Influence of the Crystal Forms of Calcium Carbonate on the Preparation and Characteristics of Indigo Carmine-Calcium Carbonate Lake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Calcium Carbonate with Different Crystalline Forms
- Calcite: A mixture of 150 mL of 0.2 mol/L CaCl2 and 150 mL of 0.2 mol/L Na2CO3 was stirred at 600 r/min at ambient temperature for 30 min. The resulting solution was centrifuged (5000 r/min for 5 min) to obtain sediment. The sediment was then dried in an oven at 45 °C for 12 h and ground to obtain IC—calcite lake.
- Aragonite: 150 mL of 0.2 mol/L CaCl2 and 150 mL of 0.2 mol/L Na2CO3 were preheated to 80 °C in a water bath, respectively. After rapid mixing, the solution was centrifuged (5000 r/min for 5 min) to obtain sediment. The sediment was dried in an oven at 45 °C for 12 h and ground to obtain IC—aragonite lake.
- Vaterite: A mixture of 150 mL of 0.1 mol/L CaCl2 (60% DI water + 40% ethanol) and 150 mL of 0.1 mol/L Na2CO3 solution (60% DI water + 40% ethanol) was stirred at 600 r/min at ambient temperature for 30 min. After centrifuging the solution at 5000 r/min for 5 min to obtain sediment, the sediment was dried in an oven at 45 °C for 12 h and ground to obtain IC—vaterite lake.
- ACC: A rapid mixture of 20 mL of 0.1 mol/L CaCl2 solution, 20 mL of 0.1 mol/L Na2CO3 solution, and 40 mL of ethanol was introduced into the filter cup of a vacuum membrane filtration set. After agitating the filter cup until many particles appeared in the reaction solution, a 250 mL of ethanol was rapidly added and vacuum-filtered. The resulting solid residue was dried in a vacuum oven at 45 °C for 12 h, and ground to obtain IC—ACC lake.
2.3. Preparation of Calcium Carbonate Colorant Lakes
2.4. Characterization of CaCO3 and Its Colorant Lakes
2.5. Kinetic Analysis of Adsorption
2.6. Isothermal Analysis of Adsorption
2.7. FTIR Analysis
2.8. Raman Spectra Analysis
2.9. TGA-DSC Analysis
2.10. Statistical Analysis
3. Results and Discussion
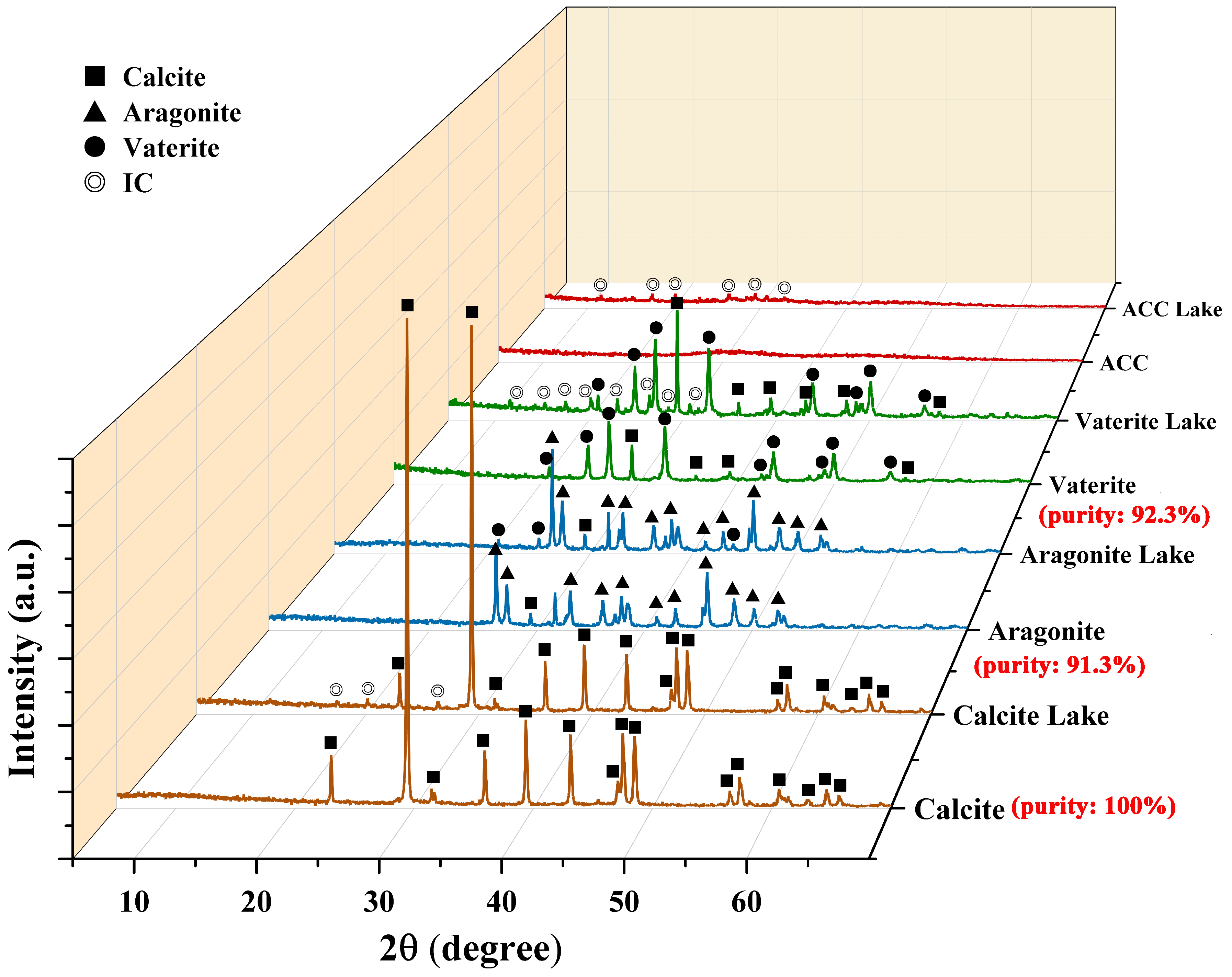
3.1. Characterization of CaCO3 and Colorant Lakes
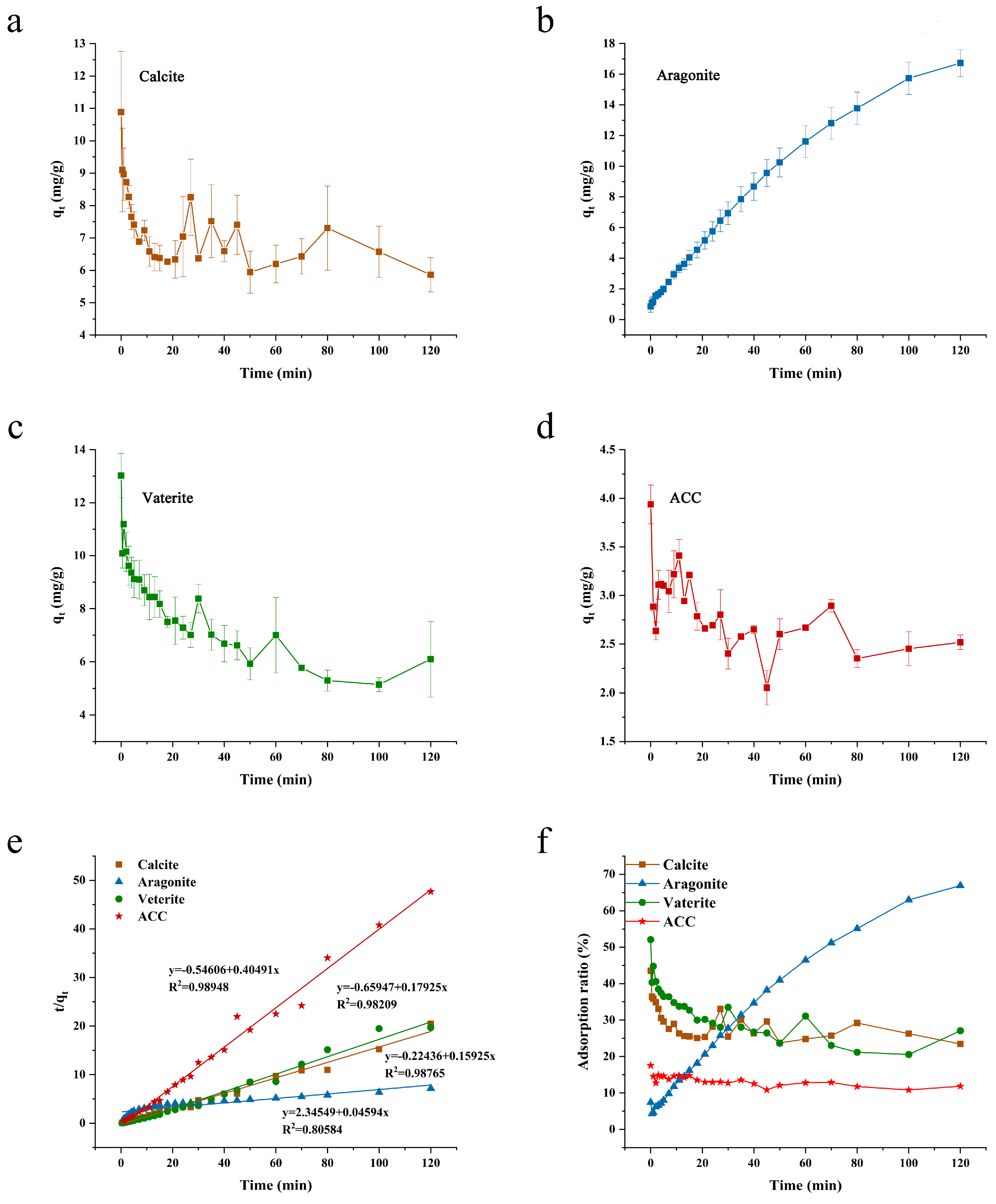
3.2. Kinetic Adsorption Analysis
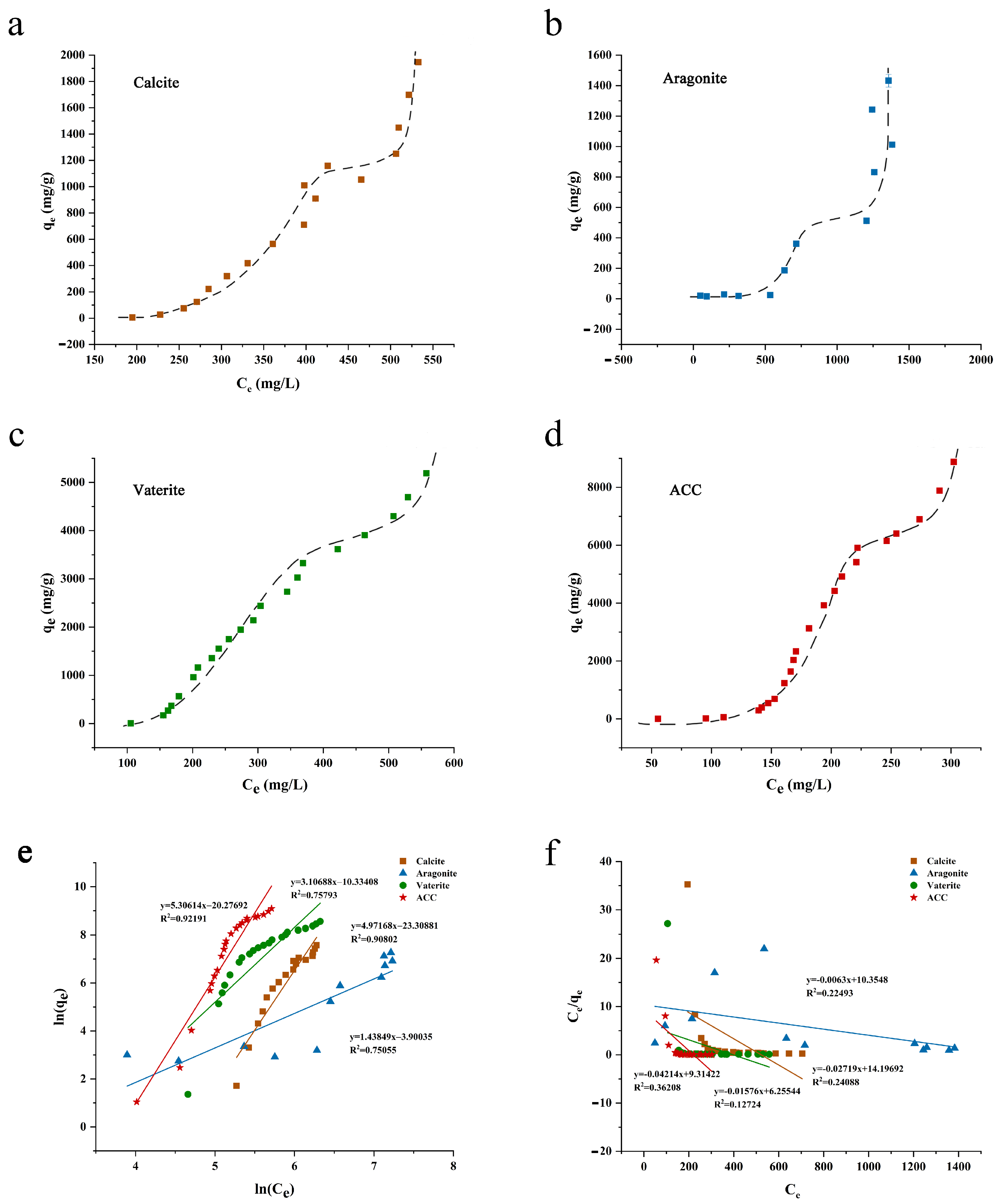
3.3. Isothermal Adsorption Analysis
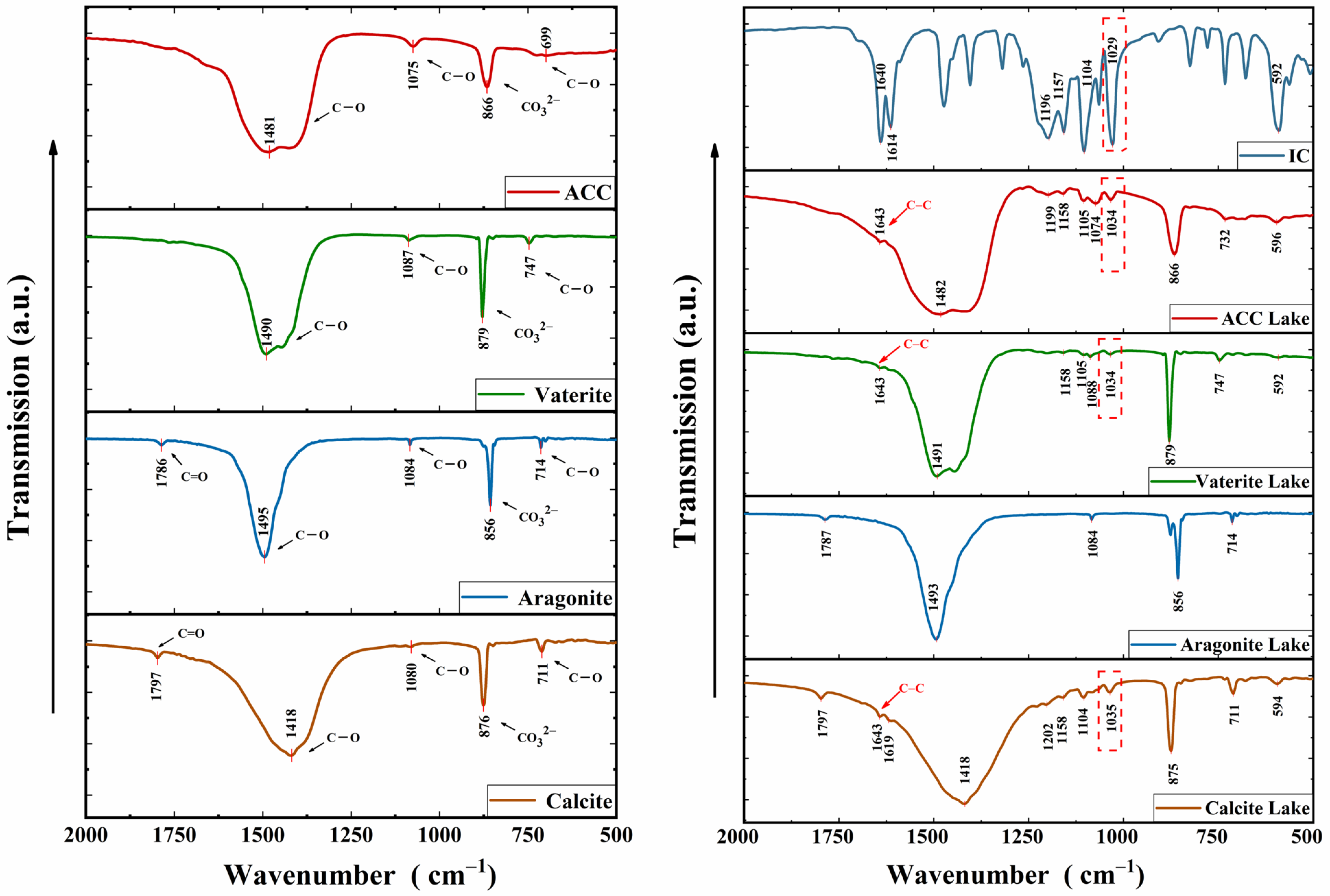
3.4. FTIR Analysis
3.5. Raman Analysis
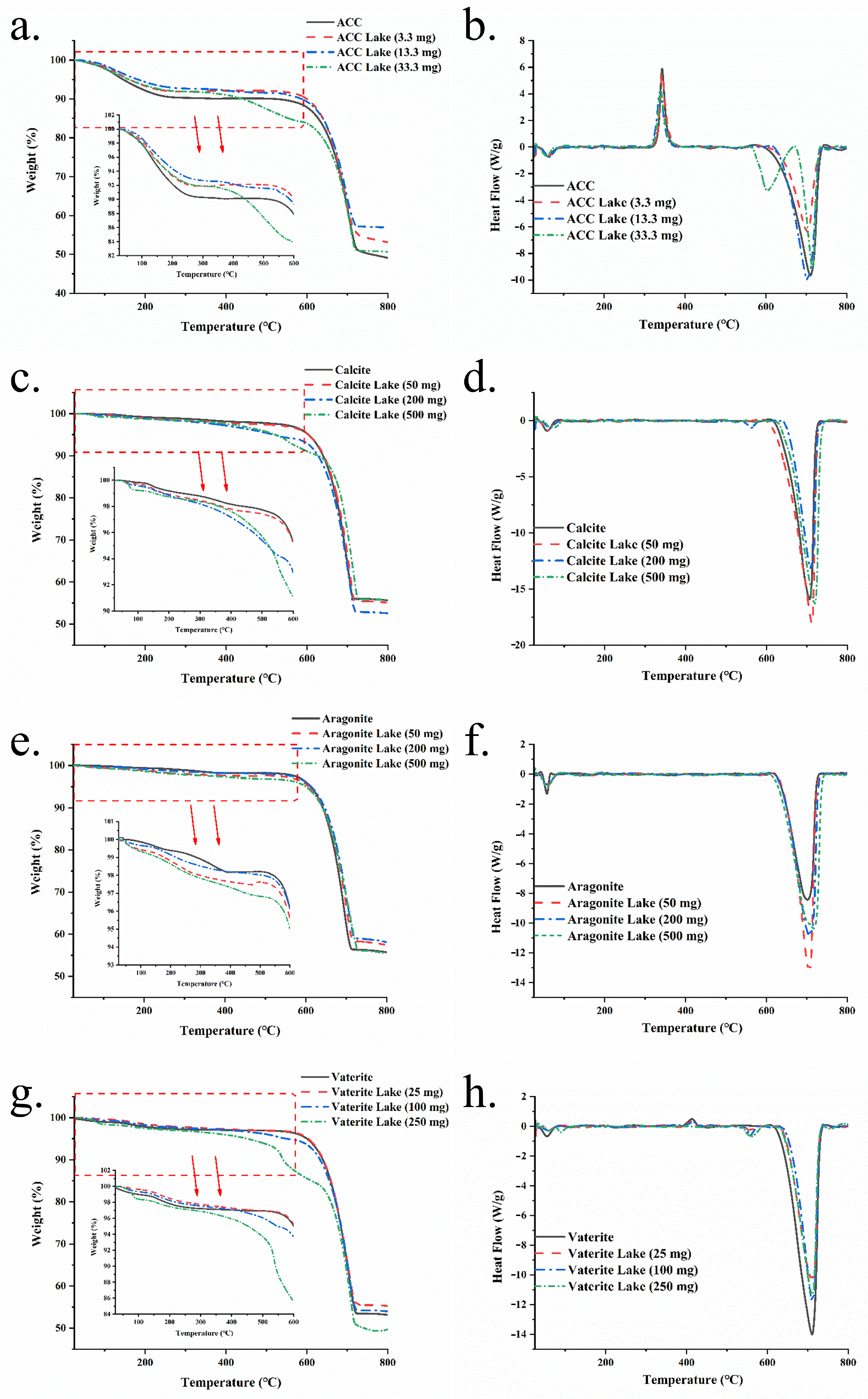
3.6. TGA-DSC Analysis
3.7. EDS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, K. A review of recent progress in the synthesis of bio-indigoids and their biologically assisted end-use applications. Dye. Pigment. 2020, 181, 108570. [Google Scholar] [CrossRef]
- Ristea, M.; Zarnescu, O. Indigo carmine: Between necessity and concern. J. Xenobiotics 2023, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Brauch, J.E.; Zapata-Porras, S.P.; Buchweitz, M.; Aschoff, J.K.; Carle, R. Jagua blue derived from Genipa americana L. fruit: A natural alternative to commonly used blue food colorants? Food Res. Int. 2016, 89, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Zhao, H.; Li, B.; Hamel, J.; Yang, Y.; Cao, D.; Natan, A.; Zhu, H. Abundant organic dye as an anolyte for aqueous flow battery with multielectron transfer. ACS Appl. Energy Mater. 2019, 2, 7425–7437. [Google Scholar] [CrossRef]
- Zosenko, O.O.; Kushch, O.V.; Kompanets, M.O.; Anishchenko, V.M.; Shendrik, A.N. Aliphatic oxime 3-(hydroxyimino) pentan-2,4-dione as new potential mediator for laccase-catalyzed decolorization of dyes. J. Environ. Chem. Eng. 2022, 10, 108149. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, C.; Liu, Y.; Lei, S.; Wen, Y. Mechanism study of the formation of indigo carmine-aluminum hydroxide lake for food use. J. Food Sci. 2019, 84, 3129–3139. [Google Scholar] [CrossRef]
- Semwal, A.D.; Padmashree, A.; Khan, M.A.; Sharma, G.K.; Bawa, A.S. Leaching of aluminium from utensils during cooking of food. J. Sci. Food Agric. 2006, 86, 2425–2430. [Google Scholar] [CrossRef]
- Soni, M.G.; White, S.M.; Flamm, W.G.; Burdock, G.A. Safety evaluation of dietary aluminum. Regul. Toxicol. Pharmacol. 2001, 33, 66–79. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, C.; Liu, Y.; Cheng, L.; Hao, S.; Sun, B. A Kind of Preparation Method of the Edible Color Lake Based on Calcium Carbonate. CN202111208947.8 30 June 2023. [Google Scholar]
- Kim, M.; Lee, J.; Jo, M.; Kim, M.; Kim, H.; Oh, J.; Song, N.W.; Choi, S. Cytotoxicity, uptake behaviors, and oral absorption of food grade calcium carbonate nanomaterials. Nanomaterials 2015, 5, 1938–1954. [Google Scholar] [CrossRef] [PubMed]
- Konar, S.K.; Gu, R.; Sain, M. Preparation and characterization of baked nitrile latex foam reinforced with biomasses. Ind. Crops Prod. 2013, 42, 261–267. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Yuan, D.; Bai, G.; Liu, Y.; Jing, L.; Wang, C.; Liu, G. A novel edible colorant lake prepared with CaCO3 and Monascus pigments: Lake characterization and mechanism study. Food Chem. 2023, 410, 135408. [Google Scholar] [CrossRef] [PubMed]
- Nebel, H.; Epple, M. Continuous preparation of calcite, aragonite and vaterite, and of magnesium-substituted amorphous calcium carbonate (Mg-ACC). Z. Anorg. Allg. Chem. 2008, 634, 1439–1443. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-controlled synthesis of vaterite calcium carbonate by the mixing method: Aiming for nanosized particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Zou, Z.; Bertinetti, L.; Politi, Y.; Jensen, A.C.S.; Weiner, S.; Addadi, L.; Fratzl, P.; Habraken, W.J.E.M. Opposite particle size effect on amorphous calcium carbonate crystallization in water and during heating in air. Chem. Mater. 2015, 27, 4237–4246. [Google Scholar] [CrossRef]
- Yuan, D.; Lei, S.; Gao, L.; Liu, Y.; Liu, G.; Wang, C. Mechanism study on the formation of complex between Ca(II) and indigo carmine for potential food use as a colorant lake. Food Chem. 2020, 322, 126709. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Ihli, J.; Kulak, A.N.; Meldrum, F.C. Freeze-drying yields stable and pure amorphous calcium carbonate (ACC). Chem. Commun. 2013, 49, 3134. [Google Scholar] [CrossRef]
- Rao, C.; Guo, X.; Li, M.; Sun, X.; Lian, X.; Wang, H.; Gao, X.; Niu, B.; Li, W. In vitro preparation and characterization of amorphous calcium carbonate nanoparticles for applications in curcumin delivery. J. Mater. Sci. 2019, 54, 11243–11253. [Google Scholar] [CrossRef]
- Moulin, P.; Roques, H. Zeta potential measurement of calcium carbonate. J. Colloid Interface Sci. 2003, 261, 115–126. [Google Scholar] [CrossRef]
- Tobler, D.J.; Rodriguez Blanco, J.D.; Dideriksen, K.; Bovet, N.; Sand, K.K.; Stipp, S.L.S. Citrate effects on amorphous calcium carbonate (ACC) structure, stability, and crystallization. Adv. Funct. Mater. 2015, 25, 3081–3090. [Google Scholar] [CrossRef]
- Cai, G.; Zhao, G.; Wang, X.; Yu, S. Synthesis of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water. J. Phys. Chem. C 2010, 114, 12948–12954. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.; Chen, F. Microwave hydrothermal transformation of amorphous calcium carbonate nanospheres and application in protein adsorption. ACS Appl. Mater. Interfaces 2014, 6, 4310–4320. [Google Scholar] [CrossRef]
- Xu, A.W.; Ma, Y.R.; Colfen, H. Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415–449. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Ke, S.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Effect of different biomass species and pyrolysis temperatures on heavy metal adsorption, stability and economy of biochar. Ind. Crops Prod. 2022, 186, 115238. [Google Scholar] [CrossRef]
- Moosavi, A.; Amooey, A.A.; Mir, A.A.; Marzbali, M.H. Extraordinary adsorption of acidic fuchsine and malachite green onto cheap nano-adsorbent derived from eggshell. Chin. J. Chem. Eng. 2020, 28, 1591–1602. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, F.; Huang, Y.; Yang, Q.; Tian, C.; Zhou, W. Preparation of peanut shell-like calcium carbonate from biowaste chicken eggshell and its application for aqueous Victoria Blue B removal. Microporous Mesoporous Mater. 2022, 329, 111549. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, P.; Bajnóczi, É.G.; Neagu, A.; Tai, C.; Persson, I.; Strømme, M.; Cheung, O. Amorphous calcium carbonate constructed from nanoparticle aggregates with unprecedented surface area and mesoporosity. ACS Appl. Mater. Interfaces 2018, 10, 21556–21564. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S. Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. J. Environ. Chem. Eng. 2014, 2, 2156–2161. [Google Scholar] [CrossRef]
- Rajoriya, S.; Saharan, V.K.; Pundir, A.S.; Nigam, M.; Roy, K. Adsorption of methyl red dye from aqueous solution onto eggshell waste material: Kinetics, isotherms and thermodynamic studies. Curr. Res. Green Sustain. Chem. 2021, 4, 100180. [Google Scholar] [CrossRef]
- Wehrmeister, U.; Soldati, A.L.; Jacob, D.E.; Haeger, T.; Hofmeister, W. Raman spectroscopy of synthetic, geological and biological vaterite: A Raman spectroscopic study. J. Raman Spectrosc. 2010, 41, 193–201. [Google Scholar] [CrossRef]
- Wehrmeister, U.; Jacob, D.E.; Soldati, A.L.; Loges, N.; Häger, T.; Hofmeister, W. Amorphous, nanocrystalline and crystalline calcium carbonates in biological materials. J. Raman Spectrosc. 2011, 42, 926–935. [Google Scholar] [CrossRef]
- Basu, S.; Das, A. Surface-enhanced Raman spectroscopy: An analytical tool for single cell analysis. J. Raman Spectrosc. 2017, 48, 6–24. [Google Scholar] [CrossRef]
- Dazzi, F.; Dazzi, C.; Ferroni, L. Infrared and Raman spectroscopy: A comparative study. J. Mol. Struct. 2016, 1105, 252–259. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Lu, Y. Raman spectroscopy for the analysis of biological samples. J. Raman Spectrosc. 2017, 48, 25–39. [Google Scholar] [CrossRef]
- Schmidt, M.P.; Ilott, A.J.; Phillips, B.L.; Reeder, R.J. Structural changes upon dehydration of amorphous calcium carbonate. Cryst. Growth Des. 2014, 14, 938–951. [Google Scholar] [CrossRef]
- Tobler, D.J.; Rodriguez Blanco, J.D.; Sørensen, H.O.; Stipp, S.L.S.; Dideriksen, K. Effect of pH on amorphous calcium carbonate structure and transformation. Cryst. Growth Des. 2016, 16, 4500–4508. [Google Scholar] [CrossRef]
- Du, Y.; Chang, H.; Yang, S.; Huang, S.; Tsai, Y.; Huang, J.J.; Chan, J.C.C. Study of binding interaction between pif80 protein fragment and aragonite. Sci. Rep. 2016, 6, 30883. [Google Scholar] [CrossRef]
- Siva, T.; Muralidharan, S.; Sathiyanarayanan, S.; Manikandan, E.; Jayachandran, M. Enhanced polymer induced precipitation of polymorphous in calcium carbonate: Calcite aragonite vaterite phases. J. Inorg. Organomet. Polym. Mater. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Maruyama, S.A.; Tavares, S.R.; Leitao, A.A.; Wypych, F. Intercalation of indigo carmine anions into zinc hydroxide salt: A novel alternative blue pigment. Dye. Pigment. 2016, 128, 158–164. [Google Scholar] [CrossRef]
- Baitalow, F.; Wolf, G.; Schmidt, H.G. Thermochemical investigations of calcium carbonate phase transitions I. thermal activated vaterite—Calcite transition. J. Therm. Anal. Calorim. 1998, 52, 5–16. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Tong, X.; Kang, X. Crystal transformation and self-assembly theory of microbially induced calcium carbonate precipitation. Appl. Microbiol. Biotechnol. 2022, 106, 3555–3569. [Google Scholar] [CrossRef]
- El-Mansy, M.A.M. Quantum chemical studies on structural, vibrational, nonlinear optical properties and chemical reactivity of indigo carmine dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Zhao, G.; Zhou, X.; Gao, J.; Zhang, Q. Preparation of pH-responsive silver nanoparticles by RAFT polymerization. J. Mater. Sci. 2008, 43, 4625–4630. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Ye, R.; Wang, W.; Li, X. Transglutaminase-induced crosslinking of gelatin-calcium carbonate composite films. Food Chem. 2015, 166, 414–422. [Google Scholar] [CrossRef] [PubMed]

| Samples | Zeta Potential (mV) | Average Diameter (μm) | Specific Surface Area (m2/g) | Δb* |
|---|---|---|---|---|
| Calcite | −21.57 ± 0.76 h | 6.116 ± 0.27 c | 3.985 ± 0.035 j | - |
| Calcite Lake (50 mg) | −21.33 ± 1.04 h | 5.794 ± 0.10 d | 3.829 ± 0.159 j | 0.01 ± 0.095 c |
| Calcite Lake (200 mg) | −15.27 ± 0.87 f | 6.459 ± 0.01 b | 2.905 ± 0.145 k | 0.07 ± 0.026 b |
| Calcite Lake (500 mg) | −10.16 ± 0.73 e | 4.549 ± 0.13 f | 3.831 ± 0.081 j | 0.07 ± 0.045 b |
| Aragonite | −5.867 ± 0.67 c | 7.564 ± 0.06 a | 3.841 ± 0.021 j | - |
| Aragonite Lake (50 mg) | 2.25 ± 0.30 a | 4.486 ± 2.31 f | 5.079 ± 0.119 h | 0.04 ± 0.005 c |
| Aragonite Lake (200 mg) | 3.53 ± 0.60 a | 4.080 ± 3.93 g | 4.449 ± 0.029 i | −0.03 ± 0.015 c |
| Aragonite Lake (500 mg) | −0.621 ± 0.91 b | 4.831 ± 0.05 e | 5.086 ± 0.074 h | −0.10 ± 0.030 c |
| Vaterite | −8.79 ± 0.54 d | 3.188 ± 0.10 h | 7.432 ± 0.082 g | - |
| Vaterite Lake (25 mg) | −10.05 ± 0.46 e | 6.655 ± 0.03 b | 8.036 ± 0.024 f | 0.08 ± 0.003 b |
| Vaterite Lake (100 mg) | −9.51 ± 0.25 e | 4.272 ± 0.02 f | 8.182 ± 0.032 f | −0.08 ± 0.015 d |
| Vaterite Lake (250 mg) | −19.07 ± 0.78 g | 2.324 ± 0.01 j | 12.042 ± 0.052 e | −0.14 ± 0.010 e |
| ACC | −1.00 ± 0.72 b | 0.368 ± 0.02 k | 36.038 ± 0.058 b | - |
| ACC Lake (3.3 mg) | 2.74 ± 0.54 a | 0.175 ± 0.01 l | 38.375 ± 0.045 a | −0.06 ± 0.105 d |
| ACC Lake (13.3 mg) | 2.96 ± 0.81 a | 0.524 ± 0.02 k | 32.029 ± 0.068 c | −0.04 ± 0.020 d |
| ACC Lake (33.3 mg) | 0.34 ± 1.21 b | 0.391 ± 0.01 k | 29.249 ± 0.259 d | −0.10 ± 0.030 d |
| IC | - | - | - | 0.14 ± 0.020 a |
| Calcite + IC * | - | - | - | −0.08 ± 0.133 d |
| Aragonite + IC * | - | - | - | 0.07 ± 0.023 b |
| Vaterite + IC * | - | - | - | −0.17 ± 0.035 e |
| ACC + IC * | - | - | - | 0.17 ± 0.017 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, L.; Liu, Y.; Cui, J.; Ma, J.; Yuan, D.; Wang, C. Influence of the Crystal Forms of Calcium Carbonate on the Preparation and Characteristics of Indigo Carmine-Calcium Carbonate Lake. Foods 2024, 13, 2607. https://doi.org/10.3390/foods13162607
Jing L, Liu Y, Cui J, Ma J, Yuan D, Wang C. Influence of the Crystal Forms of Calcium Carbonate on the Preparation and Characteristics of Indigo Carmine-Calcium Carbonate Lake. Foods. 2024; 13(16):2607. https://doi.org/10.3390/foods13162607
Chicago/Turabian StyleJing, Le, Yuhan Liu, Jiaqi Cui, Jinghan Ma, Dongdong Yuan, and Chengtao Wang. 2024. "Influence of the Crystal Forms of Calcium Carbonate on the Preparation and Characteristics of Indigo Carmine-Calcium Carbonate Lake" Foods 13, no. 16: 2607. https://doi.org/10.3390/foods13162607
APA StyleJing, L., Liu, Y., Cui, J., Ma, J., Yuan, D., & Wang, C. (2024). Influence of the Crystal Forms of Calcium Carbonate on the Preparation and Characteristics of Indigo Carmine-Calcium Carbonate Lake. Foods, 13(16), 2607. https://doi.org/10.3390/foods13162607






