Functional Cyperus esculentus L. Cookies Enriched with the Probiotic Strain Lacticaseibacillus rhamnosus SL42
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
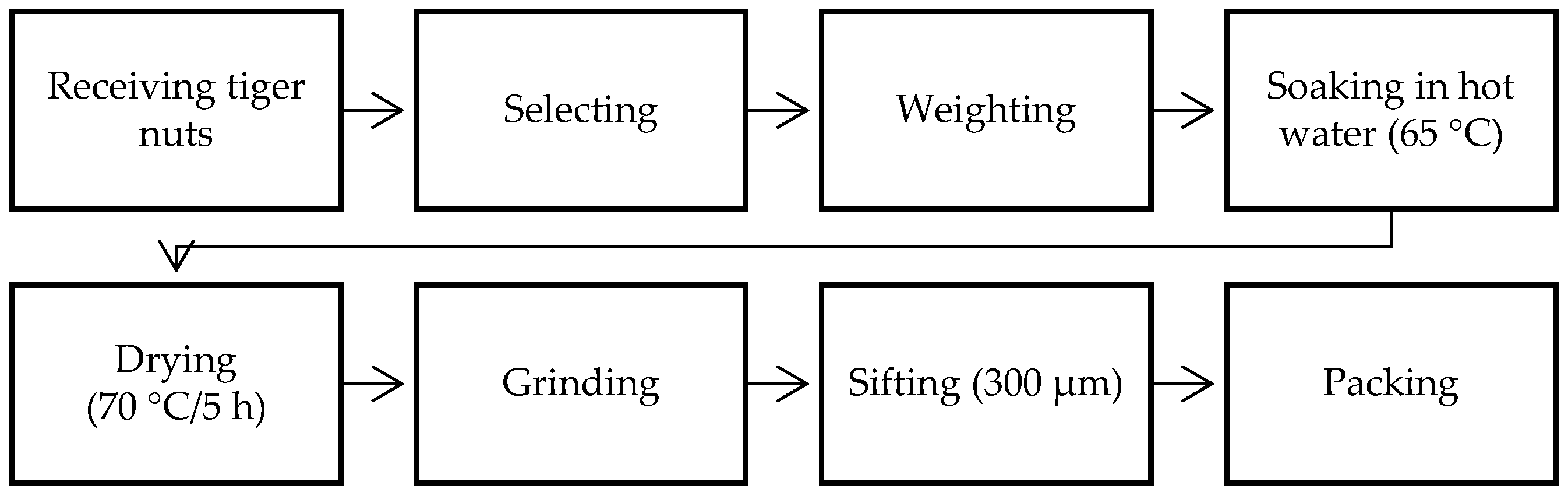
2.2. Reducing Tiger Nuts to Flour
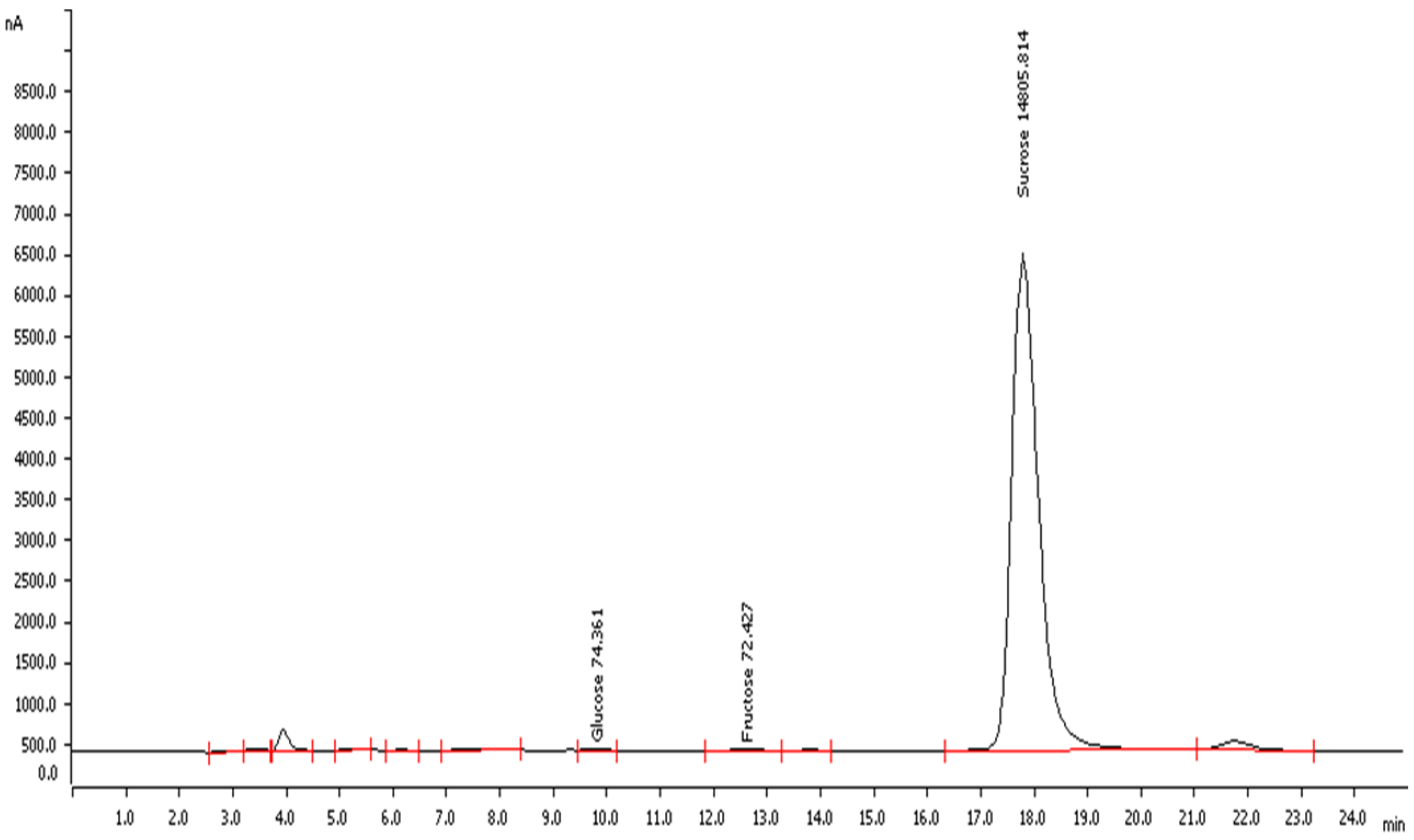
2.3. Chemical Description of Raw Tiger Nut Flour
2.4. Rheological Characteristics of Tiger Nut Flour
2.4.1. Bulk Density (BD)
2.4.2. Water Absorption Index (WAI)
2.4.3. Oil Absorption Capacity (OAC)
2.4.4. Foaming Capacity
2.4.5. Gluten Yield
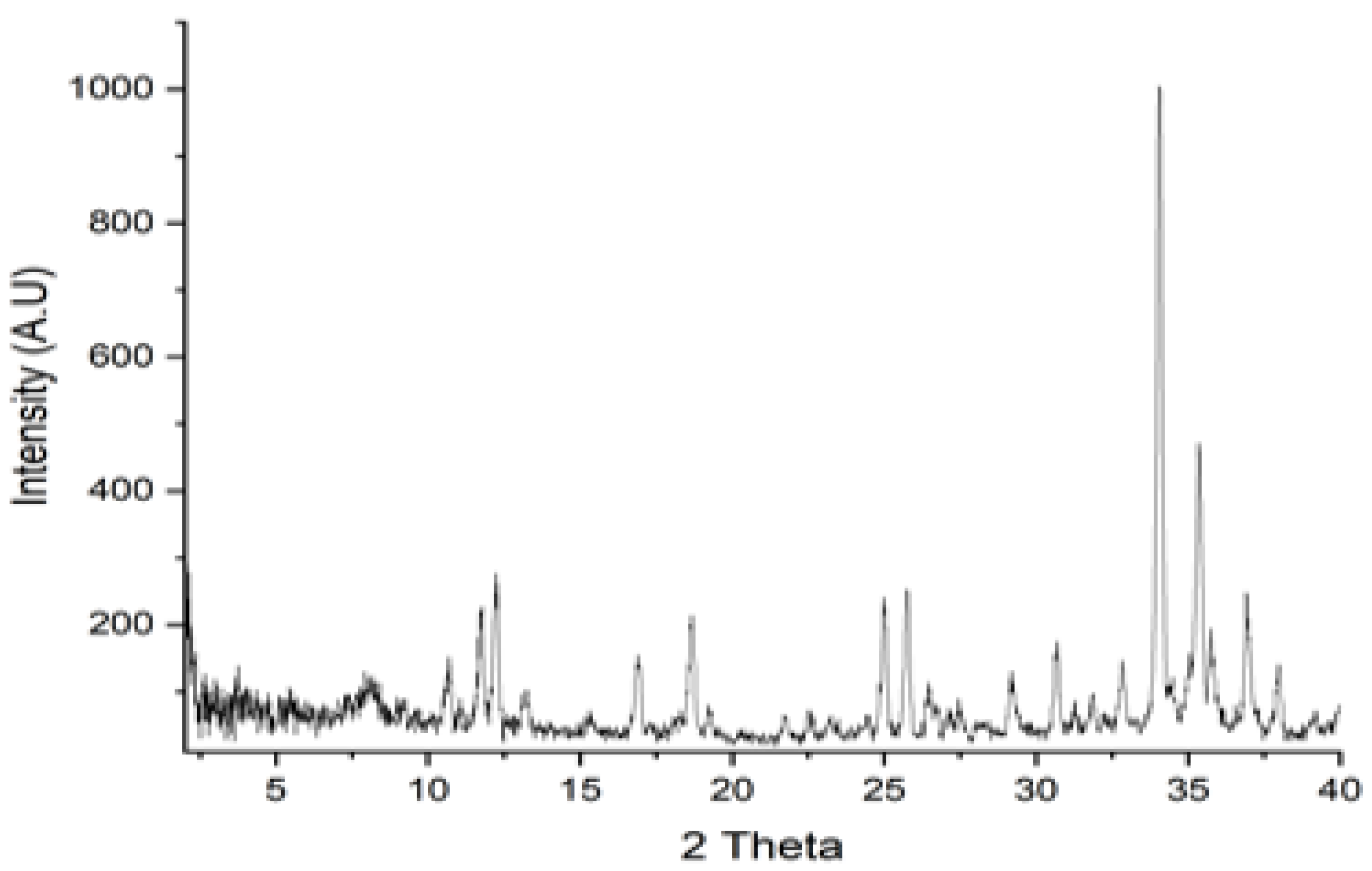
2.4.6. X-ray Diffraction Analysis (XRD)
2.5. Functional Properties of the Flour
2.5.1. Polyphenol Content
2.5.2. Flavonoid Content
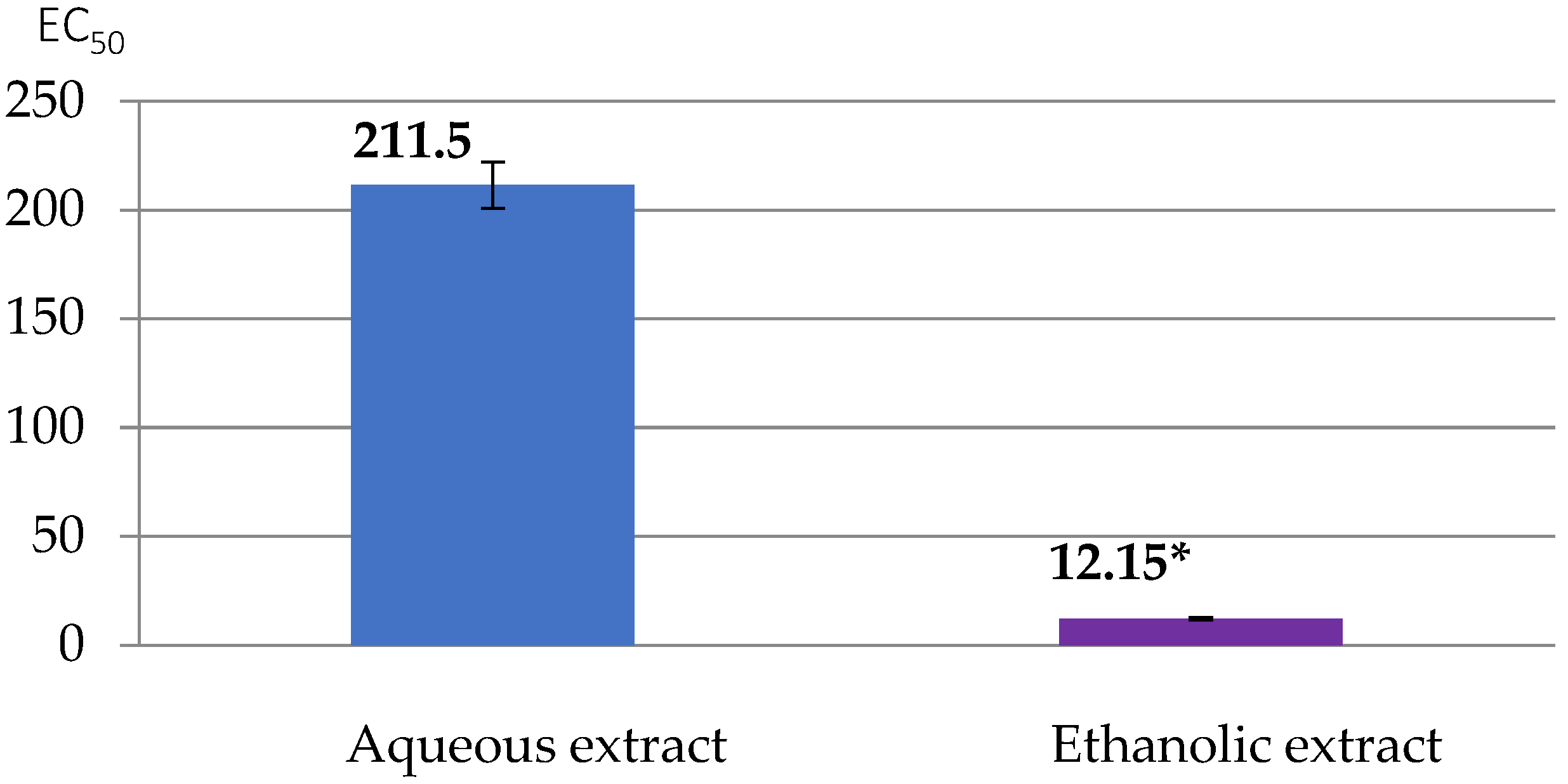
2.5.3. Antioxidant Activity
2.5.4. Antimicrobial Activity
2.6. Preparing Healthy Cookies
2.6.1. Formulation
2.6.2. Spray-Drying of SL42
2.6.3. Storage of Cookies and Assessment of Flour and Honey Effects on SL42
2.7. Appearance and Texture Analysis
2.8. Scanning Electronic Microscopy Analysis of Cookies
2.9. Sensorial Evaluation
2.10. Statistical Analysis
3. Results
3.1. Physico-Chemical Parameters Demonstrated a Rich and Nutritious Flour
3.2. Organic Tiger Nut Flour Had Good Rheological Values
3.3. Prosperous Functional Qualities of Tiger Nuts
3.4. Both Cookies Follow the International Standards
3.5. The Probiotic Strain SL42 Was Able to Grow in the Presence of Tiger Nut Flour or Honeybee Syrup
3.6. Dark Color of Tiger Nut Cookies Revealed by CIE System Analysis
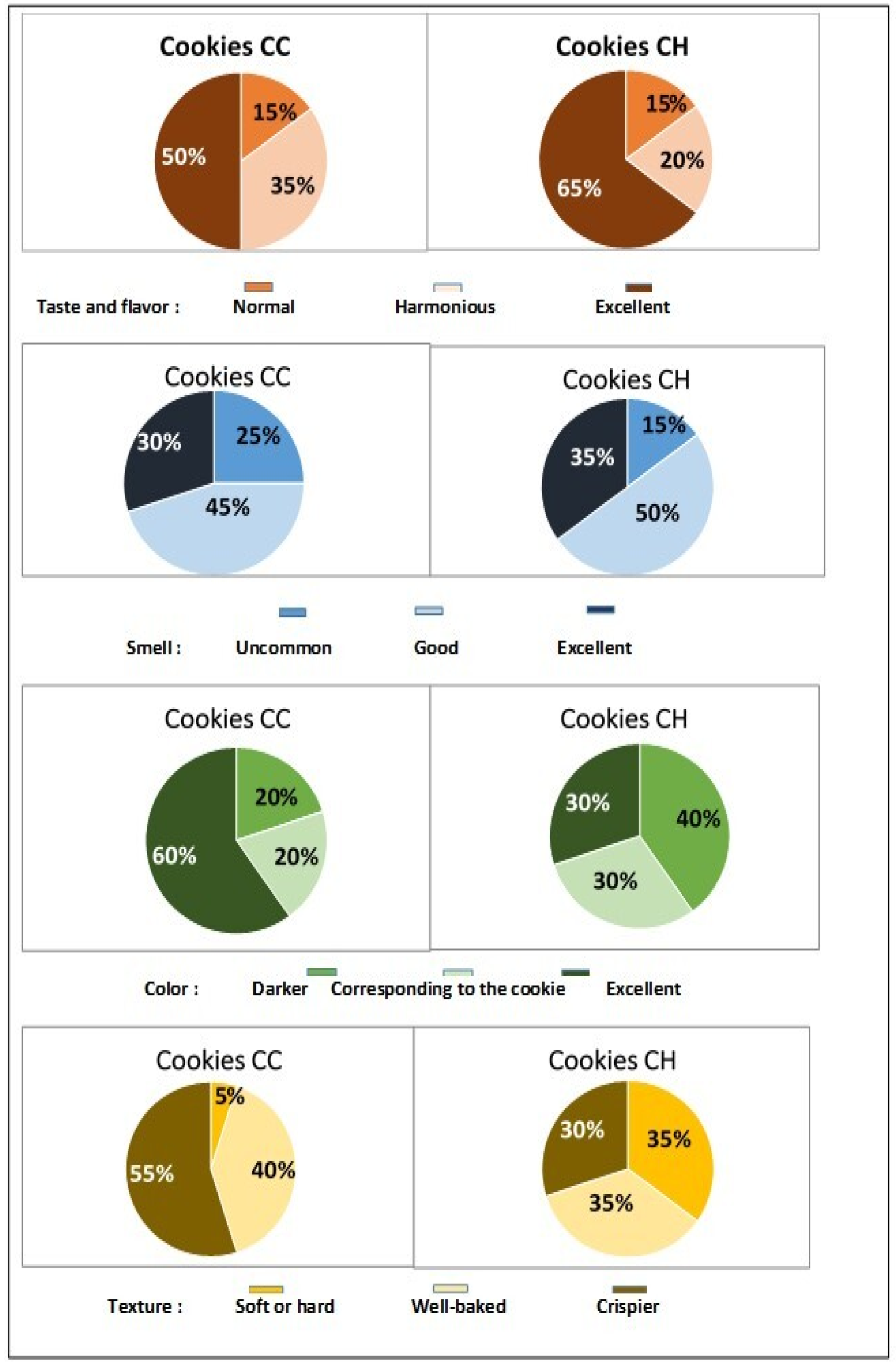
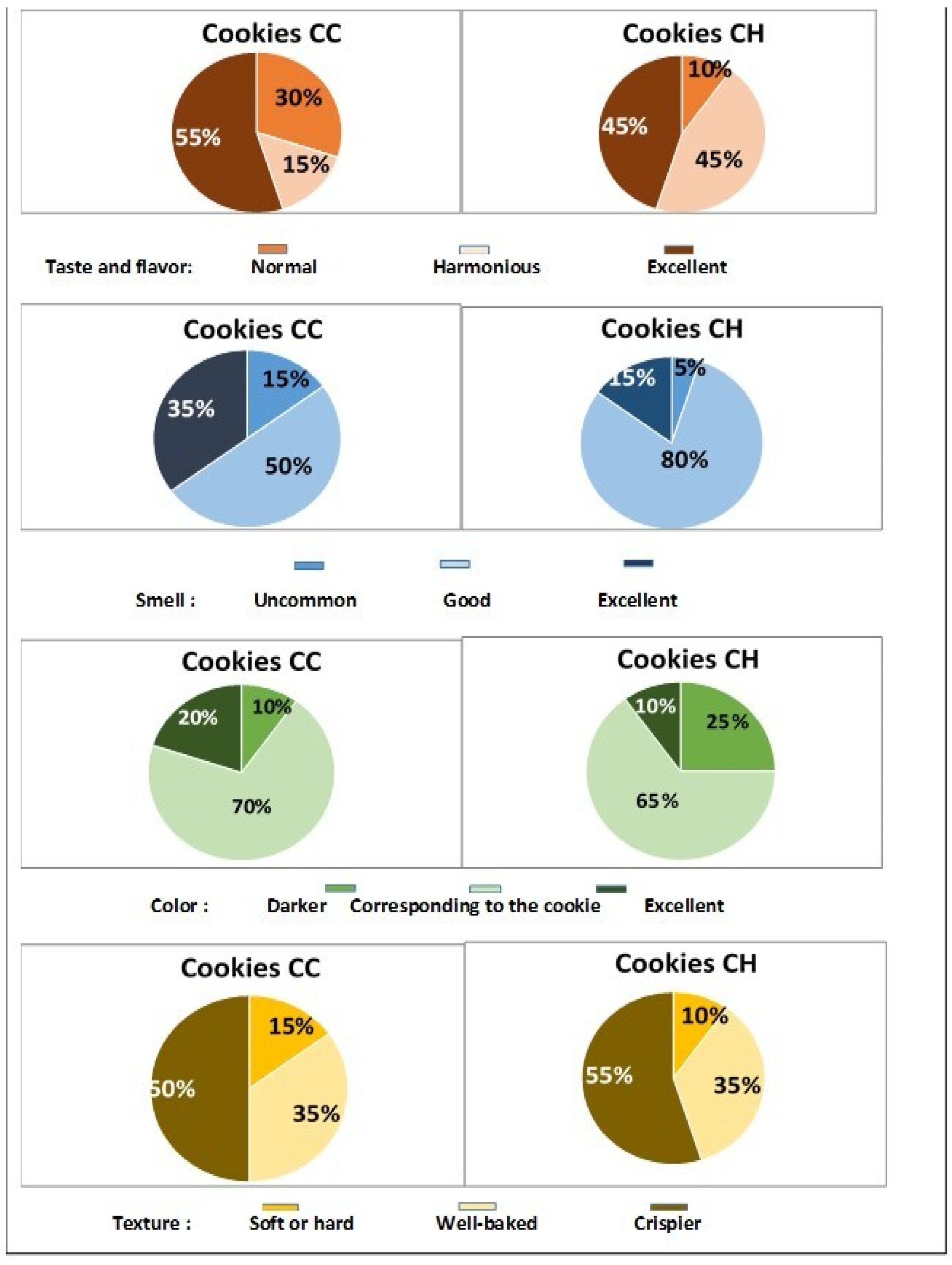
3.7. Sensory Evaluation of Both Tiger Nut Cookies Was Positive
3.8. Panel Opinion, Level of Satisfaction, and Hedonic Test
3.9. L. rhamnosus SL42 Survived at Recommended Probiotic Level in Stored Cookies
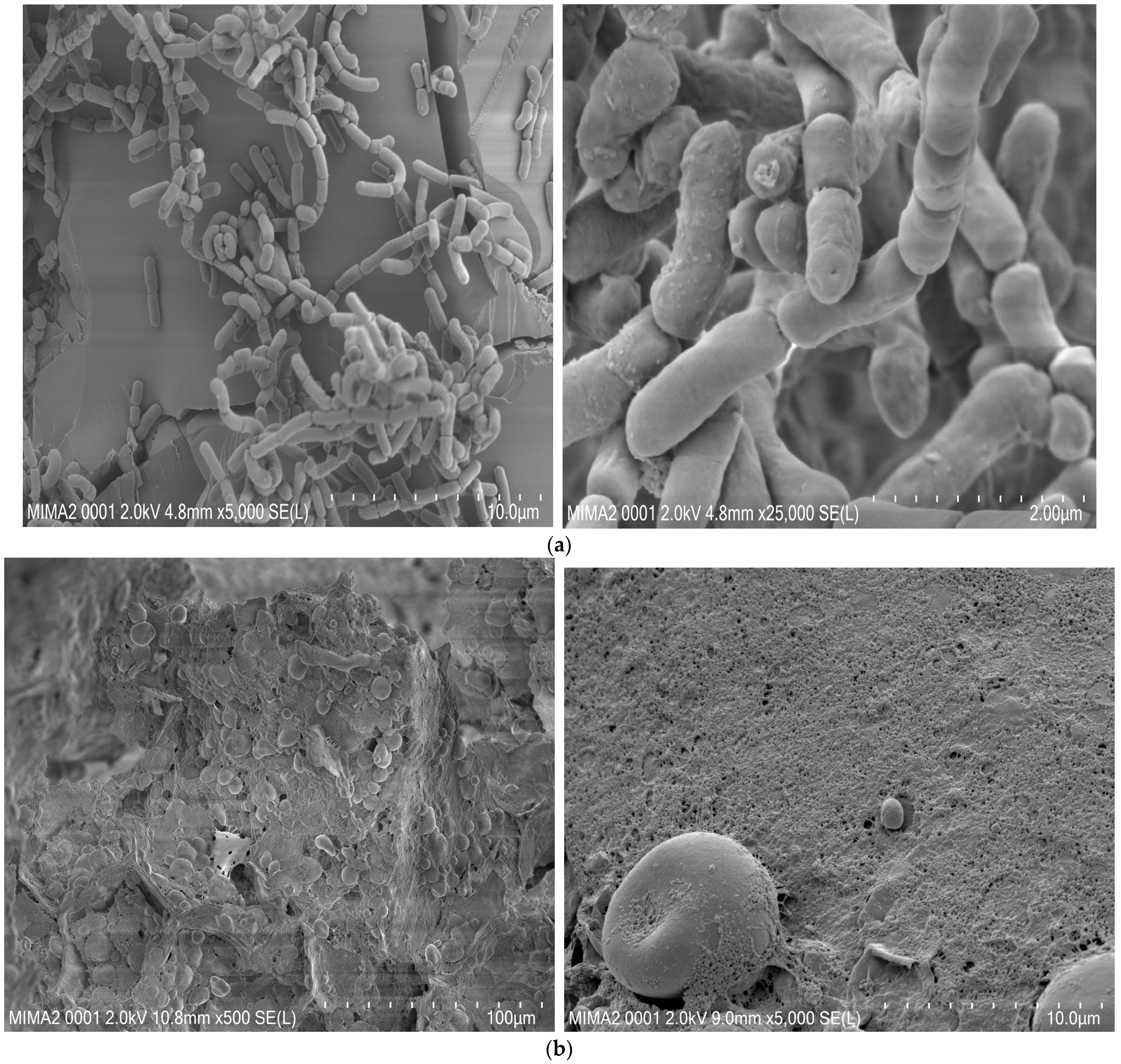
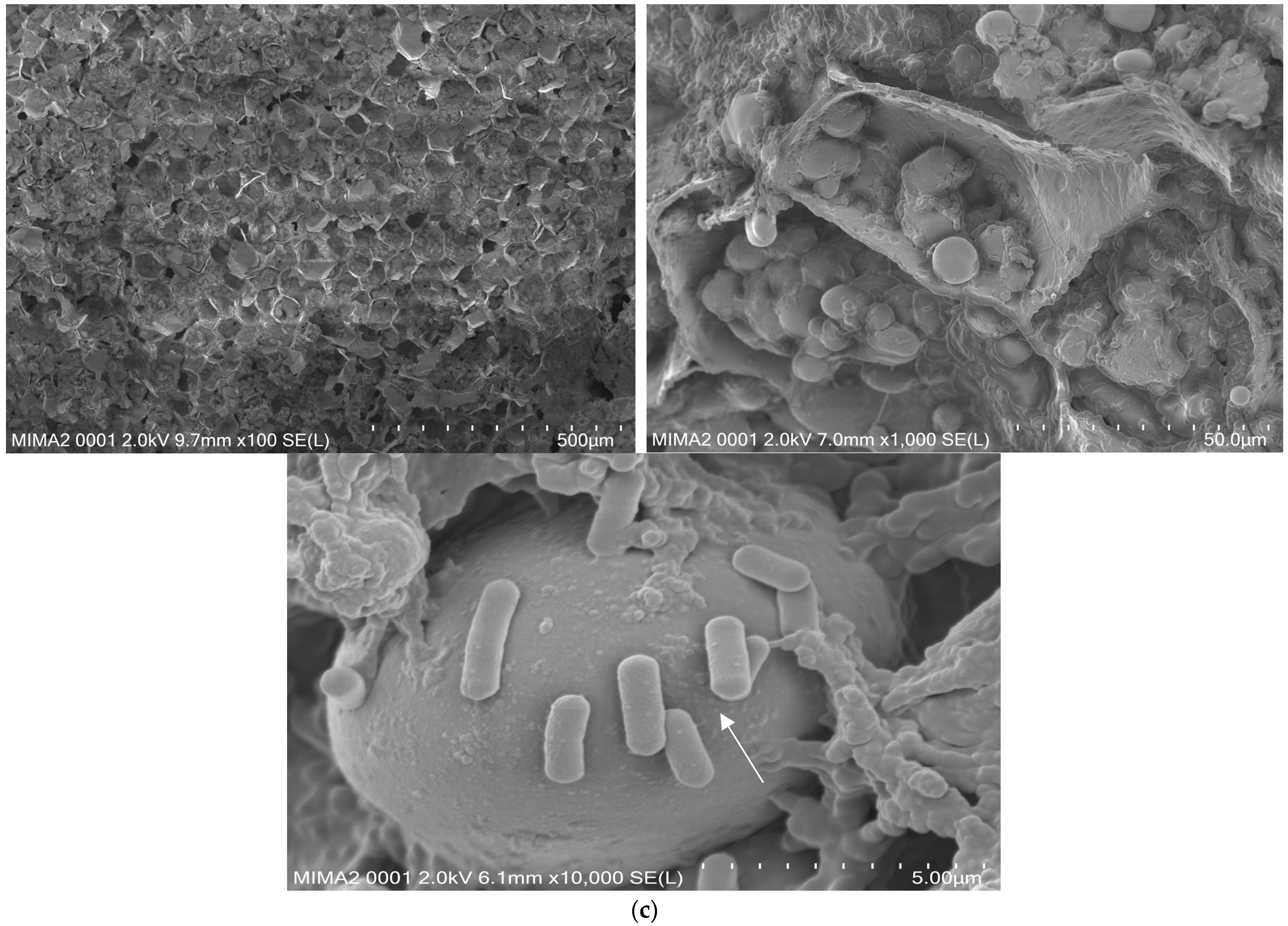
3.10. SEM Displays Distinguished Morphology between the Two Types of Cookies and Confirms SL42 Adhesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nwosu, L.C.; Edo, G.I.; Özgör, E. The phytochemical, proximate, pharmacological, GC-MS analysis of Cyperus esculentus (Tiger nut): A fully validated approach in health, food, and nutrition. Food. Biosci. 2022, 46, 101551. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Lorenzo, J.M.; Dominguez, R.; Pateiro, M.; Mañes, J.; Moltó, J.C. Evaluating the impact of supercritical-CO2 pressure on the recovery and quality of oil from “horchata” by-products: Fatty acid profile, α-tocopherol, phenolic compounds, and lipid oxidation parameters. Food. Res. Int. 2019, 120, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Olivas, E.; Asensio-Grau, A.; Calvo-Lerma, J.; García-Hernández, J.; Heredia, A.; Andrés, A. Content and bio accessibility of bioactive compounds with potential benefits for macular health in tiger nut products. Food. Biosci. 2022, 49, 101879. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G. Inhibition of carbohydrate hydrolyzing enzymes associated with type 2 diabetes and antioxidative properties of some edible seeds in vitro. Int. J. Diabetes. Dev. C 2015, 35, 516–521. [Google Scholar] [CrossRef]
- Adelakun, S.A.; Akintunde, O.W.; Ogunlade, B. Fluoride-induced testicular degeneration, and sperm quality deteriorations: Sal utary role of Cyperus esculentus tubers (tiger nut) extract in animal model. Rev. Int. Androl. 2020, 19, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Allouh, M.Z.; Daradka, H.M.; Abu Ghaida, J.H. Influence of Cyperus esculentus tubers (Tiger Nut) on male rat copulatory behavior. BMC Complement. Altern. Med. Rev. 2015, 15, 331. [Google Scholar]
- Shorinwa, O.A.; Dambani, D.T. Antidiarrheal activity of aqueous ethanol extract of Cyperus esculentus tuber in albino rats. J. Appl. Biol. Biotechnol. 2020, 8, 47–50. [Google Scholar]
- Achoribo, E.S.; Ong, M.T. Tiger nut (Cyperus esculentus): Source of natural anticancer drug? Brief review of existing literature. Euromediterr. Biomed. J. 2017, 12, 91–94. [Google Scholar]
- Delcour, J.A.; Hoseney, R.C.; Paul, M.N. Chemically leavened products. In Principles of Cereal Science And Technology; AACC International: St. Paul, MN, USA, 2010; pp. 207–228. [Google Scholar]
- Simanca-Sotelo, M.; De Paula, C.; Domínguez-Anaya, Y.; Pastrana-Puche, Y.; Álvarez-Badel, B. Physico-chemical and sensory characterization of sweet biscuits made with Yacon flour (Smallanthus sonchifolius). NFS J. 2021, 22, 14–19. [Google Scholar] [CrossRef]
- Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Advances in Probiotic Incorporation into Cereal-Based Baked Foods: Strategies, Viability, and Effects—A review. APPL. Food. Res. 2023, 3, 100330. [Google Scholar] [CrossRef]
- Heshmati, J.; Farsi, F.; Shokri, F.; Rezaeinejad, M.; Almasi-Hashiani, A.; Vesali, S.; Sepidarkish, M. A systematic review and meta-analysis of the probiotics and synbiotics effects on oxidative stress. J. Funct. Foods 2018, 46, 66–84. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Huang, X.Y.; Chen, P.X.; Li, T.; Jiang, M.M.; Wang, Y.L.; Liu, H.M. A novel non-centrifugal sugar prepared from tiger nut (Cyperus esculentus L.)meal: Preparation methods and comparison with sugarcane. Food Res. Int. 2023, 174, 113519. [Google Scholar] [CrossRef] [PubMed]
- Keddar, K.; Ziar, H.; Belmadani, N.; Monnoye, M.; Gérard, P.; Riazi, A. Probiotic bacteria from human milk can alleviate oral bovine casein sensitization in juvenile Wistar rats. Microorganisms 2023, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Methods of the American Association of Cereal Chemists, 11th ed.; American Association of Cereal Chemists International: St. Paul, MN, USA, 2010. [Google Scholar]
- Suksom, W.; Wannachai, W.; Boonchiangma, S.; Chanthai, S.; Srijaranai, S. Ion chromatographic analysis of monosaccharides and disaccharides in raw sugar. Chromatographia 2015, 78, 873–879. [Google Scholar] [CrossRef]
- Wang, J.C.; Kinsella, J.E. Functional properties of novel proteins: Alfalfa lead proteins. J. Food Sci. 1976, 41, 286–290. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; Ibanoglu, E.; Ibanoglu, S. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready-to-eat expanded snacks. J. Food Eng. 2008, 87, 554–563. [Google Scholar] [CrossRef]
- Lin, M.J.Y.; Humbert, E.S.; Sosulski, F.W. Certain functional properties of sunflower meal products. J. Food Sci. 1974, 39, 368–370. [Google Scholar] [CrossRef]
- Miranda, J.A.T.D.; Carvalho, L.M.J.D.; Vieira, A.C.D.M.; Castro, I.M.D. Scanning electron microscopy and crystallinity of starches granules from cowpea, black and carioca beans in raw and cooked forms. Food Sci. Technol.-Leb. 2019, 39, 718–724. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Poojary, M.M.; Barba, F.J.; Lorenzo, J.M.; Mañes, J.; Moltó, J.C. Tiger nut and its by-products valorization: From extraction of oil and valuable compounds to development of new healthy products. Innov. Food Sci. Emerg. 2018, 45, 306–312. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Sompach, G.; Rodklongtan, A.; Nitisinprasert, S.; Chitprasert, P. Microencapsulating role of whey protein isolate and sucrose in protecting the cell membrane and enhancing survival of probiotic lactobacilli strains during spray drying, storage, and simulated gastrointestinal passage. Food Res. Int. 2022, 159, 111651. [Google Scholar] [CrossRef]
- Oladele, A.K.; Aina, J.O. Chemical composition and functional properties of flour from two varieties of tigernut (Cyperus esculentus). Afr. J. Biotechnol. 2007, 6, 2473–2476. [Google Scholar] [CrossRef]
- Yapi, J.C.; Deffan, Z.A.B.; Koko, A.C.; Diabagate, J.R.; Kouamekan, K.B.; Kouamé, L.P. Influence de la granulométrie physicochimiques sur les caractéristiques et techno-fonctionnelles des farines de souchet (Cyperus esculentus L.). Afr. J. Agr. Res. 2021, 33, 239–250. [Google Scholar]
- Bankoffi, L.; Nemlin, G.J.; Lefevre, S.; and Kamenan, A. Caracterisation physico-chimique et potentialites therapeutiques du pois sucre (Cyperus esculentus L. cyperaceae). Agron. Afr. 2005, 17, 63–71. [Google Scholar]
- Sidohounde, A.; Pascal, C.; Dossa, A.; Nonviho, G.; Montcho, S.P.; Codjo, D.; Sohounhloue, K. Biodiesel potentials of two phenotypes of Cyperus esculentus unconventional oils. J. Pet. Technol. Altern. Fuels 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Nina, G.C.; Gontcharov, A.; Batichcheva, N.; Stanislav, V.; Okuskhanova, E.; CÃsarová, M.; Hleba, M.; Ukeyima, M.; Ogori, A.F.; Shariati, M.A. Proximate, mineral and functional properties of tiger nut flour extracted from different tiger nuts cultivars. Food Sci. 2019, 9, 653–656. [Google Scholar]
- Okpala, L.C.; Mammah, E.N. Functional properties of raw and processed pigeon pea (Cajanus cajan) flour. Int. J. Food Sci. Nutr. 2001, 52, 343–346. [Google Scholar]
- Akubor, P.I.; Badifu, G.I.O. Chemical composition, functional properties and baking potential of African breadfruit kernel and wheat flour blends. Int J. Food Sci Tech 2004, 39, 223–229. [Google Scholar] [CrossRef]
- Anand, S.S.; Hawkes, C.; de Souza, J.R.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.M.; et al. Food consumption and its impact on cardiovascular disease: Importance of solutions focused on the globalized food system. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef]
- Adegunwa, M.O.; Adelekan, E.O.; Adebowale, A.A.; Bakare, H.A.; Alamu, E.O. Evaluation of nutritional and functional properties of plantain (Musa paradisiaca L.) and tigernut (Cyperus esculentus L.) flour blends for food formulations. Cogent Chem. 2017, 3, 1383707. [Google Scholar]
- Muntean, E. Applications of ion chromatography in food analysis. In Ion-Exchange Chromatography and Related Techniques; Elsevier: Amsterdam, The Netherlands, 2024; pp. 351–369. [Google Scholar]
- Takahashi, M.; Ishmael, M.; Asikin, Y.; Hirose, N.; Mizu, M.; Shikanai, T.; Tamaki, H.; Wada, K. Composition, taste, aroma, and antioxidant activity of solidified noncentrifugal brown sugars prepared from whole stalk and separated pith of sugarcane (Saccharum officinarum L.). J. Food Sci. 2016, 81, C2647–C2655. [Google Scholar] [CrossRef] [PubMed]
- Zidan, D.; Azlan, A. Non-centrifugal sugar (NCS) and health: A Review on functional components and health benefits. Appl. Sci. 2022, 12, 460. [Google Scholar] [CrossRef]
- Menasra, A.; Fahloul, D. Quality characteristics of biscuit prepared from wheat and milk thistle seeds (Silybum marianum (l) gaertn) flour. Carpathian J. Food Technol. 2019, 11, 5–19. [Google Scholar] [CrossRef]
- Adejuyitan, J.A.; Otunola, E.T.; Akande, E.; Bolarinwa, I.F.; and Oladokun, F.M. Some Physicochemical properties of Flour Obtained from fermentation of tiger nut (Cyperus esculentus) souced from a market In Ogbomoso. Niger. Afr. J. Food Sci. 2009, 3, 51–55. [Google Scholar]
- Ohizua, E.R.; Adeola, A.A.; Idowu, M.A.; Sobukola, O.P.; Afolabi, T.A.; Ishola, R.O.; Ayansina, S.O.; Oyekale, T.O.; Falamo, A. Nutrient composition, functional, and pasting properties of unripe cooking banana, pigeon pea, and sweetpotato flour blends. Int. J. Food Sci. Nutr. 2017, 5, 750–762. [Google Scholar] [CrossRef]
- Gaiani, C.; Boyanova, P.; Hussain, R.; Murrieta Pazos, I.; Karam, M.C.; Burgain, J.; Scher, J. Morphological descriptors and colour as a tool to better understand rehydration properties of dairy powders. Int. Dairy J. 2011, 21, 461–469. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Pauls, K.P.; Fan, M.Z.; Yada, R. In vitro starch digestibility, expected glycemic index and some physicochemical properties of starch and flour from common bean (Phaseolus vulgaris L.) varieties grown in Canada. Food Res. Int. 2008, 41, 869–875. [Google Scholar] [CrossRef]
- Diallo, S.K.; Soro, D.; Kone, K.Y.; Assidjo, N.E.; Yao, K.B.; Gnakri, D. Fortification et substitution de la farine de blé par la farine de Voandzou (Vigna subterranea L. verdc) dans la production des produits de boulangerie. Int. J. Innov. 2015, 18, 440. [Google Scholar]
- Nawaza, H.; Aslam Shada, M.; Mehmood, R.; Rehman, T.; Munir, H. Comparative Evaluation of Functional Properties of Commonly Used Cereal and Legume Flours with their Blends. J. Food Allied Sci 2015, 1, 71. [Google Scholar] [CrossRef]
- Culetu, A.; Susman, I.E.; Duta, D.E.; Belc, N. Nutritional and Functional Properties of Gluten-Free Flours. Appl. Sci 2021, 11, 6283. [Google Scholar] [CrossRef]
- Žilić, S.; Janković, M.; Barać, M.; Pešić, M.; Konić-Ristić, A.; Šukalović, V.H.T. Effects of enzyme activities during steeping and sprouting on the solubility and composition of proteins, their bioactivity and relationship with the bread making quality of wheat flour. Food Funct. 2016, 7, 4323–4331. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-type starches from different plant sources. Food Hydrocolloid. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Manek, R.V.; Builders, P.F.; Kolling, W.M.; Emeje, M.; Kunle, O. Physicochemical and Binder Properties of Starch Obtained from Cyperus esculentus. Aaps Pharmscitech 2012, 13, 379–388. [Google Scholar] [CrossRef]
- Ziar, H.; Gérard, P.; Riazi, A. Effect of prebiotic carbohydrates on growth, bile survival and cholesterol uptake abilities of dairy-related bacteria. J. Sci. Food Agric. 2014, 94, 1184–1190. [Google Scholar] [CrossRef]
- Djikeng, F.T.; Djikeng, C.F.T.; Femmesi, H.M.; Ndefo, D.K.K.; Pougoué, A.A.N.; Tambo, S.T.; Esatbeyoglu, T. Effect of Different Processing Methods on the Chemical Composition, Antioxidant Activity and Lipid Quality of Tiger Nuts (Cyperus esculentus). Appl. Food Res. 2022, 2, 100124. [Google Scholar] [CrossRef]
- Oladele, A.K.; Akinwande, A.B.B.; Akinoso, R. Optimization of the antioxydants of tigernut (Cyperus esculentus L.) during roasting using response surface methodology. Elixir Food Sci. 2014, 75, 27592–27597. [Google Scholar]
- Xie, Y.; Luo, H.; Duan, J.; Hong, C.; Ma, P.; Li, G.; Zhang, T.; Wu, T.; Ji, G. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total f flavones of Hippophage rhomboids L. Fitoterapia 2014, 93, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experi mental purposes. J. Pharm. Bioallied Sci 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation ofthe use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of severinia buxifolia. J. Food Qual. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Prakash, N.; Ragavan, B. Phytochemical observation and antibacterial activity of Cyperus esculentus L. Anc. Sci. Life 2009, 28, 16–20. [Google Scholar]
- Adeola, A.; Ohizua, E.R. Physical, chemical, and sensory properties of biscuits prepared from flour blends of unripe cooking banana, pigeon pea, and sweet potato. Int. J. Food Sci. Nutr. 2018, 6, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Saxena, D.C.; Singh, S. Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. Lwt-Food Sci. Technol. 2015, 63, 939–945. [Google Scholar] [CrossRef]
- Babiker, E.E.; Özcan, M.M.; Ghafoor, K.; Juhaimi, F.A.; Ahmed, I.A.M.; Almusallam, I.A. Bioactive compounds, nutritional and sensory properties of cookies prepared with wheat and tigernut flour. Food Chem. 2021, 349, 129155. [Google Scholar] [CrossRef]
- Romani, S.; Rocculi, P.; Tappi, S.; Dalla Rosa, M. Moisture adsorption behaviour of biscuit during storage investigated by using a new Dynamic Dewpoint method. Food Chem. 2016, 195, 97–103. [Google Scholar] [CrossRef]
- Dada, M.A.; Bello, F.A.; Omobulejo, F.O.; Olukunle, F.E. Nutritional quality and physicochemical properties of biscuit from composite flour of wheat, African yam bean and tigernut. Heliyon 2023, 9, e22477. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Alade, A.E. The addition of tiger nut by-product improved the physical, nutritional and safety quality of gluten-free cookies. Food Chem. 2024, 4, 100626. [Google Scholar] [CrossRef]
- Oluwajuyitan, T.D.; Ijarotimi, O.S. Nutritional, antioxidant, glycaemic index and Antihyperglycaemic properties of improved traditional plantain-based (Musa AAB) dough meal enriched with tigernut (Cyperus esculentus) and defatted soybean (Glycine max) flour for diabetic patients. Heliyon 2019, 5, e01504. [Google Scholar] [CrossRef]
- Ziar, H.; Yahla, I.; Sadoud, M.; Keddar, K.; Dilmi-Bouras, A.; Riazi, A.; Gérard, P. Association of carob galactomannans with probiotic bacteria in synbiotic fermented milk and colon targeted-release carrier. Food Res. Int. 2022, 29, 879–891. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Sheng, Q.; Li, P.; Zhao, W.; Zhang, A.; Liu, J. The effect of heat moisture treatment times on physico chemical and digestibility properties of adzuki bean, pea, and white kidneybean flours and starches. Food Chem 2024, 440, 138228. [Google Scholar] [CrossRef]
- Ngamekaue, N.; Dumrongchai, T.; Rodklongtan, A.; Chitprasert, P. Improving probiotic survival through encapsulation in coconut oil in whey protein isolate emulsions during spray drying and gastrointestinal digestion. Lwt-Food Sci. Technol. 2024, 198, 116061. [Google Scholar] [CrossRef]
- Wang, A.; Lin, J.; Zhong, Q. Synergistic effects of whey protein isolate and amorphous sucrose on improving the viability and stability of powdered Lactobacillus salivarius NRRL B-30514. LWT-Food Sci. Technol. 2020, 118, 108722. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Bambace, M.F.; Quintana, G.; Gomez-Zavaglia, A.; Del Rosario Moreira, M. Prebiotic-alginate edible coating on fresh-cut apple as a new carrier for probiotic lactobacilli and bifidobacteria. Lwt-Food Sci. Technol. 2021, 137, 110483. [Google Scholar] [CrossRef]
- Rai, R.; Nitin, N. Apple-derived 3D scaffold for improving gastrointestinal viability and in-situ growth of probiotics. Food Res. Int. 2023, 168, 112758. [Google Scholar] [CrossRef]




| Water Content | Ash | Lipids | Carbohydrates | Fibers | Proteins | Minerals | |
|---|---|---|---|---|---|---|---|
| Tiger nut flour | 8.3 ± 0.02 | 3.04 ± 1.2 | 26.5 ± 1.2 | 55.6 ± 0.5 | 15.5 ± 0.01 1 | 5.8 ± 1.5 | 2.26 ± 0.05 |
| Bulk Density (g /mL) | Dispersibility (%) | Water Absorption Index (g/mL) | Oil Absorption Capacity (mL/g) | Foaming Capacity (%) | Wet Gluten Yield (%) | |
|---|---|---|---|---|---|---|
| Tiger nut flour | 0.74 ± 0.01 | 68 ± 0.2 | 0.086 ± 0.2 | 2.2 ± 0.03 | 12 ± 0.05 | 0 ± 0 |
| Parameter | Control Tiger Nut Cookie (CC) | Tiger Nut Cookie with Honey Syrup (CH) | Commercial Cookie Made of Wheat Flour |
|---|---|---|---|
| Weight (g) | 12.33 ± 0.81 b | 12.66 ± 0.51 b | 11.7 ± 0.4 a |
| Diameter (mm) | 50.66 ± 0.82 a | 50.5 ± 0.54 a | 53.85 ± 0.1 a |
| Thickness (mm) | 5.16 ± 0.4 a | 5.33 ± 0.51 ab | 5.71 ± 0.02 b |
| Spread ratio | 9.81 ± 2.05 b | 9.47 ± 1.05 a | 9.43 ± 0.1 a |
| Hardness (N) | 97.5 ± 0.3 a | 98.3 ± 0.4 b | 99.5 ± 0.5 c |
| Parameter | Control Tiger Nut Cookie (CC) | Tiger Nut Cookie with Honey Syrup (CH) |
|---|---|---|
| L* | 62.25 ± 4.66 a | 61.75 ± 4.94 a |
| a* | 6.95 ± 0.88 a | 7.1 ± 0.85 b |
| b* | 26.5 ± 4.61 b | 23.5 ± 4.62 a |
| Parameter | Control Tiger Nut Cookie (CC) | Tiger Nut Cookie with Honey-Syrup (CH) |
|---|---|---|
| L* | 62.75 ± 4.66 a | 61.75 ± 4.43 a |
| a* | 7.11 ± 0.48 a | 7.17 ± 0.96 a |
| b* | 25.25 ± 4.72 b | 23.25 ± 3.35 a |
| Quality Parameter | Control Tiger Nut Cookie (CC) | Tiger Nut Cookie with Honey Syrup (CH) |
|---|---|---|
| Taste | 5.5 ± 2.01 a | 6.1 ± 1.86 b |
| Color | 3.35 ± 1.53 a | 3.75 ± 1.58 b |
| Aroma | 3.7 ± 1.17 a | 3.85 ± 1.03 a |
| Texture | 1.45 ± 0.60 a | 1.45 ± 0.82 a |
| Overall acceptability | 3.5 ± 1.32 a | 3.66 ± 1.32 a |
| Quality Parameter | Control Tiger Nut Cookie (CC) | Tiger Nut Cookie with Honey Syrup (CH) |
|---|---|---|
| Taste | 5.4 ± 2.25 a | 5.55 ± 1.63 a |
| Color | 3.75 ± 0.91 b | 3.15 ± 1.26 a |
| Aroma | 3.95 ± 1.19 b | 3.3 ± 1.12 a |
| Texture | 1.35 ± 0.74 a | 1.45 ± 0.68 a |
| Overall acceptability | 3.61 ± 1.27 b | 3.36 ± 1.17 a |
| Storage Period | Tiger Nut Cookie with Honey Syrup (CH) |
|---|---|
| Day 0 | 7.15 ± 0.43 a |
| Day 1 | 6.93 ± 0.96 a |
| Day 7 | 6.66 ± 0.44 a |
| Day 15 | 6.55 ± 0.35 a |
| Day 21 | 6.43 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmadani, N.; Kassous, W.; Keddar, K.; Amtout, L.; Hamed, D.; Douma-Bouthiba, Z.; Costache, V.; Gérard, P.; Ziar, H. Functional Cyperus esculentus L. Cookies Enriched with the Probiotic Strain Lacticaseibacillus rhamnosus SL42. Foods 2024, 13, 2541. https://doi.org/10.3390/foods13162541
Belmadani N, Kassous W, Keddar K, Amtout L, Hamed D, Douma-Bouthiba Z, Costache V, Gérard P, Ziar H. Functional Cyperus esculentus L. Cookies Enriched with the Probiotic Strain Lacticaseibacillus rhamnosus SL42. Foods. 2024; 13(16):2541. https://doi.org/10.3390/foods13162541
Chicago/Turabian StyleBelmadani, Noussaiba, Wafa Kassous, Kawtar Keddar, Lamia Amtout, Djahira Hamed, Zohra Douma-Bouthiba, Vlad Costache, Philippe Gérard, and Hasnia Ziar. 2024. "Functional Cyperus esculentus L. Cookies Enriched with the Probiotic Strain Lacticaseibacillus rhamnosus SL42" Foods 13, no. 16: 2541. https://doi.org/10.3390/foods13162541
APA StyleBelmadani, N., Kassous, W., Keddar, K., Amtout, L., Hamed, D., Douma-Bouthiba, Z., Costache, V., Gérard, P., & Ziar, H. (2024). Functional Cyperus esculentus L. Cookies Enriched with the Probiotic Strain Lacticaseibacillus rhamnosus SL42. Foods, 13(16), 2541. https://doi.org/10.3390/foods13162541






