Evaluating Biocompounds in Discarded Beetroot (Beta vulgaris) Leaves and Stems for Sustainable Food Processing Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation and Drying Process
2.2.2. Proximate Chemical Characterization and Water Activity
2.2.3. Amino Acid Profile
2.2.4. Fatty Acid Profile
2.2.5. Mineral Composition and Phytic Acid Determination
2.2.6. Functional Properties and Color
2.2.7. Extraction of Bioactive Compounds
2.2.8. Determination of Betalains, Total Phenolics, and Antioxidant Capacity
2.2.9. Determination of Nitrate
2.2.10. Food Product Development and Organoleptic Analysis
2.2.11. Statistical Analysis
3. Results
3.1. Drying Characteristics
3.2. Proximate Chemical Composition and Water Activity
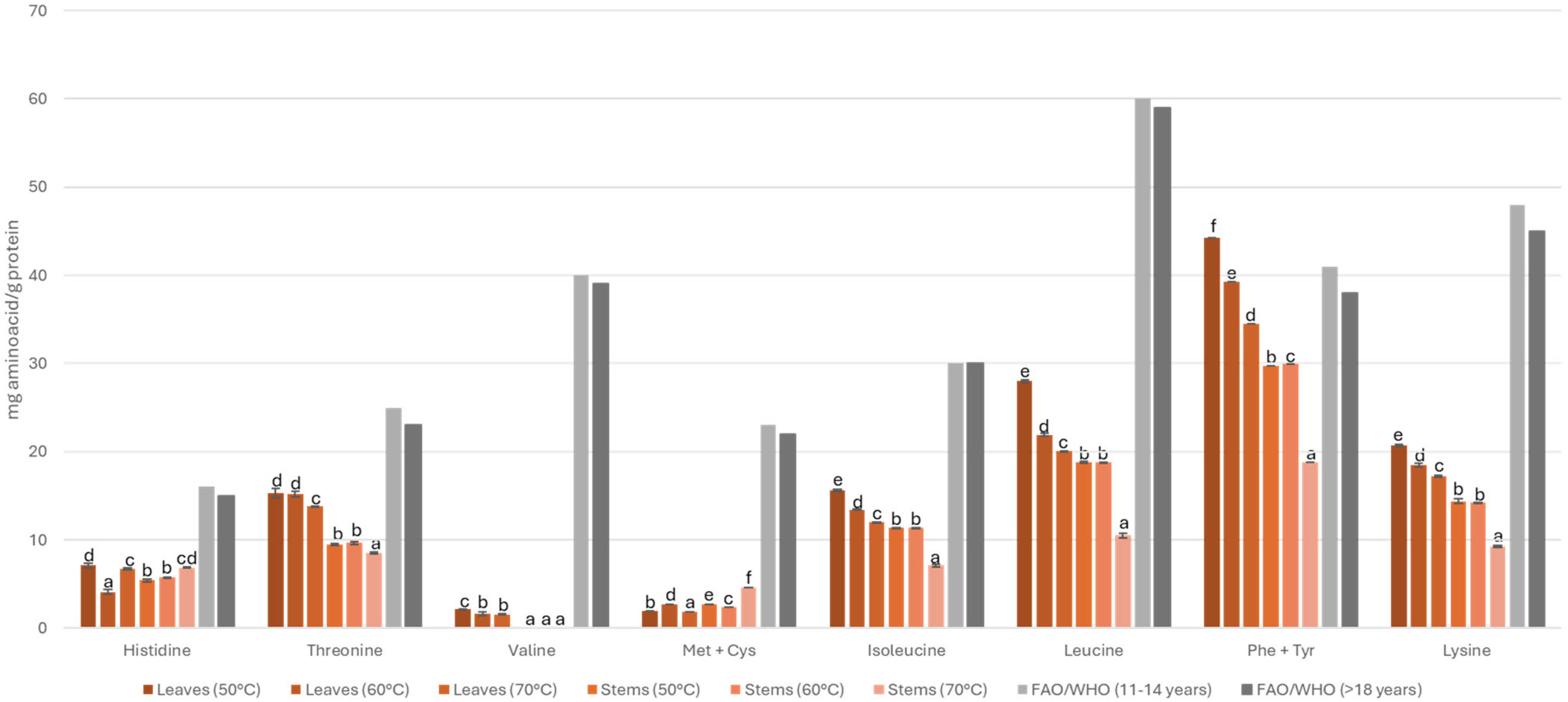
3.3. Amino Acid Profile
3.4. Fatty Acid Profile
3.5. Determination of Mineral Composition and Phytic Acid
| Minerals | Units | Stems | Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | mg/100 g | 325.00 | ± | 21.21 a | 1445.00 | ± | 7.07 b | ||||
| Mg | mg/100 g | 590.00 | ± | 28.28 a | 1310.00 | ± | 28.28 b | ||||
| Macroelements | P | mg/100 g | 315.00 | ± | 7.07 a | 300.00 | ± | 14.14 a | |||
| K | mg/100 g | 6155.00 | ± | 120.21 b | 3040.00 | ± | 0.00 a | ||||
| Cu | mg/100 g | 0.45 | ± | 0.07 a | 1.15 | ± | 0.07 b | ||||
| Mn | mg/100 g | 5.65 | ± | 0.64 a | 33.85 | ± | 3.61 b | ||||
| Microelements | Zn | mg/100 g | 1.85 | ± | 0.07 a | 2.75 | ± | 0.07 b | |||
| Fe | mg/100 g | 15.95 | ± | 0.92 a | 43.80 | ± | 1.13 b | ||||
| Flour (g) * | 1 | 5 | 10 | 1 | 5 | 10 | |||||
| Ca | DRIs 1000 | mg/day | 33 | 163 | 325 | 145 | 723 | 1445 | |||
| Mg | DRIs 350/300 | mg/day | 169/197 | 843/983 | 1689/1967 | 374/437 | 1871/2183 | 3743/4367 | |||
| Contribution to DRIs (%) * | P | DRIs 550 | mg/day | 57 | 286 | 573 | 55 | 273 | 545 | ||
| Cu | DRIs 1.6/1.3 | mg/day | 28/35 | 141/173 | 281/346 | 72/88 | 359/442 | 719/885 | |||
| Mn | DRIs 2.3/1.8 | mg/day | 246/314 | 1228/1569 | 2457/3139 | 1472/1881 | 7359/9403 | 14717 | |||
| Zn | DRIs 11/8 | mg/day | 17/23 | 9.25 | 18.5 | 2.75 | 13.75 | 18806 | |||
| Fe | DRIs 11/7 | mg/day | 145/228 | 725/1139 | 1450/2279 | 398/626 | 1991/3129 | 3982/6257 | |||
| Ratio | Ins P6/Ca < 0.24 | 21.50 | 6.68 | ||||||||
| Ins P6/Fe < 1 | 0.0006 | 0.0003 | |||||||||
| Ins P6/Zn < 15 | 0.006 | 0.006 | |||||||||
3.6. Functional Properties and Color
3.7. Determination of the Phenolic Content, Total Phenolic Content, and Antioxidant Capacity
3.8. Analysis of Nitrates
3.9. Organoleptic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamim, C.J.R.; Alves, Y.; Alves, A.; Mota de Moraes, Y.; Sakagute, S.; da Silva, G.; Santos, L.; da Silva, L.; Scoqui, C.; Arosti, M.; et al. Nitrate-rich beet juice intake on cardiovascular performance in response to exercise in postmenopausal women with arterial hypertension: Study protocol for a randomized controlled trial. Trials 2023, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Zainal Arifin, M.; Adzahan, N.M.; Abedin, N.Z.; Lasik-Kurdyś, M. Utilization of Food Waste and By-Products in the Fabrication of Active and Intelligent Packaging for Seafood and Meat Products. Foods 2023, 12, 456. [Google Scholar] [CrossRef]
- Paciulli, M.; Medina-Meza, I.G.; Chiavaro, E.; Barbosa-Cánovas, G.V. Impact of thermal and high pressure processing on quality parameters of beetroot (Beta vulgaris L.). LWT—Food Sci. Technol. 2016, 68, 98–104. [Google Scholar] [CrossRef]
- Ghazy, N.A.; El-Hafez, O.A.A.; El-Bakery, A.M.; El-Geddawy, D.I.H. Impact of silver nanoparticles and two biological treatments to control soft rot disease in sugar beet (Beta vulgaris L). Egypt. J. Biol. Pest Control 2021, 31, 3. [Google Scholar] [CrossRef]
- dos S. Baião, D.; da Silva, D.V.; Paschoalin, V.M. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, J.; Xie, S.-Y.; Zhang, T.-Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Patterson, A.J.; Macdonald-Wicks, L.; McEvoy, M. The role of inorganic nitrate and nitrite in CVD. Nutr. Res. Rev. 2017, 30, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kaimainen, M.; Järvenpää, E.; Sandell, M.; Huopalahti, R.; Yang, B.; Laaksonen, O. Red beet (Beta vulgaris) betalains and grape (Vitis vinifera) anthocyanins as colorants in white currant juice – Effect of storage on degradation kinetics, color stability and sensory properties. Food Chem. 2021, 348, 128995. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Wroblewska, M.; Juskiewicz, J.; Wiczkowski, W. Physiological properties of beetroot crisps applied in standard and dyslipidaemic diets of rats. Lipids Health Dis. 2011, 10, 178. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, D.D. Antioxidant and Antimicrobial Activities of Beet Root Pomace Extracts. Czech. J. Food Sci. 2011, 29, 575–585. [Google Scholar]
- Akan, S.; Gunes, N.T.; Erkan, M. Red beetroot: Health benefits, production techniques, and quality maintaining for food industry. J. Food Process. Preserv. 2021, 45, 10. [Google Scholar] [CrossRef]
- Battistella Lasta, H.F.; Lentz, L.; Rodrigues, L.G.G.; Mezzomo, N.; Vitali, L.; Ferreira, S.R.S. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Evaluation and characterization of nutritional, microbiological and sensory properties of beet greens. Acta Sci. Nutr. Health 2017, 3, 37–45. [Google Scholar]
- Biondo, P.B.F.; Boeing, J.S.; Barizão, É.O.; Souza, N.E.D.; Matsushita, M.; Oliveira, C.C.D.; Boroski, M.; Visentainer, J.V. Evaluation of beetroot (Beta vulgaris L.) leaves during its developmental stages: A chemical composition study. Food Sci. Technol. 2014, 34. [Google Scholar] [CrossRef]
- Asadi, S.Z.; Khan, M.A. The Effect of Beetroot (Beta vulgaris L.) Leaves Powder on Nutritional, Textural, Sensorial and Antioxidant Properties of Cookies. J. Culin. Sci. Technol. 2021, 19, 424–438. [Google Scholar] [CrossRef]
- Zzaman, W.; Biswas, R.; Hossain, M.A. Application of immersion pre-treatments and drying temperatures to improve the comprehensive quality of pineapple (Ananas comosus) slices. Heliyon 2021, 7, e05882. [Google Scholar] [CrossRef]
- AACC. Method 30-20: Crude Fat in Grain and Stock Feeds, Method 44-15A: Moisture, Method 46-13: Crude Protein-Micro-Kjeldahl Method, Method 54-21: Farinograph Method for Flour. Approved Methods of American Asociation of Cereal Chemistry; AACC: Saint Paul, MN, USA, 1995. [Google Scholar]
- AACC. Method 08-03.01: Ash—Rapid Method; American Association of Cereal Chemists (AACC): Saint Paul, MN, USA, 2000. [Google Scholar]
- Lee, S.C.; Prosky, L.; De Vries, J.W. Determination of total, soluble, and insoluble dietary fiber in foods: Enzymatic-gravimetric method, MES-TRIS buffer: Collaborative study. J. Assoc. Off. Anal. Chem. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- White, J.A.; Hart, R.J.; Fry, J.C. An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J. Autom. Chem. 1986, 8, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Vera, N.; Laguna, L.; Zura, L.; Puente, L.; Muñoz, L.A. Evaluation of the physical changes of different soluble fibres produced during an in vitro digestion. J. Funct. Foods 2019, 62, 103518. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists; American Association of Cereal Chemists: St. Paul, MN, USA, 1989. [Google Scholar]
- Segura-Campos, M.R.; Ciau-Solis, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and Functional Properties of Chia Seed (Salvia hispanica L.) Gum. Int. J. Food Sci. 2014, 2014, 241053. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Chua, M.-T.; Tung, Y.-T.; Chang, S.-T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour. Technol. 2008, 99, 1918–1925. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: New York, USA, 2010. [Google Scholar]
- Mandale, N.M.; Attkan, A.K.; Kumar, S.; Kumar, N. Drying kinetics and quality assessment of refractance window dried beetroot. J. Food Process Eng. 2023, 46, e14332. [Google Scholar] [CrossRef]
- Çetin, N. Prediction of moisture ratio and drying rate of orange slices using machine learning approaches. J. Food Process. Preserv. 2022, 46, e17011. [Google Scholar] [CrossRef]
- Qu, H.; Masud, M.H.; Islam, M.; Khan, M.I.H.; Ananno, A.A.; Karim, A. Sustainable food drying technologies based on renewable energy sources. Crit. Rev. Food Sci. Nutr. 2022, 62, 6872–6886. [Google Scholar] [CrossRef]
- Milião, G.L.; de Oliveira, A.P.H.; Soares, L.D.S.; Arruda, T.R.; Vieira, É.N.R.; Junior, B.R.D.C.L. Unconventional food plants: Nutritional aspects and perspectives for industrial applications. Future Foods 2022, 5, 100124. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Schreuders, F.K.G.; Zisopoulos, F.K.; Boom, R.M.; van der Goot, A.J. Processing concepts for the use of green leaves as raw materials for the food industry. J. Clean. Prod. 2017, 164, 736–748. [Google Scholar] [CrossRef]
- Akyüz, A.; Ersus, S. Optimization of enzyme assisted extraction of protein from the sugar beet (Beta vulgaris L.) leaves for alternative plant protein concentrate production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef] [PubMed]
- Abdo, E.; El-Sohaimy, S.; Shaltout, O.; Abdalla, A.; Zeitoun, A. Nutritional Evaluation of Beetroots (Beta vulgaris L.) and Its Potential Application in a Functional Beverage. Plants 2020, 9, 1752. [Google Scholar] [CrossRef]
- Alexandra, K.; Anne, V.; Jean-Paul, V.; Harry, G.; Alexander, W.P. Effect of Plant Age on the Quantity and Quality of Proteins Extracted from Sugar Beet (Beta vulgaris L.) Leaves. J. Agric. Food Chem. 2016, 64, 8305–8314. [Google Scholar]
- USDA. Agricultural Research Service. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov (accessed on 9 February 2024).
- Waseem, M.; Akhtar, S.; Manzoor, M.F.; Mirani, A.A.; Ali, Z.; Ismail, T.; Ahmad, N.; Karrar, E. Nutritional characterization and food value addition properties of dehydrated spinach powder. Food Sci. Nutr. 2021, 9, 1213–1221. [Google Scholar] [CrossRef]
- Cui, R.; Fei, Y.; Zhu, F. Physicochemical, structural and nutritional properties of steamed bread fortified with red beetroot powder and their changes during breadmaking process. Food Chem. 2022, 383, 132547. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, J.R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.E.; Chung, M.-S.; Min, S.C. Cold plasma treatment for the microbiological safety of cabbage, lettuce, and dried figs. Food Microbiol. 2015, 51, 74–80. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO; UNU. Protein and Aminoacid Requirements in Human Nutrition; WHO, World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Cook, R.; Lupette, J.; Benning, C. The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses. Cells 2021, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ravishankar, G.A.; Keum, Y.S. Microalgae and Thraustochytrids are Sustainable Sources of Vegan EPA and DHA with Commercial Feasibility. Indian J. Microbiol. 2023, 63, 155–158. [Google Scholar] [CrossRef]
- Rahman, N.; Hashem, S.; Akther, S.; Jothi, J.S. Impact of various extraction methods on fatty acid profile, physicochemical properties, and nutritional quality index of Pangus fish oil. Food Sci. Nutr. 2023, 11, 4688–4699. [Google Scholar] [CrossRef]
- Hiremath, S.; Fergusson, D.; Knoll, G.; Ramsay, T.; Kong, J.; Ruzicka, M. Diet or additional supplement to increase potassium intake: Protocol for an adaptive clinical trial. Trials 2022, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- O’Gallagher, K.; Cardona, S.B.; Hill, C.; Al-Saedi, A.; Shahed, F.; Floyd, C.N.; McNeill, K.; Mills, C.E.; Webb, A.J. Grapefruit juice enhances the systolic blood pressure-lowering effects of dietary nitrate-containing beetroot juice. Br. J. Clin. Pharmacol. 2021, 87, 577–587. [Google Scholar] [CrossRef]
- Khare, A.; Samudre, S.; Arora, A. Sneak-peek into iron deficiency anemia in India: The need for food-based interventions and enhancing iron bioavailability. Food Res. Int. 2022, 162, 111927. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- EFSA. Dietary Reference Values for Nutrients Summary Report; EFSA Supporting Publications: Parma, Italy, 2017; p. e15121E. [Google Scholar]
- Dahdouh, S.; Grande, F.; Espinosa, S.N.; Vincent, A.; Gibson, R.; Bailey, K.; King, J.; Rittenschober, D.; Charrondière, U.R. Development of the FAO/INFOODS/IZINCG Global Food Composition Database for Phytate. J. Food Compos. Anal. 2019, 78, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- FAO; WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Hurrell, R.F. Phytic acid degradation as a means of improving iron absorption. Int. J. Vitam. Nutr. Res. 2004, 74, 445–452. [Google Scholar] [CrossRef]
- Marolt, G.; Gričar, E.; Pihlar, B.; Kolar, M. Complex Formation of Phytic Acid With Selected Monovalent and Divalent Metals. Front. Chem. 2020, 8, 582746. [Google Scholar] [CrossRef]
- Mitrevski, J.; Pantelić, N.Đ.; Dodevska, M.S.; Kojić, J.S.; Vulić, J.J.; Zlatanović, S.; Gorjanović, S.; Laličić-Petronijević, J.; Marjanović, S.; Antić, V.V. Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods 2023, 12, 322. [Google Scholar] [CrossRef] [PubMed]
- Guillon, F.; Champ, M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Sturm, B.; Hensel, O. 12 Pigments and Nutrients during Vegetable Drying Processes, Dried Products Storage, and Their Associated Color Changes. In Handbook of Drying of Vegetables and Vegetable Products; CRC Press: Boca Raton, FL, USA, 2017; Volume 257. [Google Scholar]
- Dygas, D.; Nowak, S.; Olszewska, J.; Szymańska, M.; Mroczyńska-Florczak, M.; Berłowska, J.; Dziugan, P.; Kręgiel, D. Ability of Yeast Metabolic Activity to Reduce Sugars and Stabilize Betalains in Red Beet Juice. Fermentation 2021, 7, 105. [Google Scholar] [CrossRef]
- Kayın, N.; Atalay, D.; Akçay, T.T.; Erge, H.S. Color stability and change in bioactive compounds of red beet juice concentrate stored at different temperatures. J. Food Sci. Technol. 2019, 56, 5097–5106. [Google Scholar] [CrossRef]
- Ben Haj Koubaier, H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (Beta vulgaris L. conditiva) Roots and Stems Extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef]
- de França, P.; RCruz-Tirado, J.P.; Barbin, D.F.; Kurozawa, L.E. Hot air drying of red beet: Process and product quality monitoring by digital images and near infrared spectroscopy. Dry. Technol. 2023, 41, 1085–1095. [Google Scholar] [CrossRef]
- Bunkar, D.S.; Anand, A.; Kumar, K.; Meena, M.; Goyal, S.K.; Paswan, V.K. Development of production technology for preparation of beetroot powder using different drying methods. Ann. Phytomed. 2020, 9, 293–301. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Yang, X.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-term effects of nitrate-rich green leafy vegetables on blood pressure and arterial stiffness in individuals with high-normal blood pressure. Free. Radic. Biol. Med. 2014, 77, 353–362. [Google Scholar] [CrossRef]
- Kojić, J.; Belović, M.; Krulj, J.; Pezo, L.; Teslić, N.; Kojić, P.; Tukuljac, L.P.; Šeregelj, V.; Ilić, N. Textural, Color and Sensory Features of Spelt Wholegrain Snack Enriched with Betaine. Foods 2022, 11, 475. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]


| Formulation | Cornmeal (%) | Beetroot Stem Flour (%) | Beetroot Leaf Flour (%) |
|---|---|---|---|
| Snack 1 | 100 | - | - |
| Snack 2 | 90 | 5 | 5 |
| Snack 3 | 80 | 10 | 10 |
| Component (g/100 g) d.m | Leaves | Stems | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 50 °C | 60 °C | 70 °C | |||||||||||||
| Water Activity | 0.5036 | ± | 0.0013 c | 0.4278 | ± | 0.0008 b | 0.2011 | ± | 0.0148 a | 0.4957 | ± | 0.0021 c | 0.2851 | ± | 0.0011 b | 0.1963 | ± | 0.0066 a |
| Protein | 30.39 | ± | 0.23 c | 30.54 | ± | 0.98 c | 30.24 | ± | 0.86 c | 15.24 b | ± | 0.39 | 14.20 | ± | 0.01 a | 14.44 | ± | 0.51 a |
| Lipids | 4.0 | ± | 0.02 c | 4.1 | ± | 0.03 b | 3.5 | ± | 0.02 a | 0.46 | ± | 0.01 b | 0.53 | ± | 0.02 a | 0.44 | ± | 0.02 b |
| Phytic Acid | 0.146 | ± | 0.001 c | 0.162 | ± | 0.003 b | 0.178 | ± | 0.004 a | 0.133 | ± | 0.006 d | 0.118 | ± | 0.001 e | 0.077 | ± | 0.001 f |
| Total Dietary Fiber | 30.74 | ± | 1.68 a | 27.09 | ± | 6.73 ab | 30.42 | ± | 1.07 ab | 32.88 | ± | 2.24 b | 33.51 | ± | 0.424 b | 32.71 | ± | 2.04 b |
| Ash | 23.72 a | ± | 0.12 | 24.15 a | ± | 0.02 | 24.21 ab | ± | 0.04 | 24.88 c | ± | 0.01 | 25.43 bc | ± | 0.18 | 25.47 bc | ± | 0.07 |
| E.N.N | 12.45 | 15.36 | 12.75 | 25.53 | 25.36 | 26.10 | ||||||||||||
| Leaves (50 °C) | Leaves (60 °C) | Leaves (70 °C) | Stems (50 °C) | Stems (60 °C) | Stems (70 °C) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential aminoacids | ||||||||||||||||||
| Histidine | 0.216 | ± | 0.009 b | 0.125 | ± | 0.008 a | 0.203 | ± | 0.002 b | 0.083 | ± | 0.005 a | 0.082 | ± | 0.002 a | 0.099 | ± | 0.002 a |
| Threonine | 0.466 | ± | 0.015 b | 0.464 | ± | 0.008 b | 0.418 | ± | 0.000 a | 0.144 | ± | 0.003 b | 0.138 | ± | 0.005 ab | 0.123 | ± | 0.002 a |
| Valine | 0.065 | ± | 0.002 a | 0.051 | ± | 0.006 a | 0.047 | ± | 0.001 a | 0.000 | ± | 0.000 | 0.000 | ± | 0.000 | 0.000 | ± | 0.000 |
| Methionine | 0.009 | ± | 0.003 a | 0.022 | ± | 0.001 c | 0.018 | ± | 0.001 b | 0.003 | ± | 0.000 b | 0.002 | ± | 0.000 a | 0.002 | ± | 0.000 a |
| Cysteine | 0.049 | ± | 0.000 a | 0.060 | ± | 0.004 b | 0.039 | ± | 0.000 c | 0.039 | ± | 0.003 a | 0.032 | ± | 0.003 a | 0.065 | ± | 0.001 b |
| Isoleucine | 0.475 | ± | 0.002 c | 0.410 | ± | 0.001 b | 0.365 | ± | 0.000 a | 0.173 | ± | 0.001 b | 0.161 | ± | 0.002 b | 0.103 | ± | 0.005 a |
| Leucine | 0.851 | ± | 0.005 c | 0.670 | ± | 0.004 b | 0.608 | ± | 0.000 a | 0.287 | ± | 0.002 b | 0.266 | ± | 0.003 b | 0.151 | ± | 0.007 a |
| Phenylalanine | 0.524 | ± | 0.008 c | 0.486 | ± | 0.003 b | 0.436 | ± | 0.004 a | 0.168 | ± | 0.007 b | 0.157 | ± | 0.001 b | 0.102 | ± | 0.002 a |
| Tyrosyne | 0.821 | ± | 0.002 c | 0.713 | ± | 0.006 b | 0.608 | ± | 0.006 a | 0.286 | ± | 0.001 b | 0.269 | ± | 0.001 b | 0.169 | ± | 0.011 a |
| Lysine | 0.631 | ± | 0.003 c | 0.565 | ± | 0.006 b | 0.521 | ± | 0.004 a | 0.219 | ± | 0.009 b | 0.202 | ± | 0.001 b | 0.134 | ± | 0.003 a |
| EAAs | 4.106 | 3.565 | 3.263 | 1.402 | 1.308 | 0.947 | ||||||||||||
| Non-essential aminoacids | ||||||||||||||||||
| Alanine | 0.744 | ± | 0.002 c | 0.707 | ± | 0.001 b | 0.633 | ± | 0.006 a | 0.299 | ± | 0.010 ab | 0.336 | ± | 0.013 b | 0.259 | ± | 0.010 a |
| Arginine | 0.489 | ± | 0.001 a | 0.470 | ± | 0.014 a | 0.439 | ± | 0.002 a | 0.013 | ± | 0.000 a | 0.018 | ± | 0.000 b | 0.013 | ± | 0.001 a |
| Aspartic acid | 1.228 | ± | 0.028 c | 1.003 | ± | 0.020 b | 0.878 | ± | 0.005 a | 0.405 | ± | 0.002 c | 0.388 | ± | 0.002 b | 0.271 | ± | 0.002 a |
| Glutamic acid | 1.668 | ± | 0.020 c | 1.371 | ± | 0.020 b | 1.188 | ± | 0.004 a | 0.692 | ± | 0.025 b | 0.550 | ± | 0.013 a | 0.470 | ± | 0.019 a |
| Glycine | 0.700 | ± | 0.006 b | 0.744 | ± | 0.013 b | 0.673 | ± | 0.005 a | 0.297 | ± | 0.004 b | 0.303 | ± | 0.004 b | 0.278 | ± | 0.001 a |
| Hydroxiproline | 0.071 | ± | 0.002 b | 0.065 | ± | 0.001 a | 0.069 | ± | 0.001 ab | 0.181 | ± | 0.001 b | 0.161 | ± | 0.001 a | 0.154 | ± | 0.004 a |
| Serine | 0.511 | ± | 0.028 b | 0.508 | ± | 0.010 b | 0.464 | ± | 0.000 a | 0.228 | ± | 0.003 c | 0.205 | ± | 0.003 b | 0.186 | ± | 0.003 a |
| Parameter | Lipid Number | Units | Leaves 50 °C | Leaves 60 °C | Leaves 70 °C | Stems 50 °C | Stems 60 °C | Stems 70 °C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipids | g/100 g | 4 | 4.1 | 3.5 | 0.46 | 0.53 | 0.44 | |||||||||||||
| Total saturated fatty acids | g/100 g | 0.8 a | 0.93 c | 0.88 b | 0.10 a | 0.10 a | 0.10 a | |||||||||||||
| Total Polyunsaturated fatty acids | g/100 g | 2.9 c | 2.84 b | 2.37 a | 0.32 b | 0.39 c | 0.30 a | |||||||||||||
| Total monounsaturated fatty acids | g/100 g | 0.30 b | 0.33 c | 0.25 a | 0.04 a | 0.04 a | 0.04 a | |||||||||||||
| Fatty acid | ||||||||||||||||||||
| Myristic | C14:0 | g/100 g | 0.04 | ± | 0.00 a | 0.03 | ± | 0.00 b | 0.03 | ± | 0.00 b | Traces | Traces | Traces | ||||||
| Palmitic | C16:0 | g/100 g | 0.58 | ± | 0.01 a | 0.69 | ± | 0.00 c | 0.66 | ± | 0.00 b | 0.08 | ± | 0.00 a | 0.08 | ± | 0.00 a | 0.08 | ± | 0.00 a |
| Margarico | C17:0 | g/100 g | 0.01 | ± | 0.00 a | 0.01 | ± | 0.00 a | 0.01 | ± | 0.00 a | Traces | Traces | Traces | ||||||
| Stearic | C18:0 | g/100 g | 0.09 | ± | 0.01 a | 0.11 | ± | 0.01 c | 0.10 | ± | 0.01 b | 0.01 | ± | 0.00 a | Traces | 0.01 | ± | 0.00 a | ||
| Arachidic | C20:0 | g/100 g | 0.02 | ± | 0.00 a | 0.03 | ± | 0.00 b | 0.02 | ± | 0.00 a | Traces | Traces | Traces | ||||||
| Behenic | C22:0 | g/100 g | 0.03 | ± | 0.00 b | 0.03 | ± | 0.00 b | 0.01 | ± | 0.00 a | Traces | Traces | Traces | ||||||
| Lignocerico | C24:0 | g/100 g | 0.03 | ± | 0.00 a | 0.03 | ± | 0.00 a | 0.03 | ± | 0.00 a | 0.01 | ± | 0.00 a | 0.01 | ± | 0.00 a | Traces | ||
| Other Unidentified | - | g/100 g | 0.35 | ± | 0.01 b | 0.49 | ± | 0.03 c | 0.25 | ± | 0.03 a | 0.02 | ± | 0.00 a | 0.03 | ± | 0.00 b | 0.02 | ± | 0.00 a |
| Ʃ SFA | 0.8 a | 0.93 c | 0.88 b | 0.10 a | 0.10 a | 0.10 a | ||||||||||||||
| Palmitoleic acid | C16:1n7 | g/100 g | Traces | 0.01 | ± | 0.00 a | 0.01 | ± | 0.00 a | Traces | Traces | Traces | ||||||||
| Oleic | C18:1n9 | g/100 g | 0.29 | ± | 0.00 b | 0.31 | ± | 0.00 c | 0.24 | ± | 0.00 a | 0.04 | ± | 0.00 b | 0.04 | ± | 0.00 b | 0.03 | ± | 0.00 a |
| Eicosanoic acid | C20:1n9 | g/100 g | 0.01 | ± | 0.00 a | 0.01 | ± | 0.00 a | 0.01 | ± | 0.00 a | Traces | Traces | Traces | ||||||
| Ʃ MUFA | 2.9 c | 2.84 b | 2.37 a | 0.32 b | 0.39 c | 0.30 a | ||||||||||||||
| Linoleic acid (LA) | C18:2n6c | g/100 g | 0.54 | ± | 0.00 b | 0.57 | ± | 0.00 c | 0.51 | ± | 0.00 a | 0.19 | ± | 0.00 a | 0.24 | ± | 0.00 b | 0.19 | ± | 0.00 a |
| α-Linolenic acid (ALA) | C18:3n3 | g/100 g | 2.36 | ± | 0.03 c | 2.26 | ± | 0.03 b | 1.86 | ± | 0.03 a | 0.13 | ± | 0.00 b | 0.15 | ± | 0.00 c | 0.10 | ± | 0.00 a |
| Ʃ PUFA | 0.30 b | 0.33 c | 0.25 a | 0.04 a | 0.04 a | 0.04 a | ||||||||||||||
| Total omega 3 | g/100 g | 2.36 c | 2.26 b | 1.86 a | 0.13 b | 0.15 c | 0.10 a | |||||||||||||
| Total omega 6 | g/100 g | 0.54 b | 0.57 c | 0.51 a | 0.19 a | 0.24 b | 0.19 a | |||||||||||||
| LA/ALA | C18:2n6c/C18:3n3 | g/g | 0.22 | 0.25 | 0.27 | 1.46 | 1.6 | 1.9 | ||||||||||||
| Sample | WAdC * (g Water/g Sample) | WAbC * (g Water/g Sample) | WHC * (g Water/g Sample) | OHC * (g Oil/g Sample) | Solubility (%) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 60 °C | 70 °C | 80 °C | ||||||||||||||||||||||
| Leaves | 50 °C | 0.631 | ± | 0.036 a | 5.93 | ± | 0.75 bc | 5.17 | ± | 0.10 a | 2.29 | ± | 0.06 c | 41.63 | ± | 1.19 b | 33.10 | ± | 1.62 c | 39.53 | ± | 0.74 a | 40.70 | ± | 2.62 ab |
| 60 °C | 0.631 | ± | 0.002 a | 5.40 | ± | 0.17 ab | 5.17 | ± | 0.12 a | 2.17 | ± | 0.09 ab | 40.69 | ± | 1.44 ab | 36.00 | ± | 1.26 c | 39.82 | ± | 0.37 a | 38.88 | ± | 1.88 a | |
| 70 °C | 0.618 | ± | 0.006 a | 5.22 | ± | 0.73 a | 5.09 | ± | 0.19 a | 2.19 | ± | 0.03 bc | 37.75 | ± | 1.61 a | 36.42 | ± | 1.34 c | 38.31 | ± | 1.28 a | 42.56 | ± | 1.39 bc | |
| Stems | 50 °C | 1.28 | ± | 0.02 b | 7.27 | ± | 0.25 d | 8.15 | ± | 0.31 b | 2.55 | ± | 0.11 d | 45.14 | ± | 1.74 c | 47.84 | ± | 1.58 a | 48.98 | ± | 1.07 b | 44.78 | ± | 1.72 cd |
| 60 °C | 1.32 | ± | 0.01 b | 6.36 | ± | 0.23 c | 7.66 | ± | 0.30 c | 2.21 | ± | 0.01 bc | 47.45 | ± | 1.85 c | 48.19 | ± | 0.46 b | 48.90 | ± | 1.79 b | 48.35 | ± | 1.78 e | |
| 70 °C | 1.370 | ± | 0.04 c | 6.06 | ± | 0.14 c | 6.01 | ± | 0.20 d | 2.09 | ± | 0.04 a | 46.88 | ± | 2.31 c | 48.96 | ± | 0.87 b | 46.31 | ± | 0.88 b | 46.15 | ± | 0.86 de | |
| CieLab | Leaves | Stems | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 50 °C | 60 °C | 70 °C | |||||||||||||
| L* | 54.68 | ± | 0.31 c | 52.13 | ± | 0.50 b | 50.17 | ± | 1.47 a | 49.45 | ± | 0.31 a | 50.19 | ± | 0.60 a | 52.11 | ± | 0.33 b |
| a* | −8.79 | ± | 0.14 a | −8.53 | ± | 0.38 ab | −8.14 | ± | 0.27 b | 15.52 | ± | 0.47 e | 12.61 | ± | 0.20 d | 6.72 | ± | 0.45 c |
| b* | 15.57 | ± | 0.08 d | 15.33 | ± | 0.72 d | 15.53 | ± | 1.19 d | 0.86 | ± | 0.08 a | 3.22 | ± | 0.18 b | 6.37 | ± | 0.18 c |
| Parameters | Leaves | Stems | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 50 °C | 60 °C | 70 °C | |||||||||||||
| TPC (1) (mg GAE/100 g) | 558.9 | ± | 5.3 b | 512.3 | ± | 27.4 a | 478.2 | ± | 12.9 a | 365.3 | ± | 25.1 a | 362.2 | ± | 69.8 a | 294.7 | ± | 6.2 a |
| DPPH (2) (µmol TE/100 g) | 2057.24 | ± | 1023.8 a | 3860.53 | ± | 480.5 b | 3811.92 | ± | 198.7 b | 2561.96 | ± | 529.5 a | 3949.78 | ± | 552.2 a | 2839.92 | ± | 1108.7 a |
| Nitrate (mg/kg) | 159 | ± | 0 c | 126 | ± | 0 b | 100 | ± | 0 a | 359 | ± | 0 c | 314 | ± | 0 b | 126.5 | ± | 0.5 a |
| Parameters | Control | 10% | 20% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Color | 4.5 | ± | 0.7 c | 3.8 | ± | 1.1 b | 3 | ± | 0.9 a |
| Flavor | 3.8 | ± | 0.9 ab | 4 | ± | 1.0 c | 3.3 | ± | 1.0 a |
| Texture | 3.6 | ± | 0.8 a | 3.6 | ± | 1.2 a | 3.3 | ± | 1.0 a |
| General acceptability | 3.8 | ± | 0.8 b | 3.8 | ± | 1.1 b | 3.1 | ± | 1.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mella, C.; Rojas, N.; Calderon-Bravo, H.; Muñoz, L.A. Evaluating Biocompounds in Discarded Beetroot (Beta vulgaris) Leaves and Stems for Sustainable Food Processing Solutions. Foods 2024, 13, 2603. https://doi.org/10.3390/foods13162603
Mella C, Rojas N, Calderon-Bravo H, Muñoz LA. Evaluating Biocompounds in Discarded Beetroot (Beta vulgaris) Leaves and Stems for Sustainable Food Processing Solutions. Foods. 2024; 13(16):2603. https://doi.org/10.3390/foods13162603
Chicago/Turabian StyleMella, Carolina, Natalia Rojas, Hector Calderon-Bravo, and Loreto A. Muñoz. 2024. "Evaluating Biocompounds in Discarded Beetroot (Beta vulgaris) Leaves and Stems for Sustainable Food Processing Solutions" Foods 13, no. 16: 2603. https://doi.org/10.3390/foods13162603
APA StyleMella, C., Rojas, N., Calderon-Bravo, H., & Muñoz, L. A. (2024). Evaluating Biocompounds in Discarded Beetroot (Beta vulgaris) Leaves and Stems for Sustainable Food Processing Solutions. Foods, 13(16), 2603. https://doi.org/10.3390/foods13162603






