Critical Review of Food Colloidal Delivery System for Bioactive Compounds: Physical Characterization and Application
Abstract
:1. Introduction
2. Typical Structure of Food Colloid Delivery Carriers
2.1. Liposome
2.2. Pickering Emulsion
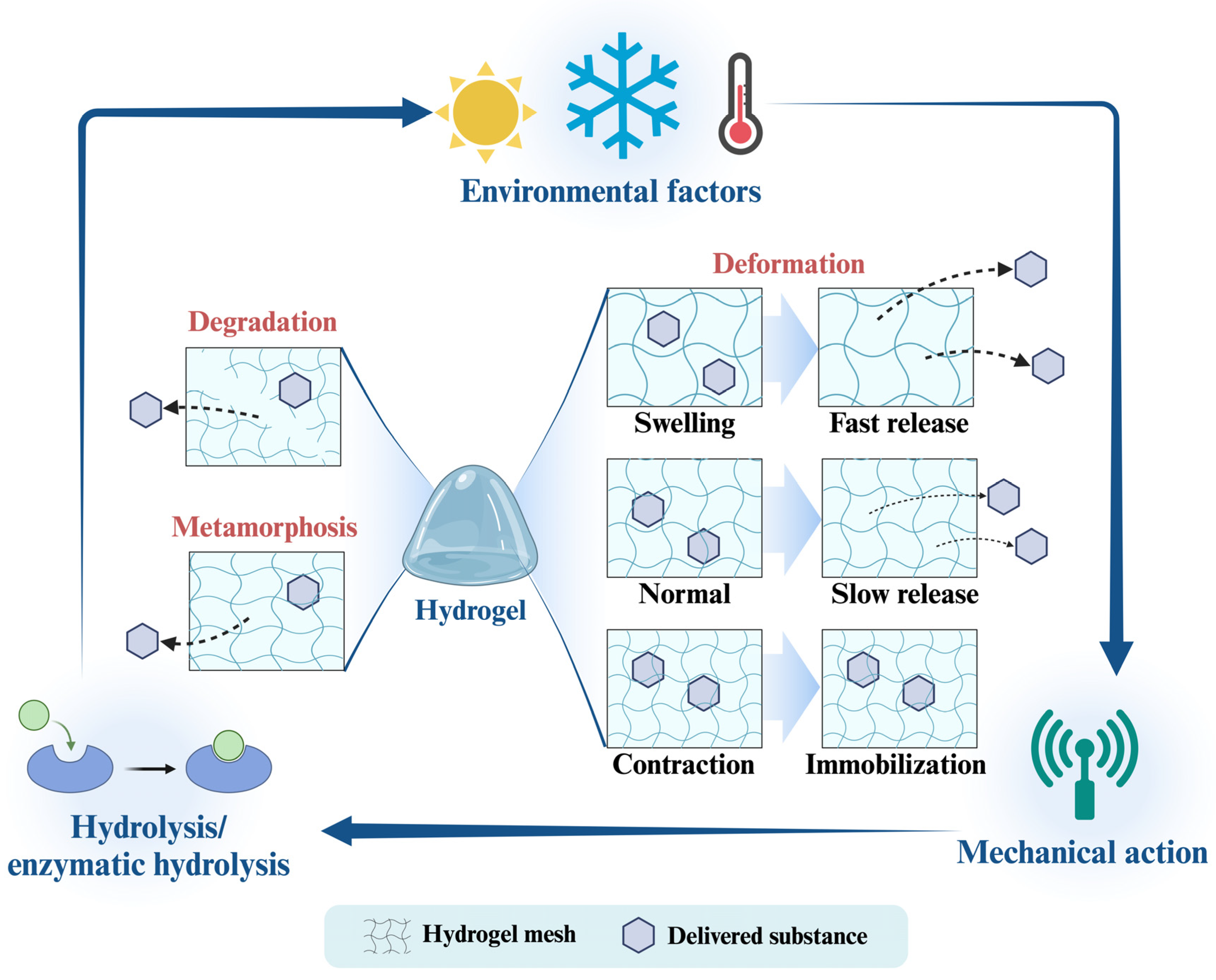
2.3. Hydrogel
2.4. Self-Assembled Microcapsules Based on Electrostatic Interaction
2.5. Self-Assembled Nanomicelle
2.6. Cyclodextrin
3. Physical Properties of Food Colloid Delivery Carriers
3.1. Size and Structure
3.2. Charged Characteristics
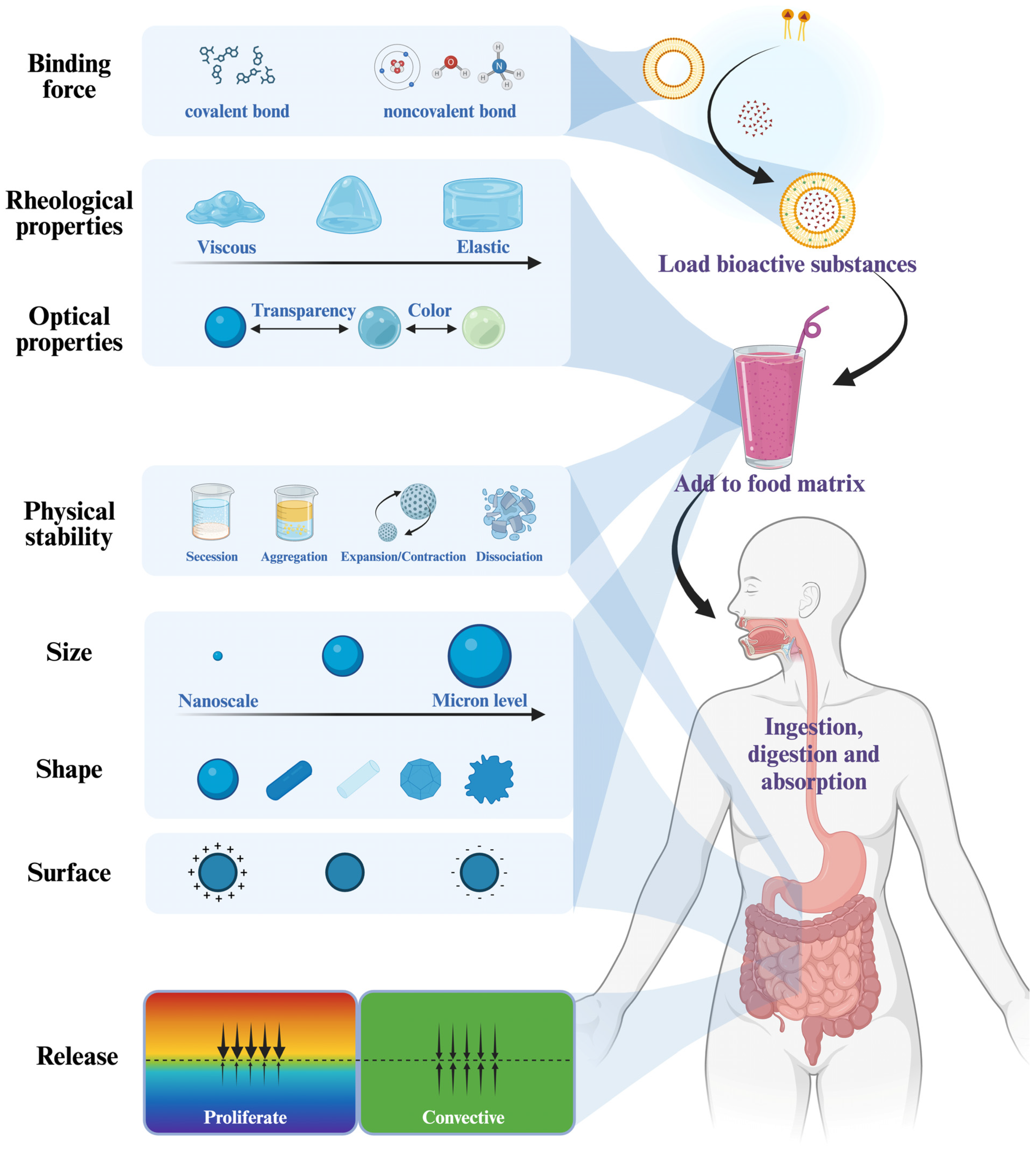
3.3. Binding Characteristics
3.4. Physical Stability
3.5. Release Behavior
3.6. Effects on the Physical Properties of Food Substrates
3.6.1. Rheological Properties
3.6.2. Optical Characterization
4. Application of Food Colloids in Delivery Systems
4.1. In Vitro Instability
4.1.1. Physical Instability
4.1.2. Chemical Instability
| Form | BACs | Functional Properties | External Influencing Factors | Body Absorption Disorders | Reference |
|---|---|---|---|---|---|
| Polyphenol | Curcumin | Used in anti-inflammatory and antioxidant tumor therapy against cardiovascular diseases, nervous disorders, inflammatory diseases, Alzheimer’s disease | Heat, light, iron | Poor solubility | [159] |
| Resveratrol | Effectively scavenging free radicals; regulating the expression of antioxidant enzymes; anti-inflammatory, anti-aging, anti-diabetic, and cardioprotective effects | Thermal, light-induced isomerization, autoxidation | Low solubility/permeability, rapid metabolism | [160] | |
| Quercetin | Antioxidant, anti-inflammatory, anti-cancer | Heat, alkali | Low solubility and bioavailability | [161] | |
| Anthocyanin | Antioxidant, anti-inflammatory, anti-cancer, and chronic disease improvement (eyes, mouth, skin, colon, etc.) | pH, heat, oxygen, light, metal ions | Low solubility and bioavailability; strong acidic environment in the GI tract | [162] | |
| Bioactive peptide | Carnosine | Antioxidant, pH buffering, chelation; anti-aging and anti-advanced glycosylation end products (AGEs) | - | Digestive tract degradation leads to low bioavailability and targeted delivery requirements | [163,164,165] |
| Bioactive polysaccharide | Lentinan | Antioxidant; combats inflammatory diseases and cancer | Heat, light, humidity | Low bioavailability, targeted delivery requirements | [166] |
| Ganoderma lucidum polysaccharides | Anti-tumor, anti-inflammatory, immunomodulatory, and anti-aging agent effects; improvement in intestinal barrier function | Heat, light, moisture | Low solubility, low bioavailability, tumor environment (oxidative and acidic conditions) | [167] | |
| Vitamin | Vitamin C | Increased collagen biosynthesis; reduced melanin synthesis; anti-aging; important for metabolic activity as a coenzyme or prosthesis | Heat, light, moisture | Low solubility, environmental degradation in the digestive tract | [168] |
| Vitamin E | Anti-cancer, anti-aging | Heat, light, humidity, alkaline | Low solubility, environmental degradation in the digestive tract | [169] | |
| Carotenoid | Lutein | Protects the retina; protects against UV-induced peroxidation; reduces lipofuscin formation and related oxidative stress-induced damage | Heat, light, oxygen | Low solubility, low bioavailability, gastrointestinal degradation | [170] |
| β-carotene | Improves vision; strengthens immune system and cognitive function; prevents coronary heart disease and cancer | Heat, light, oxygen | Low solubility, low bioavailability, gastrointestinal degradation | [171] | |
| Other | Isoflavone | Endocrine regulation, cardiovascular protection, antioxidant, anti-inflammatory, maintenance of bone health | Heat | Digestive tract degradation, low drug permeability | [172] |
| β-sitosterol | Anti-inflammatory, anti-aging, anti-tumor, lipid-lowering effect and immunomodulation, positive effects on cardiovascular and cerebrovascular diseases | Temperature (volatile) | Low bioavailability due to low solubility and structural instability | [173] |
4.2. Efficient Absorption In Vivo
4.3. Flavor Regulation
4.4. Packaging Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, A.R.; Velikov, K.P. Colloidal Delivery Systems in Foods: A General Comparison with Oral Drug Delivery. LWT Food Sci. Technol. 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Li, R.; Guo, Y.; Dong, A.; Yang, X. Protein-Based Emulsion Gels as Materials for Delivery of Bioactive Substances: Formation, Structures, Applications and Challenges. Food Hydrocoll. 2023, 144, 108921. [Google Scholar] [CrossRef]
- Boyd, B.J. Past and Future Evolution in Colloidal Drug Delivery Systems. Expert. Opin. Drug Deliv. 2008, 5, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Vishvakarma, V.; Kaur, M.; Nagpal, M.; Arora, S. Role of Nanotechnology in Taste Masking: Recent Updates. Curr. Drug Res. Rev. 2023, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Massounga Bora, A.F.; Ma, S.; Li, X.; Liu, L. Application of Microencapsulation for the Safe Delivery of Green Tea Polyphenols in Food Systems: Review and Recent Advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in Liposome Technology: Preparation Techniques and Applications in Food, Functional Foods, and Bioactive Delivery: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Hua, Y.; Wei, Z.; Xue, C. Chitosan and Its Composites-Based Delivery Systems: Advances and Applications in Food Science and Nutrition Sector. Crit. Rev. Food Sci. Nutr. 2023, 63, 4579–4598. [Google Scholar] [CrossRef] [PubMed]
- Velikov, K.P.; Pelan, E. Colloidal Delivery Systems for Micronutrients and Nutraceuticals. Soft Matter 2008, 4, 1964–1980. [Google Scholar] [CrossRef]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured Biopolymer-Based Delivery Systems for Encapsulation, Protection, and Release of Lipophilic Compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Meng, Y.; Shang, M.; Ji, H.; Li, X.; Sang, S.; Jiao, A.; Jin, Z.; Qiu, C. The Construction of Whey Protein-Coated OSA Debranched Starch Particles Used for Curcumin Steady-State Delivery and pH-Sensitive Sustained Release. Food Hydrocoll. 2024, 147, 109425. [Google Scholar] [CrossRef]
- Wang, L.; Jia, W.; Yang, Q.; Cai, H.; Zhao, X. Casein Nanoparticles as Oral Delivery Carriers for Improved Bioavailability and Hypoglycemic Activity of Apigenin. Food Hydrocoll. 2024, 146, 109194. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, L.; Li, Y.; Chen, Q.; Wang, L.; Farag, M.A.; Liu, L.; Zhan, S.; Wu, Z.; Liu, L. Soy Protein Isolate-Citrus Pectin Composite Hydrogels Induced by TGase and Ultrasonic Treatment: Potential Targeted Delivery System for Probiotics. Food Hydrocoll. 2023, 143, 108901. [Google Scholar] [CrossRef]
- Jiang, X.; Du, Z.; Zhang, X.; Zaman, F.; Song, Z.; Guan, Y.; Yu, T.; Huang, Y. Gelatin-Based Anticancer Drug Delivery Nanosystems: A Mini Review. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Feng, Y.; Deng, Y.; Yan, T.; Liang, Z.; Zhou, Y.; Zhang, W.; Xu, E.; Liu, D.; Wang, W. Continuous Flow Modulates Zein Nanoprecipitation Solvent Environment to Obtain Colloidal Particles with High Curcumin Loading. Food Hydrocoll. 2023, 134, 108089. [Google Scholar] [CrossRef]
- Sun, C.; Wei, Z.; Xue, C.; Yang, L. Development, Application and Future Trends of Starch-Based Delivery Systems for Nutraceuticals: A Review. Carbohydr. Polym. 2023, 308, 120675. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, S. Cellulose Nanocrystals Based Delivery Vehicles for Anticancer Agent Curcumin. Int. J. Biol. Macromol. 2022, 221, 842–864. [Google Scholar] [CrossRef] [PubMed]
- Singam, A.; Killi, N.; Patel, P.R.; Gundloori, R.V.N. PEGylated Ethyl Cellulose Micelles as a Nanocarrier for Drug Delivery. RSC Adv. 2021, 11, 30532–30543. [Google Scholar] [CrossRef]
- Taheri, A.; Jafari, S.M. Gum-Based Nanocarriers for the Protection and Delivery of Food Bioactive Compounds. Adv. Colloid. Interface Sci. 2019, 269, 277–295. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, M.; Kang, M.; Liao, Y.; Wang, Z.; Li, Y.; Qi, B. Novel Core-Shell Nanoparticles: Encapsulation and Delivery of Curcumin Using Guanidine Hydrochloride-Induced Oleosome Protein Self-Assembly. LWT 2023, 173, 114352. [Google Scholar] [CrossRef]
- Cengiz, B.; Gevrek, T.N.; Chambre, L.; Sanyal, A. Self-Assembly of Cyclodextrin-Coated Nanoparticles:Fabrication of Functional Nanostructures for Sensing and Delivery. Molecules 2023, 28, 1076. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Characteristics and Storage Stability of Nanoliposomes Loaded with Shrimp Oil as Affected by Ultrasonication and Microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef] [PubMed]
- Jhan, S.; Pethe, A.M. Double-Loaded Liposomes Encapsulating Lycopene β-Cyclodextrin Complexes: Preparation, Optimization, and Evaluation. J. Liposome Res. 2020, 30, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-Carotene in Wheat Gluten Nanoparticle-Xanthan Gum-Stabilized Pickering Emulsions: Enhancement of Carotenoid Stability and Bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.-H. Phytosterol Colloidal Particles as Pickering Stabilizers for Emulsions. J. Agric. Food Chem. 2014, 62, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of Double W1/O/W2 Nano-Emulsions Loaded with Oleuropein in the Internal Phase (W1) and Evaluation of Their Release Rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Wang, Z.-M.; Meng, H.-C.; Lin, J.-W.; Guo, X.-M.; Zhang, T.; Chen, H.-L.; Lei, C.-Y.; Yu, S.-J. Robust W/O/W Emulsion Stabilized by Genipin-Cross-Linked Sugar Beet Pectin-Bovine Serum Albumin Nanoparticles: Co-Encapsulation of Betanin and Curcumin. J. Agric. Food Chem. 2021, 69, 1318–1328. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Yin, J.; Chang, F.; Wang, C.; He, X.; Huang, Q.; Zhang, B. Anthocyanin-Loaded Double Pickering Emulsion Stabilized by Octenylsuccinate Quinoa Starch: Preparation, Stability and in Vitro Gastrointestinal Digestion. Int. J. Biol. Macromol. 2020, 152, 1233–1241. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (-)-Epigallocatechin-3-Gallate and Quercetin in Particle-Stabilized W/O/W Emulsion Gels: Controlled Release and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Qiu, J.; Zheng, Q.; Fang, L.; Wang, Y.; Min, M.; Shen, C.; Tong, Z.; Xiong, C. Preparation and Characterization of Casein-Carrageenan Conjugates and Self-Assembled Microcapsules for Encapsulation of Red Pigment from Paprika. Carbohydr. Polym. 2018, 196, 322–331. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of Self-Assembled Polyelectrolyte Complex Hydrogel Based on Oppositely Charged Polysaccharides for Sustained Delivery of Green Tea Polyphenols. Food Chem. 2020, 306, 125632. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, Y.; Chen, L.; Wang, T.; Zhang, S.; Li, X. Structural Changes of Layer-by-Layer Self-Assembled Starch-Based Nanocapsules in the Gastrointestinal Tract: Implications for Their M Cell-Targeting Delivery and Transport Efficiency. Int. J. Biol. Macromol. 2024, 261, 129786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, W.; Jiang, Y.; Julian McClements, D.; Liu, F.; Liu, X. Self-Assembled Nano-Micelles of Lactoferrin Peptides: Structure, Physicochemical Properties, and Application for Encapsulating and Delivering Curcumin. Food Chem. 2022, 387, 132790. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.-M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta Casein-Micelle as a Nano Vehicle for Solubility Enhancement of Curcumin; Food Industry Application. LWT Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Azzi, J.; Auezova, L.; Danjou, P.-E.; Fourmentin, S.; Greige-Gerges, H. First Evaluation of Drug-in-Cyclodextrin-in-Liposomes as an Encapsulating System for Nerolidol. Food Chem. 2018, 255, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, L.; Zhang, B.; Zhou, W.; Wang, X.; Bai, D. Photostability and Antioxidant Activity Studies on the Inclusion Complexes of Trans-Polydatin with β-Cyclodextrin and Derivatives. RSC Adv. 2018, 8, 25941–25948. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rocha, M.A.A.; Santos, L.M.N.B.F.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry Anthocyanins: β-Cyclodextrin Fortification for Thermal and Gastrointestinal Stabilization. Food Chem. 2018, 245, 426–431. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Bandral, J.D.; Gani, A.; Shams, R. Food Hydrocolloids: Functional, Nutraceutical and Novel Applications for Delivery of Bioactive Compounds. Int. J. Biol. Macromol. 2020, 165, 554–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ding, R.; Zhang, K.; Guo, H.; Lin, Y. Self-Assembled Nanocarrier Delivery Systems for Bioactive Compounds. Small 2024, 20, 2310838. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Tai, K.; Liu, F.; He, X.; Ma, P.; Mao, L.; Gao, Y.; Yuan, F. The Effect of Sterol Derivatives on Properties of Soybean and Egg Yolk Lecithin Liposomes: Stability, Structure and Membrane Characteristics. Food Res. Int. 2018, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Paulson, A.T.; Gill, T.A. Encapsulation of Bioactive Salmon Protein Hydrolysates with Chitosan-Coated Liposomes. J. Funct. Foods 2015, 19, 733–743. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research Progress on Liposomes: Application in Food, Digestion Behavior and Absorption Mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome Technology for the Food and Nutraceutical Industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Cantor, S.; Vargas, L.; Rojas A, O.E.; Yarce, C.J.; Salamanca, C.H.; Oñate-Garzón, J. Evaluation of the Antimicrobial Activity of Cationic Peptides Loaded in Surface-Modified Nanoliposomes against Foodborne Bacteria. Int. J. Mol. Sci. 2019, 20, 680. [Google Scholar] [CrossRef]
- Chai, C.; Park, J. Food Liposomes: Structures, Components, Preparations, and Applications. Food Chem. 2024, 432, 137228. [Google Scholar] [CrossRef]
- Chaves, M.A.; Ferreira, L.S.; Baldino, L.; Pinho, S.C.; Reverchon, E. Current Applications of Liposomes for the Delivery of Vitamins: A Systematic Review. Nanomaterials 2023, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Espinosa, Y.G.; Norton, I.T. Encapsulation Systems for the Delivery of Hydrophilic Nutraceuticals: Food Application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled Vesicle Self-Assembly in Microfluidic Channels with Hydrodynamic Focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef]
- Situ, W.; Song, X.; Luo, S.; Liang, Y. A Nano-Delivery System for Bioactive Ingredients Using Supercritical Carbon Dioxide and Its Release Behaviors. Food Chem. 2017, 228, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Deng, Y.; Geng, Y.; Gao, Z.; Zou, J.; Wang, Z. Preparation of Submicron Unilamellar Liposomes by Freeze-Drying Double Emulsions. Biochim. Et. Biophys. Acta (BBA) Biomembr. 2006, 1758, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, G.M.; Manohar, B. Nanocharacterization of Liposomes for the Encapsulation of Water Soluble Compounds from Cordyceps Sinensis CS1197 by a Supercritical Gas Anti-Solvent Technique. RSC Adv. 2018, 8, 34634–34649. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. A Versatile Supercritical Assisted Process for the One-Shot Production of Liposomes. J. Supercrit. Fluids 2019, 146, 136–143. [Google Scholar] [CrossRef]
- Cho, N.-J.; Hwang, L.Y.; Solandt, J.J.R.; Frank, C.W. Comparison of Extruded and Sonicated Vesicles for Planar Bilayer Self-Assembly. Materials 2013, 6, 3294–3308. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.D.; Bellato, F.; Mastrotto, F.; Garofalo, M.; Malfanti, A.; Salmaso, S.; Caliceti, P. Dexamethasone Loaded Liposomes by Thin-Film Hydration and Microfluidic Procedures: Formulation Challenges. Int. J. Mol. Sci. 2020, 21, 1611. [Google Scholar] [CrossRef] [PubMed]
- Hei, X.; Li, S.; Liu, Z.; Wu, C.; Ma, X.; Jiao, B.; Hu, H.; Zhu, J.; Adhikari, B.; Wang, Q.; et al. Characteristics of Pickering Emulsions Stabilized by Microgel Particles of Five Different Plant Proteins and Their Application. Food Chem. 2024, 449, 139187. [Google Scholar] [CrossRef]
- Estrada-Fernández, A.G.; Román-Guerrero, A.; Jiménez-Alvarado, R.; Lobato-Calleros, C.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Stabilization of Oil-in-Water-in-Oil (O1/W/O2) Pickering Double Emulsions by Soluble and Insoluble Whey Protein Concentrate-Gum Arabic Complexes Used as Inner and Outer Interfaces. J. Food Eng. 2018, 221, 35–44. [Google Scholar] [CrossRef]
- Wang, L.; Song, M.; Zhao, Z.; Chen, X.; Cai, J.; Cao, Y.; Xiao, J. Lactobacillus Acidophilus Loaded Pickering Double Emulsion with Enhanced Viability and Colon-Adhesion Efficiency. LWT 2020, 121. [Google Scholar] [CrossRef]
- Yang, S.; Qin, W.; He, F.; Zhao, X.; Zhou, Q.; Lin, F.; Gong, H.; Zhang, S.; Yu, G.; Feng, Y.; et al. Tuning Supramolecular Polymers’ Amphiphilicity via Host-Guest Interfacial Recognition for Stabilizing Multiple Pickering Emulsions. ACS Appl. Mater. Interfaces 2021, 13, 51661–51672. [Google Scholar] [CrossRef]
- Oza, K.P.; Frank, S.G. Multiple Emulsions Stabilized by Colloidal Microcrystalline Cellulose. J. Dispers. Sci. Technol. 1989, 10, 163–185. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-Grade Pickering Emulsions for Encapsulation and Delivery of Bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Boostani, S.; Sarabandi, K.; Tarhan, O.; Rezaei, A.; Assadpour, E.; Rostamabadi, H.; Falsafi, S.R.; Tan, C.; Zhang, F.; Jafari, S.M. Multiple Pickering Emulsions Stabilized by Food-Grade Particles as Innovative Delivery Systems for Bioactive Compounds. Adv. Colloid. Interface Sci. 2024, 328, 103174. [Google Scholar] [CrossRef]
- Zhu, F. Starch Based Pickering Emulsions: Fabrication, Properties, and Applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Matos, M.; Timgren, A.; Sjöö, M.; Dejmek, P.; Rayner, M. Preparation and Encapsulation Properties of Double Pickering Emulsions Stabilized by Quinoa Starch Granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 423, 147–153. [Google Scholar] [CrossRef]
- Marefati, A.; Sjöö, M.; Timgren, A.; Dejmek, P.; Rayner, M. Fabrication of Encapsulated Oil Powders from Starch Granule Stabilized W/O/W Pickering Emulsions by Freeze-Drying. Food Hydrocoll. 2015, 51, 261–271. [Google Scholar] [CrossRef]
- S, B.; M, R.; A, M.; M, R.; Smh, H. Development and Characterization of Medium and High Internal Phase Novel Multiple Pickering Emulsions Stabilized by Hordein Nanoparticles. Food Chem. 2022, 372, 131354. [Google Scholar] [CrossRef]
- Bielas, R.; Surdeko, D.; Kaczmarek, K.; Józefczak, A. The Potential of Magnetic Heating for Fabricating Pickering-Emulsion-Based Capsules. Colloids Surf. B Biointerfaces 2020, 192, 111070. [Google Scholar] [CrossRef]
- Lin, C.; Pan, P.; Shan, G.; Du, M. Microstructurally Tunable Pickering Emulsions Stabilized by Poly(Ethylene Glycol)-b-Poly(ε-Caprolactone) Diblock Biodegradable Copolymer Micelles with Predesigned Polymer Architecture. Food Chem. 2022, 374, 131827. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Binks, B.P. High Internal Phase Pickering Emulsions. Curr. Opin. Colloid. Interface Sci. 2022, 57, 101556. [Google Scholar] [CrossRef]
- Watts, S.; Chow, C.J.J.; Lim, S. Protein Nanocage Engineering for Pickering Emulsions and Potential Food Applications. Curr. Opin. Colloid. Interface Sci. 2024, 69, 101761. [Google Scholar] [CrossRef]
- Sudheer, S.; Bandyopadhyay, S.; Bhat, R. Sustainable Polysaccharide and Protein Hydrogel-Based Packaging Materials for Food Products: A Review. Int. J. Biol. Macromol. 2023, 248, 125845. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Li, C.; Sang, S.; Chen, L.; Long, J.; Qiu, C.; Jin, Z. Targeted Delivery of Hydrogels in Human Gastrointestinal Tract: A Review. Food Hydrocoll. 2023, 134, 108013. [Google Scholar] [CrossRef]
- Nie, J.; Pei, B.; Wang, Z.; Hu, Q. Construction of Ordered Structure in Polysaccharide Hydrogel: A Review. Carbohydr. Polym. 2019, 205, 225–235. [Google Scholar] [CrossRef]
- Hosseini, H.; Shirkavand Hadavand, B. Synthesis and Viscoelastic Properties of Smart Hydrogel. Polym. Sci. Ser. B 2020, 62, 394–399. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.-H.; Wu, F.-G. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Adv. Mater. 2024, 36, 2210707. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Kui, X.; Zhang, R.; Zhang, Y.; Wang, X.; Wu, Q.; Chen, T.; Sun, P. Viscoelasticity and Structures in Chemically and Physically Dual-Cross-Linked Hydrogels: Insights from Rheology and Proton Multiple-Quantum NMR Spectroscopy. Macromolecules 2017, 50, 9340–9352. [Google Scholar] [CrossRef]
- Hu, M.; Liu, G.; Zhang, W.; Du, X.; Qi, B.; Li, Y. Co-Encapsulation of (–)-Epigallocatechin-3-Gallate and Quercetin in Double Emulsion Hydrogel Beads: Microstructures, Functional Properties, and Digestion Behaviors. Food Chem. 2022, 373, 131427. [Google Scholar] [CrossRef]
- Choi, D.; Heo, J.; Park, J.H.; Jo, Y.; Jeong, H.; Chang, M.; Choi, J.; Hong, J. Nano-Film Coatings onto Collagen Hydrogels with Desired Drug Release. J. Ind. Eng. Chem. 2016, 36, 326–333. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, Q.; Liu, X.; Liu, F.; McClements, D.J. Double Network Hydrogels: Design, Fabrication, and Application in Biomedicines and Foods. Adv. Colloid. Interface Sci. 2023, 320, 102999. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Choudhary, R. Complex Coacervation: Encapsulation and Controlled Release of Active Agents in Food Systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Xiao, X.; Jia, L.; Huang, J.; Lin, Y.; Qiao, Y. Small Amphiphile-Based Coacervation. Chem. Asian J. 2022, 17, e202200938. [Google Scholar] [CrossRef] [PubMed]
- Anvari, M.; Pan, C.-H.; Yoon, W.-B.; Chung, D. Characterization of Fish Gelatin-Gum Arabic Complex Coacervates as Influenced by Phase Separation Temperature. Int. J. Biol. Macromol. 2015, 79, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Wang, Y.; Lee, J.; Ding, Y.; Huang, Q. Turbidity and Rheological Properties of Bovine Serum Albumin/Pectin Coacervates: Effect of Salt Concentration and Initial Protein/Polysaccharide Ratio. Carbohydr. Polym. 2012, 88, 838–846. [Google Scholar] [CrossRef]
- Ghorbani Gorji, S.; Ghorbani Gorji, E.; Mohammadifar, M.A.; Zargaraan, A. Complexation of Sodium Caseinate with Gum Tragacanth: Effect of Various Species and Rheology of Coacervates. Int. J. Biol. Macromol. 2014, 67, 503–511. [Google Scholar] [CrossRef]
- Aryee, F.N.A.; Nickerson, M.T. Formation of Electrostatic Complexes Involving Mixtures of Lentil Protein Isolates and Gum Arabic Polysaccharides. Food Res. Int. 2012, 48, 520–527. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The Delivery of Sensitive Food Bioactive Ingredients: Absorption Mechanisms, Influencing Factors, Encapsulation Techniques and Evaluation Models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Nurhazwani, S.; Tan, K.W.J.; Goh, K.K.T.; Sims, I.M.; Matia-Merino, L. Complex Coacervation of an Arabinogalactan-Protein Extracted from the Meryta Sinclarii Tree (Puka Gum) and Whey Protein Isolate. Food Hydrocoll. 2014, 42, 130–138. [Google Scholar] [CrossRef]
- Lemetter, C.Y.G.; Meeuse, F.M.; Zuidam, N.J. Control of the Morphology and the Size of Complex Coacervate Microcapsules during Scale-Up. AIChE J. 2009, 55, 1487–1496. [Google Scholar] [CrossRef]
- Mendanha, D.V.; Molina Ortiz, S.E.; Favaro-Trindade, C.S.; Mauri, A.; Monterrey-Quintero, E.S.; Thomazini, M. Microencapsulation of Casein Hydrolysate by Complex Coacervation with SPI/Pectin. Food Res. Int. 2009, 42, 1099–1104. [Google Scholar] [CrossRef]
- Ariga, K.; McShane, M.; Lvov, Y.M.; Ji, Q.; Hill, J.P. Layer-by-Layer Assembly for Drug Delivery and Related Applications. Expert. Opin. Drug Deliv. 2011, 8, 633–644. [Google Scholar] [CrossRef]
- Noshad, M.; Mohebbi, M.; Koocheki, A.; Shahidi, F. Influence of Interfacial Engineering on Stability of Emulsions Stabilized with Soy Protein Isolate. J. Dispers. Sci. Technol. 2016, 37, 56–65. [Google Scholar] [CrossRef]
- Kim, B.-S.; Park, S.W.; Hammond, P.T. Hydrogen-Bonding Layer-by-Layer-Assembled Biodegradable Polymeric Micelles as Drug Delivery Vehicles from Surfaces. ACS Nano 2008, 2, 386–392. [Google Scholar] [CrossRef]
- Tan, C.K.; Kan, R.Y.P. Creative Inquiry in Graphic Design: Studio Habits in an Integrated Arts Project. In Teaching and Learning the Arts in Higher Education with Technology: Vignettes from Practice; Koh, J.H.L., Kan, R.Y.P., Eds.; Springer: Singapore, 2021; pp. 129–148. ISBN 9789811649035. [Google Scholar]
- Yu, G.; Wang, X.; Zhang, C.; Chi, Z.; Chi, Z.; Liu, G. Efficient Production of Mannosylerythritol Lipids by a Marine Yeast Moesziomyces Aphidis XM01 and Their Application as Self-Assembly Nanomicelles. Mar. Life Sci. Technol. 2022, 4, 373–383. [Google Scholar] [CrossRef]
- Zhu, L.-B.; Xu, W.-L.; Zhang, W.-W.; Wu, M.-C.; Li, W.-Z.; Ge, F.; Tao, Y.-G.; Song, P. De Novosynthesis of pH-Responsive, Self-Assembled, and Targeted Polypeptide Nano-Micelles for Enhanced Delivery of Doxorubicin. Nanotechnology 2021, 32, 295707. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Ji, N.; Dai, L.; Dong, X.; Chen, M.; Xiong, L.; Sun, Q. Self-Assembled Micelles Based on Amphiphilic Biopolymers for Delivery of Functional Ingredients. Trends Food Sci. Technol. 2021, 114, 386–398. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.H.E.; Sun, L.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Lipid Digestion, Micelle Formation and Carotenoid Bioaccessibility Kinetics: Influence of Emulsion Droplet Size. Food Chem. 2017, 229, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, M.C.; Nichifor, M.; Mocanu, G.; Tuchilus, C.; Ailiesei, G.L. Block Copolymers Containing Dextran and Deoxycholic Acid Polyesters. Synthesis, Self-Assembly and Hydrophobic Drug Encapsulation. Carbohydr. Polym. 2019, 223, 115118. [Google Scholar] [CrossRef]
- Shen, F.; Ling, H.; Ge, W.; Yang, Y.; Wang, X.; Ren, J.; Wang, X. Self-Assembly Behavior and Conformation of Amphiphilic Hemicellulose-Graft-Fatty Acid Micelles. Carbohydr. Polym. 2021, 261, 117886. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Levi, M.; Lesmes, U.; Margier, M.; Reboul, E.; Livney, Y.D. Re-Assembled Casein Micelles Improve in Vitro Bioavailability of Vitamin D in a Caco-2 Cell Model. Food Funct. 2017, 8, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Jiang, T.; Yu, Y.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Shen, Y.; et al. Dual Targeting Curcumin Loaded Alendronate-Hyaluronan- Octadecanoic Acid Micelles for Improving Osteosarcoma Therapy. IJN 2019, 14, 6425–6437. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A Review on the Use of Cyclodextrins in Foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of Cyclodextrins in Food Science. A Review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Xi, Y.; Zou, Y.; Luo, Z.; Qi, L.; Lu, X. pH-Responsive Emulsions with β-Cyclodextrin/Vitamin E Assembled Shells for Controlled Delivery of Polyunsaturated Fatty Acids. J. Agric. Food Chem. 2019, 67, 11931–11941. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, A.; Fang, D.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Huang, C.; Li, W. Oregano Essential Oil/β-Cyclodextrin Inclusion Compound Polylactic Acid/Polycaprolactone Electrospun Nanofibers for Active Food Packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Zhang, W.; Ezati, P.; Khan, A.; Assadpour, E.; Rhim, J.-W.; Jafari, S.M. Encapsulation and Delivery Systems of Cinnamon Essential Oil for Food Preservation Applications. Adv. Colloid. Interface Sci. 2023, 318, 102965. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, S.; Li, Z.; Gu, Z.; Xu, S.; Ban, X.; Hong, Y.; Cheng, L.; Li, C. A Review of Controlled Release from Cyclodextrins: Release Methods, Release Systems and Application. Crit. Rev. Food Sci. Nutr. 2023, 63, 4744–4756. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, G.; Montisci, M.-J.; Dembri, A.; Durrer, C.; Duchêne, D. Mucoadhesion of Colloidal Particulate Systems in the Gastro-Intestinal Tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31. [Google Scholar] [CrossRef]
- Alai, M.S.; Lin, W.J.; Pingale, S.S. Application of Polymeric Nanoparticles and Micelles in Insulin Oral Delivery. J. Food Drug Anal. 2015, 23, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of Polymeric Nanoparticle Size to Improve Therapeutic Delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Sacanna, S.; Pine, D.J.; Yi, G.-R. Engineering Shape: The Novel Geometries of Colloidal Self-Assembly. Soft Matter 2013, 9, 8096–8106. [Google Scholar] [CrossRef]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide Colloidal Particles as Delivery Systems for Macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.-P. Parameters Influencing the Stealthiness of Colloidal Drug Delivery Systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- Effect of Surfactants on the Formation and Characterization of a New Type of Colloidal Drug Delivery System: Nanostructured Lipid Carriers. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 210–216. [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Development of Positively Charged Colloidal Drug Carriers: Chitosan-Coated Polyester Nanocapsules and Submicron-Emulsions. Colloid. Polym. Sci. 1997, 275, 46–53. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-Based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, Protection, and Release of Hydrophilic Active Components: Potential and Limitations of Colloidal Delivery Systems. Adv. Colloid. Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, W.; Patel, A.R.; Setiowati, A.D.; Van der Meeren, P. Functional Colloids from Proteins and Polysaccharides for Food Applications. Trends Food Sci. Technol. 2017, 68, 56–69. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, L.; Zhuang, Y.; Gu, Y.; Cheng, G.; Fan, X.; Ding, Y.; Liu, H. Protein-Stabilized Emulsion Gels with Improved Emulsifying and Gelling Properties for the Delivery of Bioactive Ingredients: A Review. Foods 2023, 12, 2703. [Google Scholar] [CrossRef]
- Rawel, H.M.; Frey, S.K.; Meidtner, K.; Kroll, J.; Schweigert, F.J. Determining the Binding Affinities of Phenolic Compounds to Proteins by Quenching of the Intrinsic Tryptophan Fluorescence. Mol. Nutr. Food Res. 2006, 50, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Szente, L. Elimination of Bitter, Disgusting Tastes of Drugs and Foods by Cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; Gumus, C.E.; McClements, D.J. Lutein-Enriched Emulsion-Based Delivery Systems: Influence of pH and Temperature on Physical and Chemical Stability. Food Chem. 2016, 196, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Qiu, C.; Li, X.; McClements, D.J.; Sang, S.; Jiao, A.; Jin, Z. Polysaccharide-Based Nano-Delivery Systems for Encapsulation, Delivery, and pH-Responsive Release of Bioactive Ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Colloids in Food: Ingredients, Structure, and Stability. Annu. Rev. Food Sci. Technol. 2015, 6, 211–233. [Google Scholar] [CrossRef]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-Based Nanoparticles: Fabrication and Stability of Food-Grade Colloidal Delivery Systems. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar] [CrossRef]
- Wahlgren, M.; Engblom, J.; Sjöö, M.; Rayner, M. The Use of Micro- and Nanoparticles in the Stabilisation of Pickering-Type Emulsions for Topical Delivery. Curr. Pharm. Biotechnol. 2013, 14, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent Advances of Characterization Techniques for the Formation, Physical Properties and Stability of Pickering Emulsion. Adv. Colloid. Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.-I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.-A. Coating Materials to Increase the Stability of Liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Stability Enhancement Efficiency of Surface Decoration on Curcumin-Loaded Liposomes: Comparison of Guar Gum and Its Cationic Counterpart. Food Hydrocoll. 2019, 87, 29–37. [CrossRef]
- Qiu, C.; Zhao, M.; Decker, E.A.; McClements, D.J. Influence of Anionic Dietary Fibers (Xanthan Gum and Pectin) on Oxidative Stability and Lipid Digestibility of Wheat Protein-Stabilized Fish Oil-in-Water Emulsion. Food Res. Int. 2015, 74, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable Liposome-Encapsulated Hydrogels for Biomedical Applications: A Marriage of Convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef]
- Dymek, M.; Sikora, E. Liposomes as Biocompatible and Smart Delivery Systems—The Current State. Adv. Colloid. Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.; Malone, M.E.; Homan, J.E.; Norton, I.T. A Mathematical Model of Volatile Release in Mouth from the Dispersion of Gelled Emulsion Particles. J. Control Release 2004, 98, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Neufeld, R.J. Modeling the Controllable pH-Responsive Swelling and Pore Size of Networked Alginate Based Biomaterials. Biomaterials 2009, 30, 6119–6129. [Google Scholar] [CrossRef]
- Fuchs, M.; Schweizer, K.S. Structure of Colloid-Polymer Suspensions. J. Phys. Condens. Matter 2002, 14, R239. [Google Scholar] [CrossRef]
- Genovese, D.B.; Lozano, J.E.; Rao, M.A. The Rheology of Colloidal and Noncolloidal Food Dispersions. J. Food Sci. 2007, 72, R11–R20. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, M.M.; Kaushani, K.G.; Vanniarachchy, M.P.G.; Wijesekara, I. Hydrocolloid and Water Soluble Polymers Used in the Food Industry and Their Functional Properties: A Review. Polym. Bull. 2023, 80, 3585–3610. [Google Scholar] [CrossRef]
- Xu, D.; Qi, Y.; Wang, X.; Li, X.; Wang, S.; Cao, Y.; Wang, C.; Sun, B.; Decker, E.; Panya, A. The Influence of Flaxseed Gum on the Microrheological Properties and Physicochemical Stability of Whey Protein Stabilized β-Carotene Emulsions. Food Funct. 2017, 8, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Paximada, P.; Howarth, M.; Dubey, B.N. Double Emulsions Fortified with Plant and Milk Proteins as Fat Replacers in Cheese. J. Food Eng. 2021, 288, 110229. [Google Scholar] [CrossRef]
- Penninkhof, J.J.; Moroz, A.; van Blaaderen, A.; Polman, A. Optical Properties of Spherical and Oblate Spheroidal Gold Shell Colloids. J. Phys. Chem. C 2008, 112, 4146–4150. [Google Scholar] [CrossRef]
- Huang, S.; Yang, C.; Huang, J.; Wang, X.; Wang, M. Near-Infrared Fluorescent Pyrrolopyrrole Cyanine Derivatives and Colloidal Nanoparticles with Tunable Optical Properties for in Vivo Bioimaging. Dye. Pigment. 2018, 154, 269–274. [Google Scholar] [CrossRef]
- Bogatyrev, V.A.; Dykman, L.A.; Khlebtsov, B.N.; Khlebtsov, N.G. Measurement of Mean Size and Evaluation of Polydispersity of Gold Nanoparticles from Spectra of Optical Absorption and Scattering. Opt. Spectrosc. 2004, 96, 128–135. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Krause, M.E.; Sahin, E. Chemical and Physical Instabilities in Manufacturing and Storage of Therapeutic Proteins. Curr. Opin. Biotechnol. 2019, 60, 159–167. [Google Scholar] [CrossRef]
- Diarrassouba, F.; Garrait, G.; Remondetto, G.; Alvarez, P.; Beyssac, E.; Subirade, M. Improved Bioavailability of Vitamin D3 Using a β-Lactoglobulin-Based Coagulum. Food Chem. 2015, 172, 361–367. [Google Scholar] [CrossRef]
- Le Maux, S.; Giblin, L.; Croguennec, T.; Bouhallab, S.; Brodkorb, A. β-Lactoglobulin as a Molecular Carrier of Linoleate: Characterization and Effects on Intestinal Epithelial Cells in Vitro. J. Agric. Food Chem. 2012, 60, 9476–9483. [Google Scholar] [CrossRef]
- Banun, V.J.; Rewatkar, P.; Chaudhary, Z.; Qu, Z.; Janjua, T.; Patil, A.; Wu, Y.; Ta, H.T.; Bansal, N.; Miles, J.A.; et al. Protein Nanoparticles for Enhanced Oral Delivery of Coenzyme-Q10: In Vitro and in Silico Studies. ACS Biomater. Sci. Eng. 2023, 9, 2846–2856. [Google Scholar] [CrossRef]
- Hategekimana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Encapsulation of Vitamin E: Effect of Physicochemical Properties of Wall Material on Retention and Stability. Carbohydr. Polym. 2015, 124, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lee, S.; Han, Y.-S.; Park, K.-C.; Choy, J.-H. Efficient Transdermal Penetration and Improved Stability of L-Ascorbic Acid Encapsulated in an Inorganic Nanocapsule. Bull. Korean Chem. Soc. 2003, 24, 499–503. [Google Scholar] [CrossRef]
- Zimet, P.; Livney, Y.D. Beta-Lactoglobulin and Its Nanocomplexes with Pectin as Vehicles for ω-3 Polyunsaturated Fatty Acids. Food Hydrocoll. 2009, 23, 1120–1126. [Google Scholar] [CrossRef]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano Formulation Approaches for Curcumin Delivery- a Review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent Progress in Nanotechnology-Based Drug Carriers for Resveratrol Delivery. Drug Deliv. 2023, 30, 2174206. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Papakyriakopoulou, P.; Saitani, E.-M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, L.; Zhao, F.; Yu, A.; Zhou, Y.; Wen, Q.; Wang, J.; Zheng, T.; Chen, P. Protein and Peptide-Based Nanotechnology for Enhancing Stability, Bioactivity, and Delivery of Anthocyanins. Adv. Healthc. Mater. 2023, 12, 2300473. [Google Scholar] [CrossRef]
- Nagarajan, U.; Monica Denise, R.; Kanth, S.V.; Natarajan, S. An Eco Friendly Approach for the Development of a Dipeptide Based Anti-TB Drug Nanocomposites: A Greener Approach in Drug Delivery System for Pulmonary Delivery. Sustain. Mater. Technol. 2024, 41, e01037. [Google Scholar] [CrossRef]
- Katsogiannis, I.; Naziris, N.; Sarika, A.; Gardikis, K.; Hatziantoniou, S.; Boukos, N.; Dallas, P.; Fikioris, N.; Demetzos, C. Development and in Vitro Evaluation of Liposomal Carnosine for Dermatological and Cosmeceutical Applications. J. Drug Deliv. Sci. Technol. 2024, 96, 105654. [Google Scholar] [CrossRef]
- Beňovič, P.; Sokol, J.; Purdešová, A.; Maliarová, M. Biological Properties and Methods for Determination of Carnosine. Monatsh Chem. 2023, 154, 1045–1060. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Yuan, Y.; Wang, Q.; Wang, L.; Wu, J. Lentinan Progress in Inflammatory Diseases and Tumor Diseases. Eur. J. Med. Res. 2024, 29, 8. [Google Scholar] [CrossRef]
- Kou, F.; Ge, Y.; Wang, W.; Mei, Y.; Cao, L.; Wei, X.; Xiao, H.; Wu, X. A Review of Ganoderma Lucidum Polysaccharides: Health Benefit, Structure–Activity Relationship, Modification, and Nanoparticle Encapsulation. Int. J. Biol. Macromol. 2023, 243, 125199. [Google Scholar] [CrossRef] [PubMed]
- Caritá, A.C.; Resende de Azevedo, J.; Chevalier, Y.; Arquier, D.; Buri, M.V.; Riske, K.A.; Ricci Leonardi, G.; Bolzinger, M.-A. Elastic Cationic Liposomes for Vitamin C Delivery: Development, Characterization and Skin Absorption Study. Int. J. Pharm. 2023, 638, 122897. [Google Scholar] [CrossRef]
- Wijekoon, M.M.J.O.; Mahmood, K.; Ariffin, F.; Mohammadi Nafchi, A.; Zulkurnain, M. Recent Advances in Encapsulation of Fat-Soluble Vitamins Using Polysaccharides, Proteins, and Lipids: A Review on Delivery Systems, Formulation, and Industrial Applications. Int. J. Biol. Macromol. 2023, 241, 124539. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Nanostructured Lipid Carriers (NLCs) Stabilized by Natural or Synthetic Emulsifiers for Lutein Delivery: Improved Physicochemical Stability, Antioxidant Activity, and Bioaccessibility. Food Chem. 2023, 403, 134465. [Google Scholar] [CrossRef]
- Qiang, S.; Zhou, J.; Yang, T.; Wang, J.; Chen, Y.; Chen, G.; Li, S. Structure, Stability and in Vitro Digestion of a Novel Zein-Based Oil Gel Delivery System Loaded β-Carotene. J. Food Eng. 2024, 366, 111848. [Google Scholar] [CrossRef]
- Hu, M.; Gao, Y.; Wen, W.; Zhang, P.; Zhang, F.; Fan, B.; Wang, F.; Li, S. The Aggregation Behavior between Soybean Whey Protein and Polysaccharides of Diverse Structures and Their Implications in Soybean Isoflavone Delivery. Food Chem. 2024, 439, 138061. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, G. Controlled Volatile Release from β-Sitosterol-Based Oleogels Based on Different Self-Assembly Mechanisms. Food Chem. 2023, 425, 136506. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In Vitro Gastrointestinal Digestion Study of Broccoli Inflorescence Phenolic Compounds, Glucosinolates, and Vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, Protection, and Delivery of Bioactive Proteins and Peptides Using Nanoparticle and Microparticle Systems: A Review. Adv. Colloid. Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhu, X.; Liu, M.; Li, L.; Zhong, J.; Sun, W.; Zhang, Z.; Huang, Y. Overcoming the Diffusion Barrier of Mucus and Absorption Barrier of Epithelium by Self-Assembled Nanoparticles for Oral Delivery of Insulin. ACS Nano 2015, 9, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Dodane, V.; Vilivalam, V.D. Pharmaceutical Applications of Chitosan. Pharm. Sci. Technol. Today 1998, 1, 246–253. [Google Scholar] [CrossRef]
- Jiang, P.; Huang, J.; Bao, C.; Jiao, L.; Zhao, H.; Du, Y.; Fazheng, R.; Li, Y. Enzymatically Partially Hydrolyzed α-Lactalbumin Peptides for Self-Assembled Micelle Formation and Their Application for Coencapsulation of Multiple Antioxidants. J. Agric. Food Chem. 2018, 66, 12921–12930. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, M.; Xiao, H.; Young Quek, S.; Ogawa, Y.; Ma, G.; Zhang, C. Advances in Spray-Dried Probiotic Microcapsules for Targeted Delivery: A Review. Crit. Rev. Food Sci. Nutr. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Kim, H.W.; Park, H.J. Customized Oral Mucosal Adhesive Film-Based Functional-Substance Delivery System Using Embedded 3D Printing Method. Food Hydrocoll. 2022, 131, 107762. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. A Human Gastric Simulator (HGS) to Study Food Digestion in Human Stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef]
- Mao, L.; Roos, Y.H.; Biliaderis, C.G.; Miao, S. Food Emulsions as Delivery Systems for Flavor Compounds: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3173–3187. [Google Scholar] [CrossRef]
- Karaiskou, S.; Blekas, G.; Paraskevopoulou, A. Aroma Release from Gum Arabic or Egg Yolk/Xanthan-Stabilized Oil-in-Water Emulsions. Food Res. Int. 2008, 41, 637–645. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Bortnowska, G. Effects of pH and Ionic Strength of NaCl on the Stability of Diacetyl and (−)-α-Pinene in Oil-in-Water Emulsions Formed with Food-Grade Emulsifiers. Food Chem. 2012, 135, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Mu, H.; Liu, Y.; Zhang, Y.; Wang, Q.; Erazo Quintero, L.E.; Li, X.; Chen, S.; Gong, Y.; et al. The Stability and Spicy Taste Masking Effect of Capsaicin Loaded α-Lactalbumin Micelles Formulated in Defatted Cheese. Food Funct. 2022, 13, 12258–12267. [Google Scholar] [CrossRef]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Antioxidant Packaging with Encapsulated Green Tea for Fresh Minced Meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the Antimicrobial Activity of Chitosan and Its Quaternized Derivative on E. coli and S. aureus Growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Recent Progress in Nanoformulations of Silver Nanoparticles with Cellulose, Chitosan, and Alginic Acid Biopolymers for Antibacterial Applications. Appl. Microbiol. Biotechnol. 2019, 103, 8669–8676. [Google Scholar] [CrossRef]
- Su Cha, D.; Choi, J.H.; Chinnan, M.S.; Park, H.J. Antimicrobial Films Based on Na-Alginate and κ-Carrageenan. LWT 2002, 35, 715–719. [Google Scholar] [CrossRef]
- Franz, R. Migration Modelling from Food-Contact Plastics into Foodstuffs as a New Tool for Consumer Exposure Estimation. Food Addit. Contam. 2005, 22, 920–937. [Google Scholar] [CrossRef]
- Li, Z.; Peng, S.; Chen, X.; Zhu, Y.; Zou, L.; Liu, W.; Liu, C. Pluronics Modified Liposomes for Curcumin Encapsulation: Sustained Release, Stability and Bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef]


| Structure | Delivered Substances | Colloidal Materials | Processing Technology | Function | References |
|---|---|---|---|---|---|
| Liposome | Curcumin | Phosphatidylcholine, cholesterol | Detergent removal method | Improved stability, solubility in aqueous medium | [7] |
| Shrimp oil | Soy lecithin (L-α-Phosphatidylcholine) | Ultrasonication and microfluidization method | Prevents oxidation of oil during storage and masks the undesirable fishy odor | [7,22] | |
| Lycopene | Soy lecithin, cholesterol, β-CD | Thin-film hydration method | Enhances in vivo activity; controls release | [23] | |
| Green tea polyphenols | - | Microencapsulation | Improve the stability of polyphenols in food processing | [6] | |
| Pickering emulsion | Carotenoids (β-carotene) | Wheat gluten nanoparticles | - | Improve stability to chemical degradation during storage and enhances its bioaccessibility in a simulated gastrointestinal tract | [24] |
| Water-insoluble phytosterols | Phytosterols, whey protein concentrate | Anti-solvent method | Improved stability, bioavailability | [25] | |
| Oleuropein | Pectin, whey protein | Sonication | Encapsulation efficiency of 91% is achieved | [26] | |
| Betanin, curcumin | Sugar beet pectin, bovine serum albumin nanoparticles | Genipin cross-linking strategy | Improve their thermal stability and bioavailability | [27] | |
| Anthocyanin | Octenylsuccinate quinoa starch | - | Improves storage stability | [28] | |
| Epigallocatechin-3-gallate, quercetin | Gelatin, gliadin nanoparticles | Two-step procedure | Improve EGCG chemical stability and quercetin solubility under simulated gastrointestinal conditions | [29] | |
| Hydrogel | Lactobacillus plantarum, Lactobacillus salivarius | Peach gum polysaccharide and Auricularia polytricha β-glucans | Inverse emulsion cross-linking method | Potential application as intestinal-targeted delivery systems | [30] |
| Green tea polyphenols | Salecan and N,N,N-trimethyl chitosan | Self-assembly | Efficiently encapsulated into PEC hydrogels and liberated in a sustained pattern | [31] | |
| Self-assembled microcapsules based on electrostatic interactions | Red pigment from paprika | Casein, carrageenan | Ultrasonic Maillard dry treatment | Enhances thermal, enteric, and storage stability for free release and emulsification | [30] |
| Ovalbumin | RGD peptide-grafted carboxymethyl starch, cationic quaternary ammonium starch | - | Improves intestinal stability and delivery transportation efficiency | [32] | |
| Self-assembled nanomicelle | Curcumin | Lactoferrin hydrolysate | - | Improves thermal, dilution, and storage stability as well as improved in vivo conversion and bioavailability | [33] |
| Curcumin | Beta-casein | - | The antioxidant activity of curcumin encapsulated in B-CN is higher than that of both free B-CN and curcumin | [34] | |
| Cyclodextrin | Quercetin | SBE-β-CD | Complexation in water | Significantly increases the solubility and bioavailability of quercetin | [35] |
| Trans-polydatin | β-CD | Complexation in water/ethanol solution and freeze-dried | Increases solubility, thermal stability, photostability, and antioxidant activities of trans-polydatin after forming CICs. | [36] | |
| Blackberry anthocyanins | β-CD | Complexation in water and freeze-dried | Enhance thermal stability and bioaccessibility of blackberry anthocyanins after forming CICs | [37] |
| Carrier Classification | Size | Charged Characteristics | Binding Characteristics | Physical Stability | Release Behavior | References |
|---|---|---|---|---|---|---|
| Liposome | 20 nm to a few microns | Surface charging properties are mainly dependent on the lipid composition contained (categorized as cationic/negative/neutral liposomes) | Adhesive interactions and chemical bonding between phospholipid molecules | Physical instability (thermodynamic instability), chemical instability (susceptibility to oxidation and hydrolysis), and biological instability | Mainly concerned with structural changes in liposome membranes and kinetic processes of drug release | [48] |
| Pickering emulsion | Micron level (depending on the process) | - | Capillary force and adsorption of solid particles at the interface | Surface wettability of solid particles, concentration, electrolytes in the aqueous phase, pH, and the volume ratio of the oil–water phase affect the adsorption behavior of solid particles at the oil–water interface | Stabilization of solid particles at the oil–water interface to control emulsions | [57] |
| Hydrogel | Size of hydrogel is a flexible and variable concept; there are large-size hydrogels at the macroscopic scale and nanoscale hydrogels at the microscopic scale | The situation is complex and is usually related to the charged groups of the polymer chain, surface ion adsorption, pH, and ion concentration in the environment | It can be formed by chemical cross-linking (covalent bonding) or physical cross-linking (non-covalent bonding) of macromolecules. Chemical cross-linking has been widely used in food macromolecule modification, food packaging, microsphere preparation, and other applications. | Dehydration-induced hardening and softening phenomena, polymer aggregation, and stabilizer selection and use | Primarily concerned with their physical and chemical properties and how they affect drug release | [74] |
| Complex coacervates | Usually about 10–200 nm | Mainly in the nature and distribution of its surface charge | Electrostatic force | The pH and ionic concentration of the surrounding medium, temperature, etc., affect its stability | Usually depends on disintegration mediated by external conditions | [83] |
| Layer-by-layer | Nanometer scale | Mainly in the nature and distribution of its surface charge | Weak interactions (e.g., electrostatic interactions, hydrogen bonding, coordination bonding, etc.) | The pH and ionic concentration of the surrounding medium, temperature, etc., affect its stability | Precise control and release of substances can be achieved by controlling the interactions between layers | [93] |
| Self-assembled nanomicelles | Between a few nanometers and a few tens of nanometers | Depends mainly on the type of surfactant it contains | Interactions between non-covalently bonded molecules such as hydrogen bonding, hydrophobic interactions, electrostatic interactions, etc. | Mainly concerned with their dynamic properties and preparation methods | May be related to the dissociation of micelles, which may occur when external environmental conditions change | [33] |
| Cyclodextrin | Depends on its specific type | Electrically neutral | - | This is mainly related to its structural characteristics and some unfavorable factors in the application environment | Decomposition of the inclusion by adjusting its structure and properties | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; LvYe, J.; Yang, S.; Shi, Y.; Chen, Q. Critical Review of Food Colloidal Delivery System for Bioactive Compounds: Physical Characterization and Application. Foods 2024, 13, 2596. https://doi.org/10.3390/foods13162596
Wang B, LvYe J, Yang S, Shi Y, Chen Q. Critical Review of Food Colloidal Delivery System for Bioactive Compounds: Physical Characterization and Application. Foods. 2024; 13(16):2596. https://doi.org/10.3390/foods13162596
Chicago/Turabian StyleWang, Bijie, Jiayi LvYe, Shaoming Yang, Ying Shi, and Qihe Chen. 2024. "Critical Review of Food Colloidal Delivery System for Bioactive Compounds: Physical Characterization and Application" Foods 13, no. 16: 2596. https://doi.org/10.3390/foods13162596







