Oral Yak Whey Protein Can Alleviate UV-Induced Skin Photoaging and Modulate Gut Microbiota Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Yak Whey Protein (YWP) Preparation
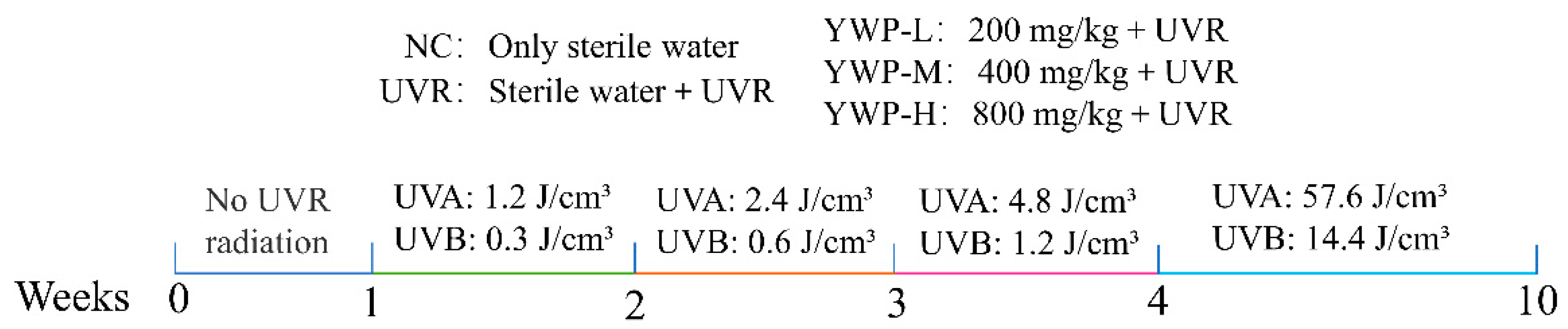
2.3. Animal Model Construction
2.4. HE Staining
2.5. Biochemical Detection
2.6. Western Blotting Analysis
2.7. Real-Time Fluorescence Quantitative PCR (RT-qPCR)
2.8. 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
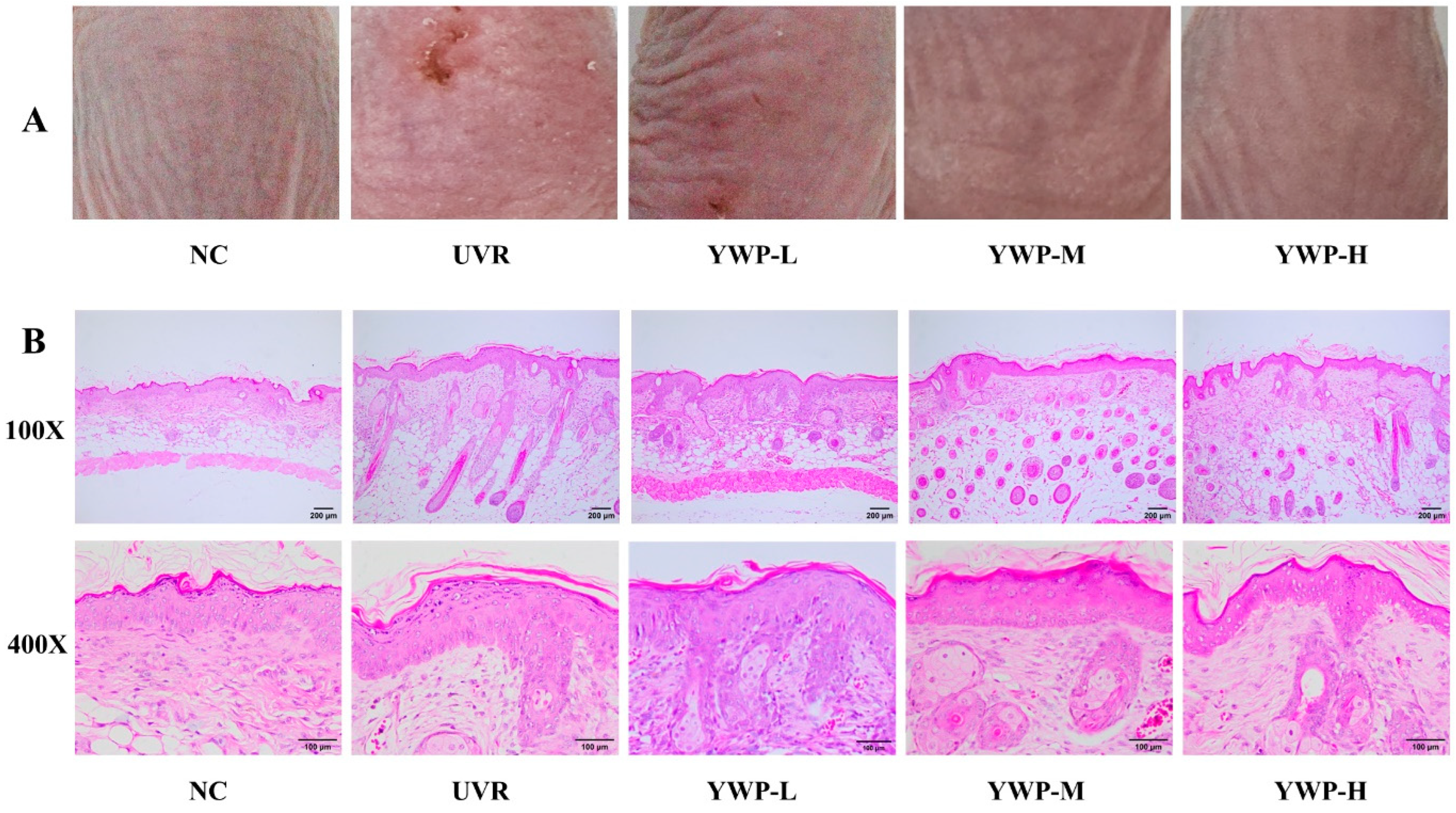
3.1. Macroscopic and Histologic Effects of YWP on Mouse Skin
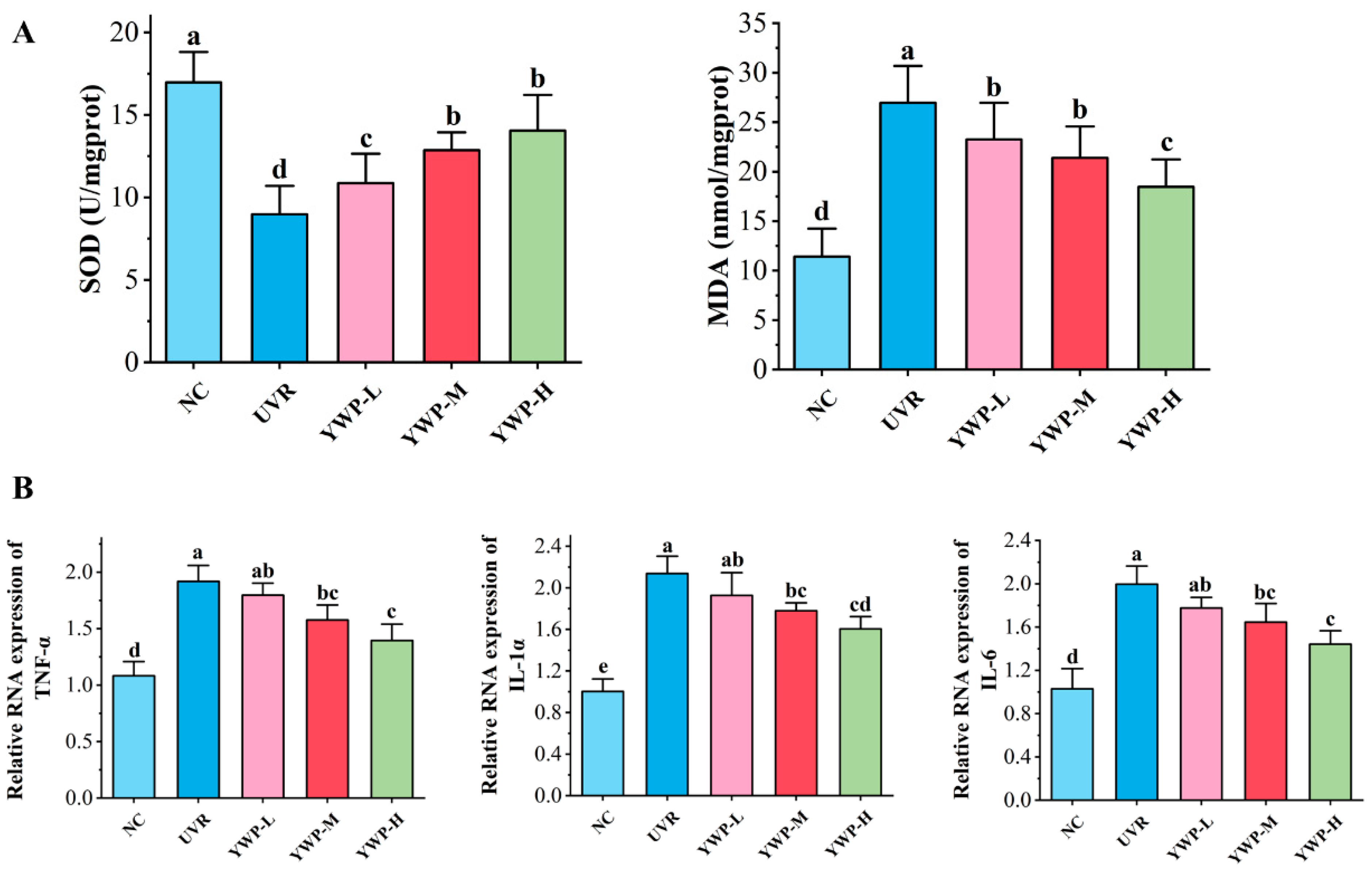
3.2. Effect of YWP on Oxidative Stress and Inflammation Levels in Mouse Skin
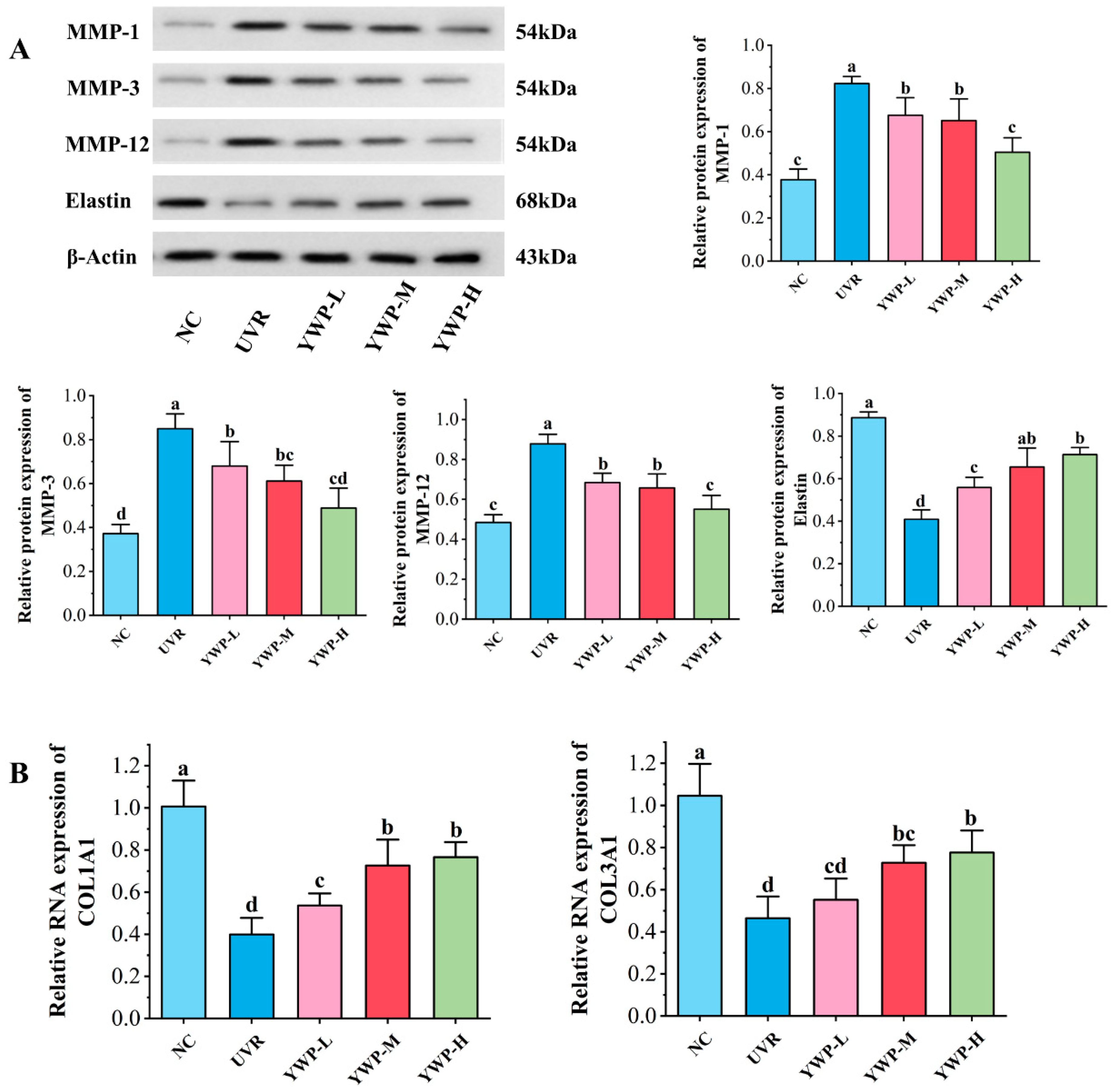
3.3. Effect of YWP on MMPs, Elastin, and Collagen in Mouse Skin
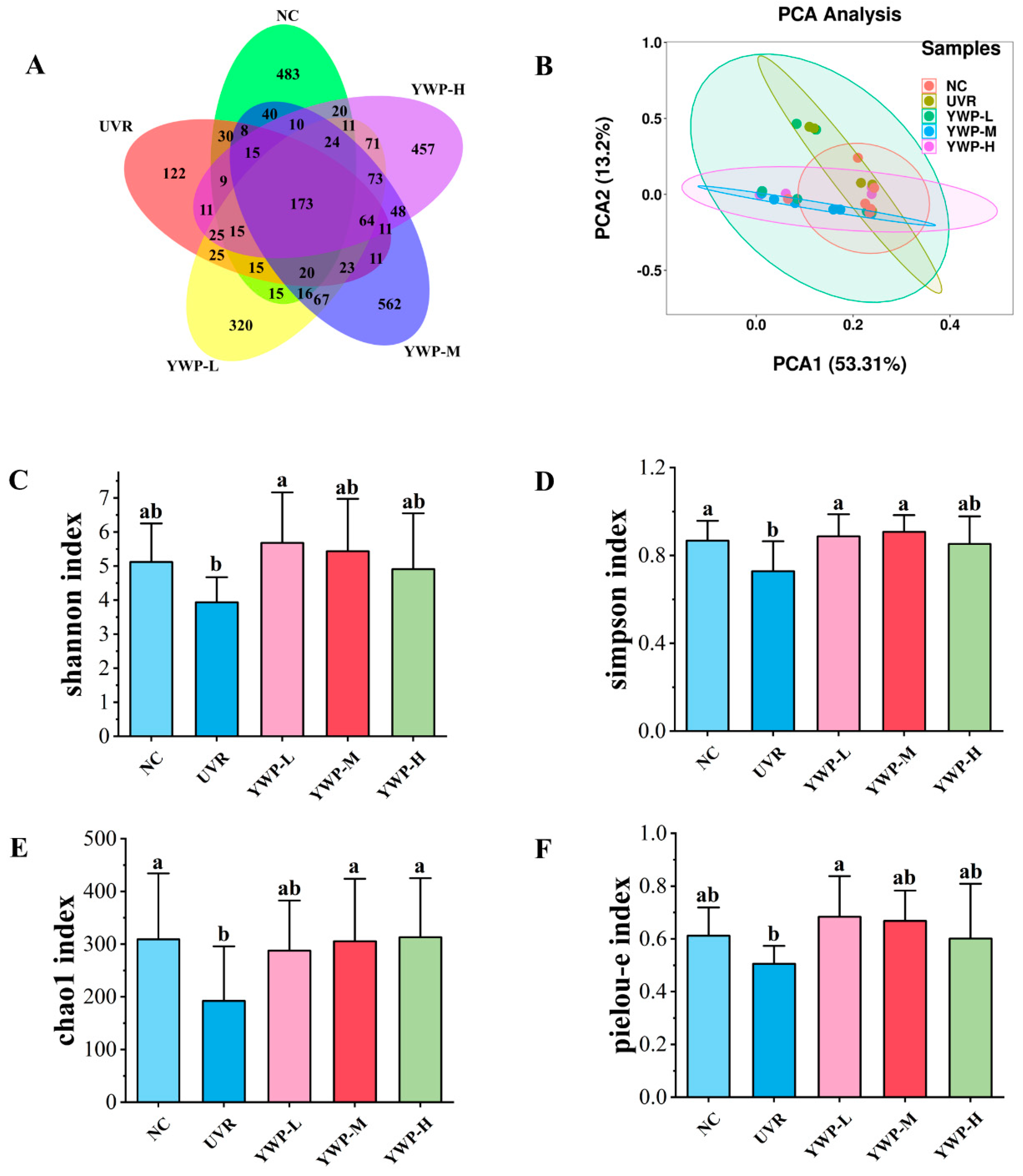
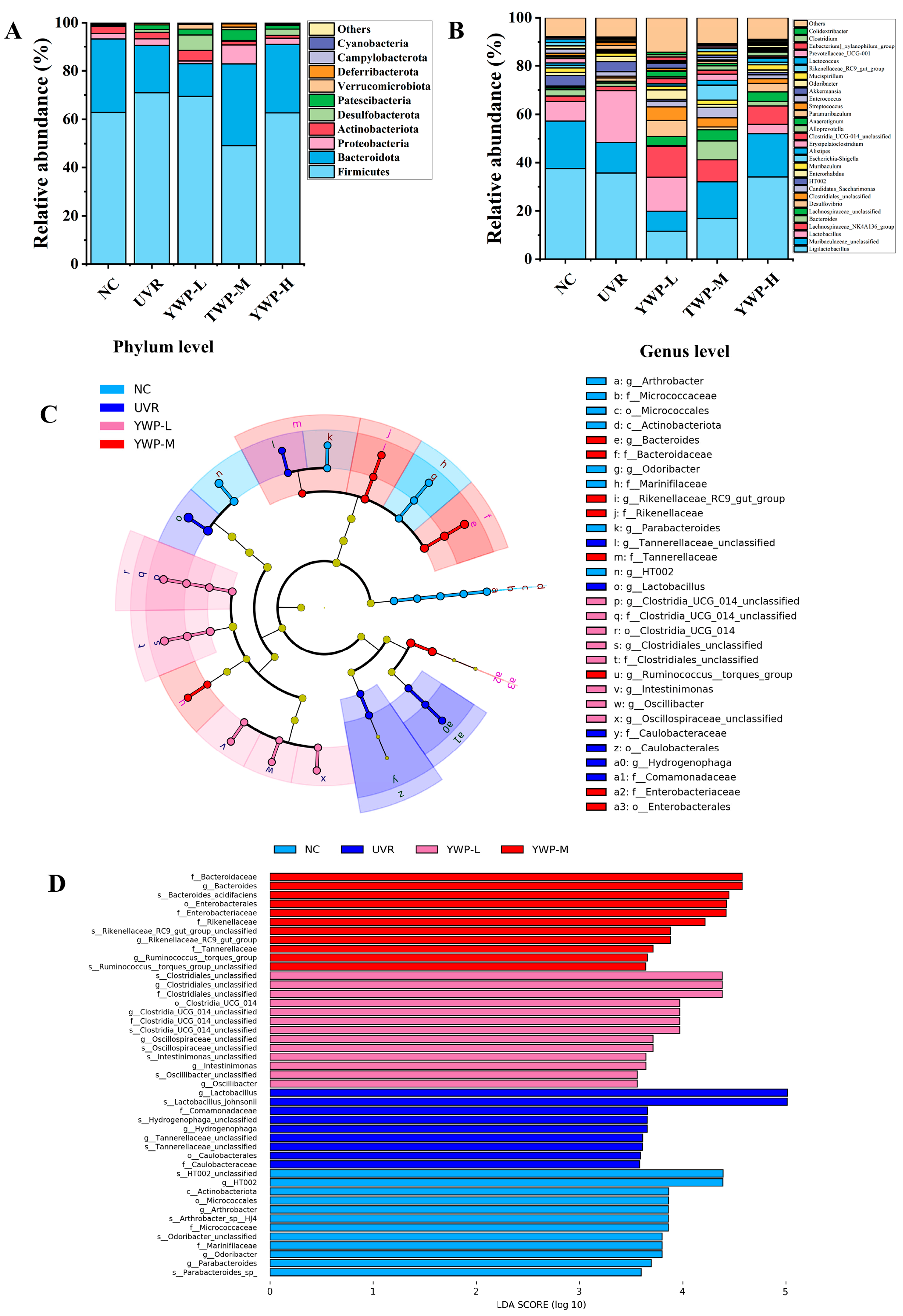
3.4. Effect of YWP on the Structure of Intestinal Flora in Photoaged Mice
4. Discussion
4.1. YWP Ameliorates Clinical Signs and Reduces Oxidation and Inflammation Levels in Skin Tissues in Photoaged Mice
4.2. YWP Promotes Skin Elastin and Collagen Production by Inhibiting the Expression of MMPs
4.3. YWP Exerts Anti-Photoaging Activity by Promoting the Production of Beneficial Intestinal Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, S.H.; Lee, Y.H.; Rho, N.-K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Sample, A.; He, Y.-Y. Autophagy in UV Damage Response. Photochem. Photobiol. 2017, 93, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Xu, X.; Sun, Z.; Wang, F.; Ma, R.; Feng, K.; Li, T.; Sun, L. Protective Effects of Ginseng Proteins on Photoaging of Mouse Fibroblasts Induced by UVA. Photochem. Photobiol. 2020, 96, 113–123. [Google Scholar] [CrossRef]
- de Moura Paiva, J.; de Moura Fernandes Paiva, É.; Lustoza Rodrigues Carolliny Moreira, T.; Messias Monteiro France, A.; de Sousa Ferreira, N.; dos Santos Ferreira Aline, M.; Scotti Tullius, M.; Scotti, L. Targets Involved in Skin Aging and Photoaging and their Possible Inhibitors: A Mini-review. Curr. Drug Targets 2023, 24, 797–815. [Google Scholar] [CrossRef]
- Bang, J.S.; Choung, S.-Y. Inhibitory effect of oyster hydrolysate on wrinkle formation against UVB irradiation in human dermal fibroblast via MAPK/AP-1 and TGFβ/Smad pathway. J. Photochem. Photobiol. B Biol. 2020, 209, 111946. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Zhao, M. Attenuation of UV-induced skin photoaging in rats by walnut protein hydrolysates is linked to the modulation of MAPK/AP-1 and TGF-β/Smad signaling pathways. Food Funct. 2022, 13, 609–623. [Google Scholar] [CrossRef]
- Pereira, M.S.; Redanz, S.; Kriegel, M.A. Skin Deep: The Role of the Microbiota in Cutaneous Autoimmunity. J. Investig. Dermatol. 2022, 142, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yan, S.; Ma, X.; Zhao, J.; Han, Y.; Pan, Y.; Zhao, H. The pivotal role of Bifida Ferment Lysate on reinforcing the skin barrier function and maintaining homeostasis of skin defenses in vitro. J. Cosmet. Dermatol. 2023, 22, 3427–3435. [Google Scholar] [CrossRef]
- Ge, H.; Qi, F.; Shen, Z.; Wang, H.; Zhu, S.; Zhou, S.; Xie, Z.; Li, D. Large-leaf yellow tea protein derived-peptides alleviated dextran sodium sulfate-induced acute colitis and restored intestinal microbiota balance in C57BL/6 J mice. Food Chem. 2024, 456, 139936. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 2021, 18, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gao, G.; Kwok, L.-Y.; Sun, Z. Gut microbiome-targeted therapies for Alzheimer’s disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef]
- Aguwa, C.; Enwereji, N.; Santiago, S.; Hine, A.; Kels, G.G.; McGee, J.; Lu, J. Targeting dysbiosis in psoriasis, atopic dermatitis, and hidradenitis suppurativa: The gut-skin axis and microbiome-directed therapy. Clin. Dermatol. 2023, 41, 640–649. [Google Scholar] [CrossRef]
- Tang, L.; Cao, X.; Chen, S.; Jiang, X.; Li, D.; Chen, G. Dietary Galacto-oligosaccharides Ameliorate Atopic Dermatitis-like Skin Inflammation and Behavioral Deficits by Modulating Gut Microbiota–Brain–Skin Axis. J. Agric. Food Chem. 2024, 72, 7954–7968. [Google Scholar] [CrossRef]
- Zhu, W.; Hamblin, M.R.; Wen, X. Role of the skin microbiota and intestinal microbiome in rosacea. Front. Microbiol. 2023, 14, 1108661. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, Y.; Danfeng, X.; Tao, X.; Fan, Y. The Role of Probiotics in Skin Photoaging and Related Mechanisms: A Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Min, J.-W.; Gye, S.-B.; Kim, Y.-W.; Kang, H.-C.; Choi, Y.-S.; Seo, W.-S.; Lee, B.-Y. Suppression of UVB-Induced MMP-1 Expression in Human Skin Fibroblasts Using Lysate of Lactobacillus iners Derived from Korean Women’s Skin in Their Twenties. Curr. Issues Mol. Biol. 2024, 46, 513–526. [Google Scholar] [CrossRef]
- Song, H.H.; Hong, K.-B.; Kim, S.; Kim, B.-Y.; Hyung Shik, S.; Suh, H.J.; Ahn, Y. Effects of fish collagen on hairless mice skin photoaging induced by ultraviolet irradiation via regulation of the TGF- β signaling pathway: Anti-photoaging effect of fish collagen in UVB-induced hairless mice. J. Funct. Foods 2023, 105, 105554. [Google Scholar] [CrossRef]
- Amer, M.; Farag, F.; Amer, A.; ElKot, R.; Mahmoud, R. Dermapen in the treatment of wrinkles in cigarette smokers and skin aging effectively. J. Cosmet. Dermatol. 2018, 17, 1200–1204. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yao, W.; Chang, S.; You, L.; Zhao, M.; Chi-Keung Cheung, P.; Hileuskaya, K. Structural characterization and anti-photoaging activity of a polysaccharide from Sargassum fusiforme. Food Res. Int. 2022, 157, 111267. [Google Scholar] [CrossRef]
- Maia Campos, P.M.; Franco, R.S.; Kakuda, L.; Cadioli, G.F.; Costa, G.M.; Bouvret, E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Molecules 2021, 26, 4880. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, Y.; Wang, X.; Duan, H.; Guo, J.; Zheng, Y.; Yan, W. Functions of whey proteins and their applications in functional foods. Food Sci. 2024, 1–20. Available online: https://link.cnki.net/urlid/11.2206.ts.20240523.1702.026 (accessed on 23 July 2024).
- Kimura, Y.; Sumiyoshi, M.; Kobayashi, T. Whey Peptides Prevent Chronic Ultraviolet B Radiation–Induced Skin Aging in Melanin-Possessing Male Hairless Mice1, 2, 3. J. Nutr. 2014, 144, 27–32. [Google Scholar] [CrossRef]
- Katayoshi, T.; Kusano, Y.; Shibata, T.; Uchida, K.; Tsuji-Naito, K. Low-molecular-weight whey proteins promote collagen production in dermal fibroblasts via the TGF-β receptor/Smad pathway. Biosci. Biotechnol. Biochem. 2021, 85, 2232–2240. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Zheng, X.; Guo, J.; Duan, H.; Zhou, S.; Yan, W. Yak Milk: Nutritional Value, Functional Activity, and Current Applications. Foods 2023, 12, 2090. [Google Scholar] [CrossRef] [PubMed]
- Ruiping, G.; Qi, L.; Lili, B. Effect of Protein Content on Functional Properties of Yak Whey Protein Concentrate. Food Sci. 2021, 42, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Silkworm pupa protein peptide improved DSS-induced colitis in C57BL/6 mice through the MAPK/NF-κB signaling pathway. J. Funct. Foods 2023, 110, 105852. [Google Scholar] [CrossRef]
- Song, B.; Liu, D.; Liu, T.C.; Li, K.; Wang, S.; Liu, J.; Regenstein, J.M.; Wu, Y.; Zhou, P. The combined effect of commercial tilapia collagen peptides and antioxidants against UV-induced skin photoaging in mice. Food Funct. 2023, 14, 5936–5948. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meng, J.; Chen, S.; Li, C. Integrative analysis of the gut microbiota and metabolome in rats treated with rice straw biochar by 16S rRNA gene sequencing and LC/MS-based metabolomics. Sci. Rep. 2019, 9, 17860. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Walker, M. Human skin through the ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Q.; Xu, Q.; Zheng, L.; Zhang, S.; Lu, S.; Xiao, G.; Zhao, M. Effects of collagen hydrolysates on UV-induced photoaging mice: Gly-Pro-Hyp as a potent anti-photoaging peptide. Food Funct. 2024, 15, 3008–3022. [Google Scholar] [CrossRef]
- Woo, Y.R.; Kim, H.S. Interaction between the microbiota and the skin barrier in aging skin: A comprehensive review. Front. Physiol. 2024, 15, 1322205. [Google Scholar] [CrossRef]
- Yasheng, D.; Siyin, H.; Hui, H.; Tianwei, L.; Yiqing, Z.; Yanping, F.; Jiang, L. Regulating effect of south pearl powder on intestinal flora in skin photoaging mice. Mod. J. Integr. Tradit. Chin. West. Med. 2023, 32, 2675–2686. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, R.; Huang, Y.; Li, W.; Chen, J.; Cheng, Z.; Wu, X.; Diao, Y. The anti-photoaging effect of C-phycocyanin on ultraviolet B-irradiated BALB/c-nu mouse skin. Front. Bioeng. Biotechnol. 2023, 11, 1229387. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Baek, J.; Lee, M.-G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Geng, R.; Kang, S.-G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Abu Kassim, N.H.; Czernuszka, J.; Rahman, M.T. Molecular Mechanisms of Stress-Responsive Changes in Collagen and Elastin Networks in Skin. Ski. Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Taddese, S.; Weiss, A.S.; Jahreis, G.; Neubert, R.H.H.; Schmelzer, C.E.H. In vitro degradation of human tropoelastin by MMP-12 and the generation of matrikines from domain 24. Matrix Biol. 2009, 28, 84–91. [Google Scholar] [CrossRef]
- Fligiel, S.E.G.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Collagen Degradation in Aged/Photodamaged Skin In Vivo and After Exposure to Matrix Metalloproteinase-1 In Vitro. J. Investig. Dermatol. 2003, 120, 842–848. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Tong, H.; Liu, Y.; Wu, Y.; Ge, C. Oral Intake of Chicken Bone Collagen Peptides Anti-Skin Aging in Mice by Regulating Collagen Degradation and Synthesis, Inhibiting Inflammation and Activating Lysosomes. Nutrients 2022, 14, 1622. [Google Scholar] [CrossRef]
- Qu, L.; Ma, X.; Wang, F. The roles of gut microbiome and metabolites associated with skin photoaging in mice by intestinal flora sequencing and metabolomics. Life Sci. 2024, 341, 122487. [Google Scholar] [CrossRef] [PubMed]
- Hyun Mee, K.; Dong Eun, L.; Soo Dong, P.; Yong-Tae, K.; Yu Jin, K.; Ji Woong, J.; Sung Sik, J.; Young-Tae, A.; Jae-Hun, S.; Chul-Sung, H.; et al. Oral Administration of Lactobacillus plantarum HY7714 Protects Hairless Mouse Against Ultraviolet B-Induced Photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Kim, G.; Jang, M.; Hwang, I.; Cho, J.; Kim, S. Radish sprout alleviates DSS-induced colitis via regulation of NF-kB signaling pathway and modifying gut microbiota. Biomed. Pharmacother. 2021, 144, 112365. [Google Scholar] [CrossRef]
- Heiman, M.L.; Greenway, F.L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Wu, C.-Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Kambal, A.; Zale, E.A.; Pierre, J.F.; Hubert, N.; Miyoshi, S.; Miyoshi, J.; Ringus, D.L.; Harris, D.; Yang, K.; et al. High-fat diet disrupts REG3γ and gut microbial rhythms promoting metabolic dysfunction. Cell Host Microbe 2022, 30, 809–823.e806. [Google Scholar] [CrossRef]
- Han, K.; Hong, K.-B.; Ahn, Y.; Jo, K.; Jung, J.; Suh, H.J. Effects of Collagen-Tripeptide and Galacto-oligosaccharide Mixture on Skin Photoaging Inhibition in UVB-exposed Hairless Mice. Photochem. Photobiol. 2022, 98, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Kang, S.-G.; Huang, K.; Tong, T. Dietary Isoeugenol Supplementation Attenuates Chronic UVB-Induced Skin Photoaging and Modulates Gut Microbiota in Mice. Nutrients 2024, 16, 481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, M.; Yang, M.; Jin, C.; Song, Y.; Chen, J.; Gao, M.; Ai, Z.; Su, D. Pulsatilla chinensis Saponins Ameliorate Inflammation and DSS-Induced Ulcerative Colitis in Rats by Regulating the Composition and Diversity of Intestinal Flora. Front. Cell. Infect. Microbiol. 2021, 11, 728929. [Google Scholar] [CrossRef]
- Jin, M.; Kalainy, S.; Baskota, N.; Chiang, D.; Deehan, E.C.; McDougall, C.; Tandon, P.; Martínez, I.; Cervera, C.; Walter, J.J.L.I. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019, 39, 1437–1447. [Google Scholar] [CrossRef]
| Amino Acid Type | Content (g/100 g) | Amino Acid Type | Content (g/100 g) |
|---|---|---|---|
| aspartic acid (Asp) | 9.92 | leucine (Leu) * | 7.98 |
| threonine (Thr) * | 4.24 | tyrosine (Tyr) | 2.56 |
| serine (Ser) | 4.38 | phenylalanine (Phe) * | 2.92 |
| glutamic acid (Glu) | 13.38 | lysine (Lys) * | 7.24 |
| glycine (Gly) | 1.698 | histidine (His) | 2.14 |
| alanine (Ala) | 2.44 | arginine (Arg) | 1.926 |
| valine (Val) * | 3.28 | proline (Pro) | 3.52 |
| methionine (Met) * | 1.178 | essential amino acid | 30.985 |
| isoleucine (Ile) * | 4.12 | total amino acid | 72.922 |
| Gene Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| COL1A1 | GAGAACATCCGCAGCCCCGAAG | CGATCCAGTACTCTCCGCTCT |
| COL3A1 | CCCAACCCAGAGATCCCATT | GGTCACCATTTCTCCCAGGA |
| IL-1α | CCGTGTTGCTGAAGGAGTTG | GTGCACCCGACTTTGTTCTT |
| IL-6 | ACTTCCATCCAGTTGCC | ATGTGTAATTAAGCCTCCGAC |
| TNF-α | ACGGCATGGATCTCAAAGACAAC | AGATAGCAAATCGGCTGACGG |
| β-Actin | CTCCTGAGCGCAAGTACTCT | TACTCCTGCTTGCTGATCCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhou, Y.; Zhao, J.; Ren, C.; Yan, W. Oral Yak Whey Protein Can Alleviate UV-Induced Skin Photoaging and Modulate Gut Microbiota Composition. Foods 2024, 13, 2621. https://doi.org/10.3390/foods13162621
Wang D, Zhou Y, Zhao J, Ren C, Yan W. Oral Yak Whey Protein Can Alleviate UV-Induced Skin Photoaging and Modulate Gut Microbiota Composition. Foods. 2024; 13(16):2621. https://doi.org/10.3390/foods13162621
Chicago/Turabian StyleWang, Diandian, Yaxi Zhou, Jian Zhao, Chao Ren, and Wenjie Yan. 2024. "Oral Yak Whey Protein Can Alleviate UV-Induced Skin Photoaging and Modulate Gut Microbiota Composition" Foods 13, no. 16: 2621. https://doi.org/10.3390/foods13162621






