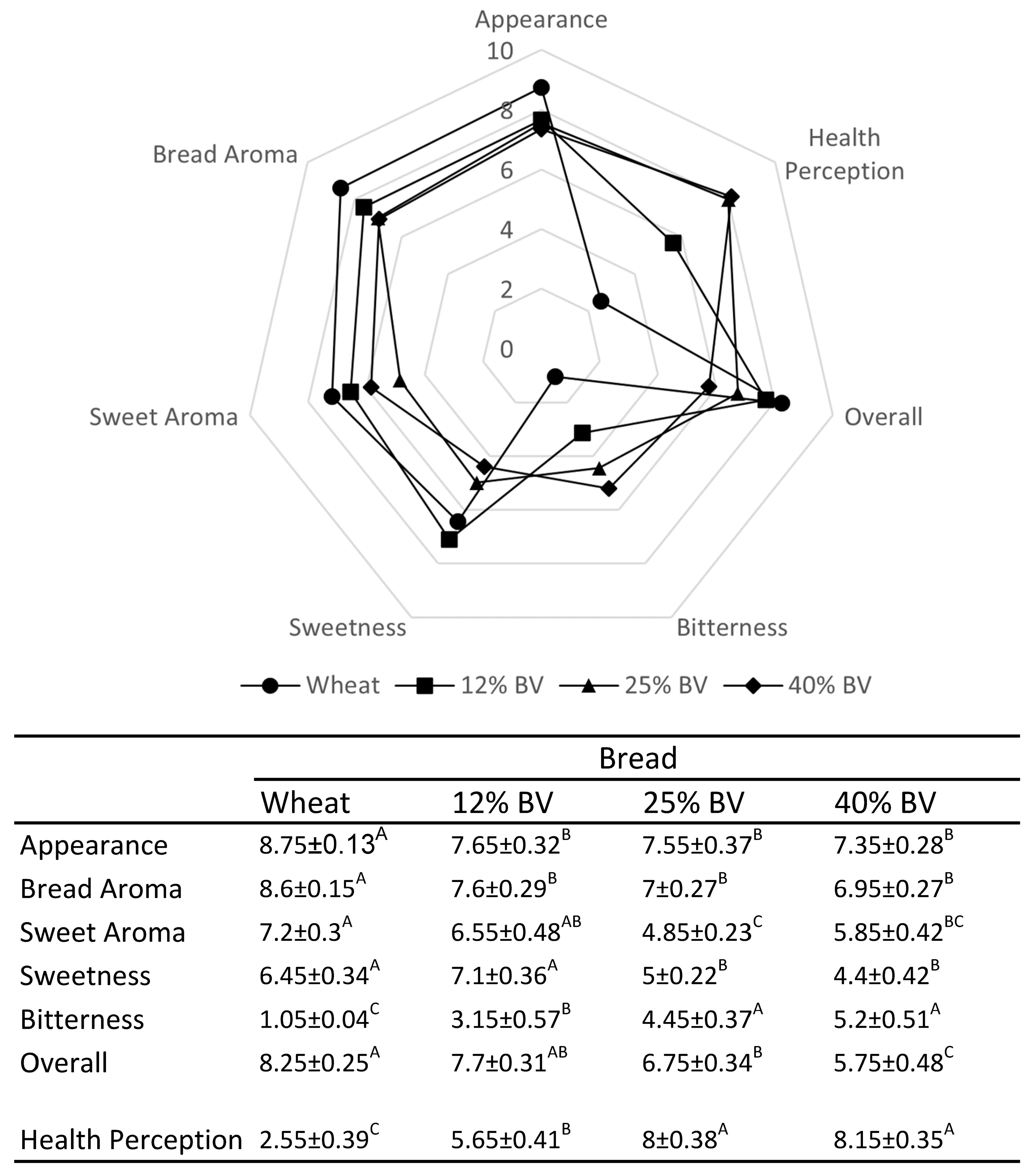
3.5. Sensory Evaluation of Bitter Vetch Breads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, E.; Tirimanna, A. Associations of amino acids and related compounds in the seeds of forty-seven species of Vicia: Their taxonomic and nutritional significance. Biochem. J. 1965, 97, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hedges, J.B.; Ryan, K.S. Biosynthetic pathways to nonproteinogenic α-amino acids. Chem. Rev. 2020, 120, 3161–3209. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.A. l-Canavanine: A higher plant insecticidal allelochemical. Amino Acids 2001, 21, 319–330. [Google Scholar] [CrossRef]
- Rosenthal, G.A. The biological effects and mode of action of L-canavanine, a structural analogue of L-arginine. Q. Rev. Biol. 1977, 52, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Karatsai, O.; Shliaha, P.; Jensen, O.N.; Stasyk, O.; Rędowicz, M.J. Combinatory treatment of canavanine and arginine deprivation efficiently targets human glioblastoma cells via pleiotropic mechanisms. Cells 2020, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Green, M.H.; Brooks, T.L.; Mendelsohn, J.; Howell, S.B. Antitumor activity of l-canavanine against L1210 murine leukemia. Cancer Res. 1980, 40, 535–537. [Google Scholar] [PubMed]
- European Food Safety Authority (EFSA). Opinion on the safety of ‘Alfalfa protein concentrate’ as food. EFSA J. 2009, 7, 997. [Google Scholar] [CrossRef]
- Enneking, D. The Toxicity of Vicia Species and Their Utilisation as Grain Legumes. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 1994. Available online: https://digital.library.adelaide.edu.au/dspace/handle/2440/37799 (accessed on 8 August 2024).
- Hatamikia, M.; Elhamirad, A.H.; Heydari, R.; Sharayei, P.; Azarpazhooh, E. Investigating the effect of various methods of soaking from Vicia ervilia in water and alkaline, acid and salt solutions on reduction of anti-nutritional compounds. Int. J. Biol. Chem. 2019, 12, 48–59. [Google Scholar] [CrossRef]
- Golchin-Gelehdooni, S.; Shawrang, P.; Nikkhah, A.; Sadeghi, A.A.; Teimouri-Yansari, A. Effect of Extrusion and Conventional Processing Methods on the Levels of Anti-Nutrients Factors and Digestibility of Bitter Vetch (Vicia ervilia) Seeds in Broilers. Iran. J. Appl. Anim. Sci. 2014, 4, 835–842. [Google Scholar]
- Enneking, D.; Leulou, R.; Nouttir, N.; Nouneimate, M. A note on Vicia ervilia cultivation utilisation and toxicity in Morocco. Al Awamia 1995, 89, 141–148. [Google Scholar]
- Gavériaux, F.; Motta, L.; Bailey, P.; Brilli, M.; Sadori, L. Crop husbandry at Gabii during the Iron Age and Archaic period: The archaeobotanical and stable isotope evidence. Environ. Archaeol. 2022, 1–14. [Google Scholar] [CrossRef]
- Abbo, S.; Lev-Yadun, S.; Gopher, A. Yield stability: An agronomic perspective on the origin of Near Eastern agriculture. Veg. Hist. Archaeobotany 2010, 19, 143–150. [Google Scholar] [CrossRef]
- Ficiciyan, A.; Loos, J.; Sievers-Glotzbach, S.; Tscharntke, T. More than yield: Ecosystem services of traditional versus modern crop varieties revisited. Sustainability 2018, 10, 2834. [Google Scholar] [CrossRef]
- Harlan, J.R. The Living Fields: Our Agricultural Heritage; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Sultan, B.; Defrance, D.; Iizumi, T. Evidence of crop production losses in West Africa due to historical global warming in two crop models. Sci. Rep. 2019, 9, 12834. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.A.; Mayes, S.; Massawe, F. Crop diversification through a wider use of underutilised crops: A strategy to ensure food and nutrition security in the face of climate change. In Sustainable Solutions for Food Security: Combating Climate Change by Adaptation; Sarkar, A., Sensarma, S.R., vanLoon, G.W., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 125–149. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Wezel, A.; Herren, B.G.; Kerr, R.B.; Barrios, E.; Gonçalves, A.L.R.; Sinclair, F. Agroecological principles and elements and their implications for transitioning to sustainable food systems. A review. Agron. Sustain. Dev. 2020, 40, 40. [Google Scholar] [CrossRef]
- Ekanayake, S.; Skog, K.; Asp, N.-G. Canavanine content in sword beans (Canavalia gladiata): Analysis and effect of processing. Food Chem. Toxicol. 2007, 45, 797–803. [Google Scholar] [CrossRef]
- Melcion, J.-P.; Kodaira, M.; León, A.; Michelangeli, C.; Vargas, R.E.; Picard, M. Detoxification of the jackbean (Canavalia ensiformis L.) with pilot scale roasting I.: Technological conditions and analytical data. Anim. Feed. Sci. Technol. 1998, 73, 217–230. [Google Scholar] [CrossRef]
- Flint-Hamilton, K.B. Legumes in ancient Greece and Rome: Food, medicine, or poison? Hesperia J. Am. Sch. Class. Stud. Athens 1999, 68, 371–385. [Google Scholar] [CrossRef]
- Valamoti, S.M. Ground cereal food preparations from Greece: The prehistory and modern survival of traditional Mediterranean ‘fast foods’. Archaeol. Anthropol. Sci. 2011, 3, 19–39. [Google Scholar] [CrossRef]
- Noort, M.W.J.; Renzetti, S.; Linderhof, V.; du Rand, G.E.; Marx-Pienaar, N.J.M.M.; de Kock, H.L.; Magano, N.; Taylor, J.R.N. Towards sustainable shifts to healthy diets and food security in sub-Saharan Africa with climate-resilient crops in bread-type products: A food system analysis. Foods 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Romano, A.; Miralles, B.; Ferranti, P.; Masi, P.; Santos-Hernández, M.; Recio, I. Physicochemical properties, structure and digestibility in simulated gastrointestinal environment of bread added with green lentil flour. LWT 2022, 154, 112713. [Google Scholar] [CrossRef]
- Kotsiou, K.; Sacharidis, D.-D.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and functional aspects of composite wheat-roasted chickpea flours in relation to dough rheology, bread quality and staling phenomena. Food Hydrocoll. 2022, 124, 107322. [Google Scholar] [CrossRef]
- Cacho, J.; Angeles Garcia, M.; Ferrando, I. Selective spectrophotometric determination of canavanine. Analyst 1989, 114, 965–968. [Google Scholar] [CrossRef]
- Kounnoun, A.; ELMaadoudi, M.; Cacciola, F.; Mondello, L.; Bougtaib, H.; Alahlah, N.; Amajoud, N.; ELBaaboua, A.; Louajri, A. Development and validation of a high-performance liquid chromatography method for the determination of histamine in fish samples using fluorescence detection with pre-column derivatization. Chromatographia 2020, 83, 893–901. [Google Scholar] [CrossRef]
- Negro, A.; Garbisa, S.; Gotte, L.; Spina, M. The use of reverse-phase high-performance liquid chromatography and precolumn derivatization with dansyl chloride for quantitation of specific amino acids in collagen and elastin. Anal. Biochem. 1987, 160, 39–46. [Google Scholar] [CrossRef]
- Megías, C.; Cortés-Giraldo, I.; Girón-Calle, J.; Vioque, J.; Alaiz, M. Determination of l-canavanine and other free amino acids in Vicia disperma (Fabaceae) seeds by precolumn derivatization using diethyl ethoxymethylenemalonate and reversed-phase high-performance liquid chromatography. Talanta 2015, 131, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Thiex, N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Nudel, A.; Cohen, R.; Abbo, S.; Kerem, Z. Developing a nutrient-rich and functional wheat bread by incorporating Moringa oleifera leaf powder and gluten. LWT 2023, 187, 115343. [Google Scholar] [CrossRef]
- Horwitz, W.; The Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 1997; Available online: https://repositorioinstitucional.ceu.es/handle/10637/3158 (accessed on 8 August 2024).
- World Health Organization; Food and Agriculture Organization of the United Nations; United Nations University. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO technical report series 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [CrossRef]
- Vioque, J.; Girón-Calle, J.; Torres-Salas, V.; Elamine, Y.; Alaiz, M. Characterization of Vicia ervilia (bitter vetch) seed proteins, free amino acids, and polyphenols. J. Food Biochem. 2020, 44, e13271. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.A.; Tirapegui, J.; Silva, R.S.S. Protein mixtures and their nutritional properties optimized by response surface methodology. Nutr. Res. 2000, 20, 1341–1353. [Google Scholar] [CrossRef]
- Yamada, E.A.; Sgarbieri, V.C. Yeast (Saccharomyces cerevisiae) protein concentrate: Preparation, chemical composition, and nutritional and functional properties. J. Agric. Food Chem. 2005, 53, 3931–3936. [Google Scholar] [CrossRef] [PubMed]
- Olesik, J.W. Elemental analysis using icp-oes and icp/ms. Anal. Chem. 1991, 63, 12A–21A. [Google Scholar] [CrossRef]
- Peryam, D.R.; Pilgrim, F.J. Hedonic scale method of measuring food preferences. Food Technol. 1957, 11, 9–14. [Google Scholar]
- Food Data Central. USDA’s Comprehensive Source of Food Composition Data with Multiple Distinct Data Types. Available online: https://fdc.nal.usda.gov/index.html (accessed on 8 August 2024).
- Romano, A.; Gallo, V.; Ferranti, P.; Masi, P. Lentil flour: Nutritional and technological properties, in vitro digestibility and perspectives for use in the food industry. Curr. Opin. Food Sci. 2021, 40, 157–167. [Google Scholar] [CrossRef]
- Shrestha, S.; van ’t Hag, L.; Haritos, V.S.; Dhital, S. Lentil and mung bean protein isolates: Processing, functional properties, and potential food applications. Food Hydrocoll. 2023, 135, 108142. [Google Scholar] [CrossRef]
- Elamine, Y.; Alaiz, M.; Girón-Calle, J.; Guine, R.; Vioque, J. Nutritional characteristics of the seed protein in 23 Mediterranean legumes. Agronomy 2022, 12, 400. [Google Scholar] [CrossRef]
- Lam van, T.V.; Heindl, M.R.; Schlutt, C.; Böttcher-Friebertshäuser, E.; Bartenschlager, R.; Klebe, G.; Brandstetter, H.; Dahms, S.O.; Steinmetzer, T. The basicity makes the difference: Improved canavanine-derived inhibitors of the proprotein convertase furin. ACS Med. Chem. Lett. 2021, 12, 426–432. [Google Scholar] [CrossRef]
- Sadeghi, G.; Mohammadi, L.; Ibrahim, S.A.; Gruber, K.J. Use of bitter vetch (Vicia ervilia) as a feed ingredient for poultry. World’s Poult. Sci. J. 2009, 65, 51–64. [Google Scholar] [CrossRef]
- Pycia, K.; Ivanišová, E. Physicochemical and antioxidant properties of wheat bread enriched with hazelnuts and walnuts. Foods 2020, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, P.; Nydahl, M.; Kihlberg, I.; Marklinder, I. Consumers’ health-related perceptions of bread–implications for labeling and health communication. Appetite 2018, 121, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Angioloni, A.; Collar, C. High legume-wheat matrices: An alternative to promote bread nutritional value meeting dough viscoelastic restrictions. Eur. Food Res. Technol. 2012, 234, 273–284. [Google Scholar] [CrossRef]
- Schaafsma, G. The protein digestibility-corrected amino acid score (PDCAAS)—A concept for describing protein quality in foods and food ingredients: A critical review. J. AOAC Int. 2005, 88, 988–994. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Reference Amounts Customarily Consumed: List of Products for Each Product Category: Guidance for Industry; US Food and Drug Administration: Washington, DC, USA, 2018. Available online: https://www.fda.gov/media/102587/download (accessed on 21 July 2024).
- Miñarro, B.; Albanell, E.; Aguilar, N.; Guamis, B.; Capellas, M. Effect of legume flours on baking characteristics of gluten-free bread. J. Cereal Sci. 2012, 56, 476–481. [Google Scholar] [CrossRef]
- Kabukcu, C.; Hunt, C.; Hill, E.; Pomeroy, E.; Reynolds, T.; Barker, G.; Asouti, E. Cooking in caves: Palaeolithic carbonised plant food remains from Franchthi and Shanidar. Antiquity 2023, 97, 12–28. [Google Scholar] [CrossRef]
| Source of Protein | Limiting AA | Amount (mg/g of Protein) | FAO/WHO Ref. (mg/g of Protein) | AAS | Protein Digestibility | PDCAAS | Reference |
|---|---|---|---|---|---|---|---|
| BVF | - | - | - | 1 | 0.5 | 0.50 | [36] |
| Gluten | Lysine | 83.2 | 362.5 | 0.23 | 0.983 | 0.23 | [37] |
| Yeast | Leucine | 60 | 66 | 0.91 | 0.68 | 0.62 | [38] |
| Legume | ||||
|---|---|---|---|---|
| Nutritional Value (g/100 g) | Bitter Vetch | Green Lentils | Chickpeas | Dried Peas |
| Calories/100 g | 356 ± 4 | 356 | 378 | 364 |
| Carbohydrates | 63.6 ± 1.2 | 61.1 | 63 | 61.6 |
| Lipids | 1.1 ± 0.1 | 1.6 | 6.1 | 3. 9 |
| Moisture | 9.3 ± 0.6 | 9.8 | 7.7 | 8.7 |
| Protein | 22.9 ± 0.9 | 24.3 | 21.3 | 23.1 |
| Ash | 3.1 ± 0.1 | 3.3 | 2.9 | 2. 7 |
| Percentage BV in Flour (%) | ||||
|---|---|---|---|---|
| Nutritional Value (g/100 g) | 0 | 12 | 25 | 40 |
| Energy (kCal) | 243.33 ± 0.87 A | 251.33 ± 0.32 B | 258.33 ± 0.87 BC | 255 ± 1.52 C |
| Carbohydrates | 48.76 ± 0.08 | 49.86 ± 0.06 | 50.06 ± 0.11 | 49.5 ± 0.15 |
| Total Fiber | 3.83 ± 0.17 A | 5.43 ± 0.08 B | 6.36 ± 0.17 C | 8.36 ± 0.14 D |
| Lipids | 3.25 ± 0.01 | 3.33 ± 0.03 | 3.03 ± 0.03 | 3 ± 0.15 |
| Moisture | 39.94 ± 0.09 A | 37.01 ± 0.05 B | 34.3 ± 0.19 C | 33.77 ± 0.2 C |
| Protein | 6.69 ± 0.01 A | 8.32 ± 0.02 B | 8.97 ± 0.01 C | 11.82 ± 0.03 D |
| Ash | 1.36 ± 0.03 A | 1.6 ± 0.01 B | 1.7 ± 0 C | 2 ± 0 D |
| Mineral content (mg/Kg) | ||||
| Ca | 126.69 ± 2.37 A | 195.13 ± 6.1 B | 228.67 ± 0.63 BC | 251.52 ± 13.9 C |
| Fe | 5.12 ± 0.04 A | 14.87 ± 0.29 B | 20.67 ± 0.19 C | 23.75 ± 1.67 C |
| K | 1680.33 ± 24.83 A | 1760.52 ± 34.54 A | 2376.48 ± 21.82 B | 2678.76 ± 120.81 C |
| Mg | 168.54 ± 1.88 A | 239.69 ± 7.23 B | 284.01 ± 5.73 C | 315.27 ± 11.22 C |
| Mn | 3.28 ± 0.01 A | 5.74 ± 0.1 B | 6.94 ± 0.07 C | 7.76 ± 0.36 C |
| P | 634.29 ± 13.97 A | 1114.82 ± 31.23 B | 1303 ± 23.45 C | 1475.2 ± 58.15 D |
| S | 715.05 ± 12.98 A | 899.47 ± 33.23 B | 908.32 ± 14.21 B | 988.85 ± 35.21 B |
| Zn | 5.77 ± 0.23 A | 11.85 ± 0.35 B | 12.87 ± 0.12 BC | 14.91 ± 0.87 C |
| Source of Protein | Wheat Bread | 12% BVF Bread | 25% BVF Bread | 40% BVF Bread | |
|---|---|---|---|---|---|
| Protein’s Contribution to the Recipe (%) * | BVF | 0.00 | 15.32 | 31.15 | 41.99 |
| Gluten | 94.81 | 80.29 | 65.28 | 55.00 | |
| Yeast | 5.19 | 4.39 | 3.57 | 3.01 | |
| Recipe’s Protein Digestibility | 96.73 | 89.57 | 82.17 | 77.11 | |
| Recipe’s PDCAAS | 24.55 | 28.45 | 32.48 | 35.24 |
| Canavanine (mg/100 g) | 12% BVF Bread | 25% BVF Bread | 40% BVF Bread |
|---|---|---|---|
| Flour Mix | 10.75 ± 0.2 A | 22.03 ± 0.91 A | 35.52 ± 1.21 A |
| Bread Crust | 1.84 ± 0.01 C | 3.78 ± 0.25 C | 6.07 ± 0.64 C |
| Bread Crumb | 6.04 ± 0.71 B | 12.31 ± 0.92 B | 20.07 ± 1.13 B |
| Bread * | 3.87 ± 0.08 D | 7.89 ± 0.66 D | 12.86 ± 1.03 D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nudel, A.; Abbo, S.; Kerem, Z. Canavanine Content Quantification in Processed Bitter Vetch (Vicia ervilia) and Its Application as Flour in Breads: An Analysis of Nutritional and Sensory Attributes. Foods 2024, 13, 2528. https://doi.org/10.3390/foods13162528
Nudel A, Abbo S, Kerem Z. Canavanine Content Quantification in Processed Bitter Vetch (Vicia ervilia) and Its Application as Flour in Breads: An Analysis of Nutritional and Sensory Attributes. Foods. 2024; 13(16):2528. https://doi.org/10.3390/foods13162528
Chicago/Turabian StyleNudel, Adi, Shahal Abbo, and Zohar Kerem. 2024. "Canavanine Content Quantification in Processed Bitter Vetch (Vicia ervilia) and Its Application as Flour in Breads: An Analysis of Nutritional and Sensory Attributes" Foods 13, no. 16: 2528. https://doi.org/10.3390/foods13162528
APA StyleNudel, A., Abbo, S., & Kerem, Z. (2024). Canavanine Content Quantification in Processed Bitter Vetch (Vicia ervilia) and Its Application as Flour in Breads: An Analysis of Nutritional and Sensory Attributes. Foods, 13(16), 2528. https://doi.org/10.3390/foods13162528





